Open Access Article
Open Access ArticleSynthesis of 3-aryl-2-phosphinoimidazo[1,2-a]pyridine ligands for use in palladium-catalyzed cross-coupling reactions†
Ryan Q. Tran,
Seth A. Jacoby,
Kaitlyn E. Roberts,
William A. Swann,
Nekoda W. Harris,
Long P. Dinh ,
Emily L. Denison and
Larry Yet
,
Emily L. Denison and
Larry Yet *
*
University of South Alabama, Department of Chemistry, Mobile, AL 36618, USA. E-mail: lyet@southalabama.edu
First published on 5th June 2019
Abstract
3-Aryl-2-phosphinoimidazo[1,2-a]pyridine ligands were synthesized from 2-aminopyridine via two complementary routes. The first synthetic route involves the copper-catalyzed iodine-mediated cyclizations of 2-aminopyridine with arylacetylenes followed by palladium-catalyzed cross-coupling reactions with phosphines. The second synthetic route requires the preparation of 2,3-diiodoimidazo[1,2-a]pyridine or 2-iodo-3-bromoimidazo[1,2-a]pyridine from 2-aminopyridine followed by palladium-catalyzed Suzuki/phosphination or a phosphination/Suzuki cross-coupling reactions sequence, respectively. Preliminary model studies on the Suzuki synthesis of sterically-hindered biaryl and Buchwald–Hartwig amination compounds are presented with these ligands.
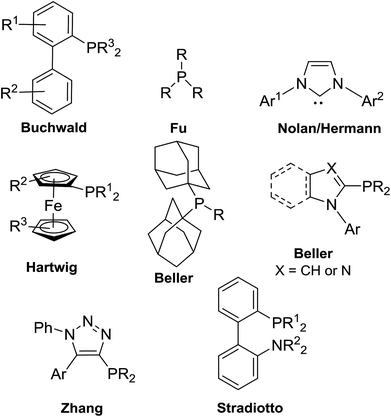
Palladium-catalyzed cross-coupling reactions have revolutionized the formation of C–C and C–X bond formation in the academic and industrial synthetic organic chemistry sectors.1,2 Applications such as synthesis of natural products,3 active pharmaceutical ingredients (API),4 agrochemicals,5 and materials for electronic applications6 are showcased. Snieckus described in his 2010 Nobel Prize review that privileged ligand scaffolds represented the “third wave” in the cross-coupling reactions where the “first wave” was the investigation of the metal catalyst-the rise of palladium and the “second wave” was the exploration of the organometallic coupling partner.1 In the last twenty years, it was recognized that the choice of ligand facilitated the oxidative addition and reductive-elimination steps of the catalytic cycle of transition metal-catalyzed cross-coupling reactions, increasing the overall rate of the reaction. For example, bulky trialkylphosphines facilitated the oxidative addition processes of electron-rich, unactivated substrates such as aryl chlorides.7,8 Sterically demanding ligands also provided enhanced rates of reductive elimination from [(L)nPd(aryl)(R), R = aryl, amido, phenoxo, etc.] species by alleviation of steric congestion.9 Privileged ligands such as Buchwald's biarylphosphines,10,11 Fu's trialkylphosphines,7,8,12 Nolan–Hermann's N-heterocyclic carbenes (NHC),13–15 Hartwig's ferrocenes,16,17 Beller's bis(adamantyl)phosphines18,19 and N-aryl(benz)imidazolyl or N-pyrrolylphosphines,20,21 Zhang's ClickPhos ligands,22,23 and Stradiotto's biaryl P–N phosphines,24,25 to mention a few, have found wide-spread use in Suzuki–Miyaura, Corriu–Kumada, Heck, Negishi, Sonogashira, C–X (X = S, O, P) cross-coupling and Buchwald–Hartwig amination reactions (Fig. 1). Preformed catalysts with these ligands attached to the palladium metal center are also recognized as well-defined entities in cross-coupling reactions.26
The term privileged structure was first coined by Evans et al. in 1988 and was defined as “a single molecular framework able to provide ligands for diverse receptors”.27 In the last three decades, it is clear that privileged structures are exploited as opportunities in drug discovery programs.28–31 For example, imidazo[1,2-a]pyridines are privileged structures in medicinal chemistry programs (Fig. 2).32 Imidazo[1,2-a]pyridines are a represented motif in several drugs on the market such as zolpidem, marketed as Ambien™ for the treatment of insomnia,33 minodronic acid, marketed as Bonoteo™ for oral treatment of osteoporosis,34 and olprinone, sold as Coretec™ as a cardiotonic agent.35
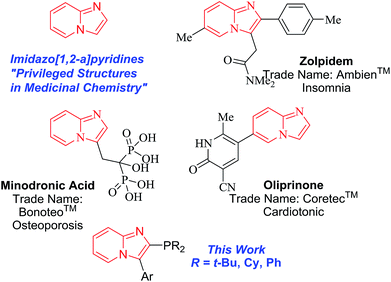 | ||
| Fig. 2 Imidazo[1,2-a]pyridines as privileged structures in medicinal chemistry and in our cross-coupling reactions approach. | ||
Our group is interested in a long-term research program directed at the use of key privileged structures that are employed in drug discovery programs as potential phosphorus ligands for cross-coupling reactions. In our entry into the use of privileged structures from the medicinal chemistry literature for our investigation into new phosphorus ligands, we have developed two complementary synthetic routes for the preparation of 3-aryl-2-phosphinoimidazo[1,2-a]pyridine ligands from 2-aminopyridine as our initial substrate.
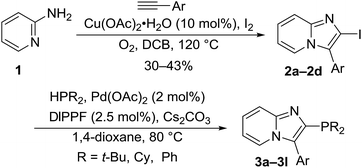
Our first synthetic route for the preparation of 3-aryl-2-phosphinoimidazo[1,2-a]pyridine ligands 3a–3l required the copper(II) acetate iodine-mediated double oxidative C–H amination of 2-aminopyridine (1) with arylacetylenes under an oxygen atmosphere to give 3-aryl-2-iodoimidazo[1,2-a]pyridines 2a–2d (Scheme 1).36,37
Phenylacetylene and 2-/3-/4-methoxyphenylacetylenes were commercially available reagents. With intermediates 2a–d in hand, we explored several cross-coupling phosphination reactions and we found that palladium-catalyzed phosphination with DIPPF ligand in the presence of cesium carbonate as the base in 1,4-dioxane under reflux provided twelve new ligands 3a–3l as shown in Table 1.38 Moderate to good yields were obtained under these cross-coupling conditions. There are few commercially available dimethoxyphenylacetylenes, and most are prohibitively expensive, and so an alternative synthetic strategy was explored.
| Entry | Ar | R | 3 (% yield) |
|---|---|---|---|
| a Reaction conditions: 2a–2d (1 equiv.), HPR2 (1 equiv.), Pd(OAc)2 (2 mol%), Cs2CO3 (1.2 equiv.), DIPPF (2.5 mol%), 1,4-dioxane, 80 °C. | |||
| 1 | Ph (2a) | t-Bu | 3a (41) |
| 2 | Ph (2a) | Cy | 3b (50) |
| 3 | Ph (2a) | Ph | 3c (61) |
| 4 | 2-OMeC6H4 (2b) | t-Bu | 3d (53) |
| 5 | 2-OMeC6H4 (2b) | Cy | 3e (83) |
| 6 | 2-OMeC6H4 (2b) | Ph | 3f (69) |
| 7 | 3-OMeC6H4 (2c) | t-Bu | 3g (62) |
| 8 | 3-OMeC6H4 (2c) | Cy | 3h (72) |
| 9 | 3-OMeC6H4 (2c) | Ph | 3i (79) |
| 10 | 4-OMeC6H4 (2d) | t-Bu | 3j (73) |
| 11 | 4-OMeC6H4 (2d) | Cy | 3k (55) |
| 12 | 4-OMeC6H4 (2d) | Ph | 3l (59) |
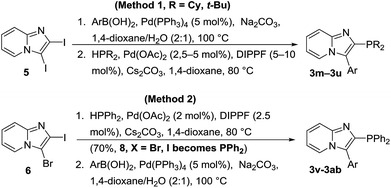
2-Iodoimidazo[1,2-a]pyridine (4) was conveniently prepared in three steps from 2-aminopyridine (1) following literature procedures, which was then converted into either iodo 5 or bromo 6 with NIS or NBS, respectively (Scheme 2).39,40
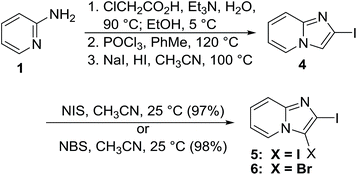 | ||
| Scheme 2 Preparation of 2,3-diiodoimidazo[1,2-a]pyridine (5) and 3-bromo-2-iodoimidazo[1,2-a]pyridine (6). | ||
When the phosphorus ligands 3 contained tert-butyl or cyclohexyl groups, method 1 was followed where 2,3-diiodoimidazo[1,2-a]pyridine (5) underwent Suzuki cross-coupling reactions with arylboronic acids to yield aryl intermediates 7a–7f, which was followed by palladium-catalyzed cross-coupling phosphination reactions with di-tert-butylphosphine or dicyclohexylphosphine to give C-2 substituted phosphorus ligands 3m–3u in low to moderate yields (Scheme 3, Table 2).38 The phosphorus ligands 3v–3ab were prepared from 3-bromo-2-iodoimidazo[1,2-a]pyridine (6) via a palladium-catalyzed phospination with diphenylphosphine (method 2) to give intermediate 8 (X = Br, I becomes PPh2) followed by Suzuki palladium-catalyzed cross-coupling reactions with arylboronic acids. Note that the change in reactivity of the core when switching between bromo and iodo at C3 results in a change in the order of cross-coupling steps.
| Entry | R | Ar | Method/substrate | Step 1 (% yield) | Step 2 (% yield) |
|---|---|---|---|---|---|
a Reaction conditions: 5, ArB(OH)2, Pd(PPh3)4 (5 mol%), Na2CO3 (2 equiv.), 1,4-dioxane/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and HPR2 (1 equiv.), Pd(OAc)2 (2.5–5 mol%), Cs2CO3 (1.2 equiv.), DIPPF (2.5–10 mol%), 1,4-dioxane, 80 °C or 6, reverse sequence of reactions. 1) and HPR2 (1 equiv.), Pd(OAc)2 (2.5–5 mol%), Cs2CO3 (1.2 equiv.), DIPPF (2.5–10 mol%), 1,4-dioxane, 80 °C or 6, reverse sequence of reactions. |
|||||
| 1 | t-Bu | 2,3-diOMeC6H3 | 1, 5 | 7a (59) | 3m (64) |
| 2 | t-Bu | 3,4-diOMeC6H3 | 1, 5 | 7b (54) | 3n (31) |
| 3 | t-Bu | 2,5-diOMeC6H3 | 1, 5 | 7c (58) | 3o (61) |
| 4 | t-Bu | 3,4,5-triOMeC6H2 | 1, 5 | 7d (50) | 3p (62) |
| 5 | Cy | 2,3-diOMeC6H3 | 1, 5 | 7a (59) | 3q (46) |
| 6 | Cy | 2,6-diOMeC6H3 | 1, 5 | 7e (40) | 3r (52) |
| 7 | Cy | 3,4-diOMeC6H3 | 1, 5 | 7b (54) | 3s (52) |
| 8 | Cy | 2,3,4-triOMeC6H2 | 1, 5 | 7f (58) | 3t (21) |
| 9 | Cy | 3,4,5-triOMeC6H2 | 1, 5 | 7d (50) | 3u (55) |
| 10 | Ph | 2,3-diOMeC6H3 | 2, 6 | 8 (70) | 3v (52) |
| 11 | Ph | 2,5-diOMeC6H3 | 2, 6 | 8 (70) | 3w (68) |
| 12 | Ph | 3,4-diOMeC6H3 | 2, 6 | 8 (70) | 3x (67) |
| 13 | Ph | 2,3,4-triOMeC6H2 | 2, 6 | 8 (70) | 3y (52) |
| 14 | Ph | 3,4,5-triOMeC6H2 | 2, 6 | 8 (70) | 3z (64) |
| 15 | Ph | 4-FC6H4 | 2, 6 | 8 (70) | 3aa (40) |
| 16 | Ph | 3-F,5-OMeC6H3 | 2, 6 | 8 (70) | 3ab (39) |
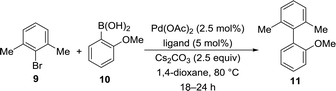
With our library of functionalized imidazo[1,2-a]pyridine phosphorus ligands 3a–3ab in hand, we began to screen these ligands in Suzuki–Miyaura cross-coupling reactions to prepare sterically-hindered biaryl compounds. We chose the Suzuki–Miyaura cross-coupling reactions of m-bromo-xylene (9) and 2-methoxyphenylboronic acid (10) to give 2,6-dimethyl-(2-methoxy)biphenyl (11) as our model reaction as outlined in Table 3. Our initial screening conditions included 5.0 mol% ligand, 2.5 mol% palladium(II) acetate with 2.5 equivalents of base in 1,4-dioxane at 80 °C for 12–24 h. As expected, SPhos and XPhos were employed as our initial ligands to confirm our GC analyses of >99% conversion in our chosen model reaction (Entries 14–15). With the GC conditions validated, we screened selected ligands from 3a–3ab. It was clearly evident that the di-tert-butyl phosphorus ligands represented by 3a, 3m, and 3p were ineffective ligands in our model reactions (Entries 1–3). Furthermore, the diphenyl phosphorus ligands such as 3w, 3y, 3z, and 3ab showed low to moderate conversions in the model cross-coupling reactions (Entries 6–9). However, the dicyclohexyl phosphorus ligands shown by 3r and 3t showed greater than 99% conversions by GC analyses (Entries 4–5). Further exploration of ligand 3r with K3PO4 as the base, stirring the reaction overnight at room temperature or for 3 h at 80 °C showed inferior conversions (Entries 10–12). There was no conversion when a ligand was not used in the model reaction (Entry 13).
| Entry | Ligand | Conditions | Conversiona (%) |
|---|---|---|---|
| a Based on GC analyses of consumed 9.b Isolated yield of 96% was obtaisned. | |||
| 1 | 3a | 12 | |
| 2 | 3m | 20 | |
| 3 | 3p | 14 | |
| 4 | 3r | >99b | |
| 5 | 3t | >99 | |
| 6 | 3w | 21 | |
| 7 | 3y | 55 | |
| 8 | 3z | 46 | |
| 9 | 3ab | 11 | |
| 10 | 3r | K3PO4 was used as base reaction was performed at 25 °C reaction was stirred for 3 h no ligand | 91 |
| 11 | 3r | 4 | |
| 12 | 3r | 39 | |
| 13 | — | 0 | |
| 14 | SPhos | >99 | |
| 15 | XPhos | >99 | |
Furthermore, a Buchwald–Hartwig amination model study was investigated with our new imidazo[1,2-a]pyridine phosphorus ligands 3a–3ab. The Buchwald–Hartwig amination reaction of 4-chlorotoluene (12) with aniline (13) to give 4-methyl-N-phenylaniline (14) was screened with our ligands (Table 4). Our screening conditions were exactly as used in the optimization of the Suzuki cross-coupling reactions of m-bromo-xylene (9) and 2-methoxyphenylboronic acid (10) to give 2,6-dimethyl-(2-methoxy)biphenyl (11). Tert-butyl phosphine ligands 3a, 3d, 3g, 3n, and 3p were all ineffective in the amination reactions (Entries 1–2, 4, 7–8). However, as expected, the dicyclohexyl phosphorus ligands 3e, 3q, and 3s showed >99% conversion (Entries 3, 9, and 11) in the model screening reaction conditions. Phosphorus ligand 3s were screening against other bases such as K3PO4, K2CO3, KOt-Bu, and NaOt-Bu (Entries 12–15) where all gave >99% conversions except for K2CO3 which was ineffective. Finally, ligands 3h and 3k showed moderate conversions (Entries 5–6).
| Entry | Ligand | Conditions | Conversiona (%) |
|---|---|---|---|
| a Based on GC analyses of consumed 13.b Isolated yield of 76% was obtained. | |||
| 1 | 3a | 38 | |
| 2 | 3d | 26 | |
| 3 | 3e | >99b | |
| 4 | 3g | 29 | |
| 5 | 3h | 54 | |
| 6 | 3k | 71 | |
| 7 | 3n | 0 | |
| 8 | 3p | 0 | |
| 9 | 3q | >99 | |
| 10 | 3r | 92 | |
| 11 | 3s | >99 | |
| 12 | 3s | K3PO4 was used as base | 83 |
| 13 | 3s | K2CO3 was used as base | 0 |
| 14 | 3s | KOt-Bu was used as base | >99 |
| 15 | 3s | NaOt-Bu was used as base | >99 |
In summary, we have disclosed two complementary synthetic routes to 3-aryl-2-phosphinoimidazo[1,2-a]pyridine ligands 3a–3ab from 2-aminopyridine (1). In one method, 2-aminopyridine (1) underwent a copper-catalyzed iodine-mediated cyclization with arylacetylenes followed by palladium-catalyzed cross-coupling reactions with phosphines. In the second protocol, 2,3-diiodoimidazo[1,2-a]pyridine (5) or 3-bromo-2-iodoimidazo[1,2-a]pyridine (6) were prepared from 2-aminopyridine (1) followed by palladium-catalyzed phosphination/Suzuki or Suzuki/phosphination reactions sequences, respectively. We are currently exploring the scope and limitations of the 3-aryl-2-phosphinoimidazo[1,2-a]pyridine ligand 3r and 3e in our Suzuki–Miyaura and Buchwald–Hartwig amination cross-coupling reactions, respectively.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the University of South Alabama, Chemistry Department for financial support.Notes and references
- C. C. C. J. Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CrossRef PubMed.
- Palladium-Catalyzed Coupling Reactions: Practical Aspects and Future Development, ed. A. Molnar, Wiley-VCH, Weinheim, Germany, 2013 Search PubMed.
- K. C. Nicolaou, P. G. Bulger and D. Sariah, Angew. Chem., Int. Ed., 2005, 44, 4442–4489 CrossRef CAS PubMed.
- J. Magano and J. R. Dunetz, Chem. Rev., 2011, 111, 2177–2250 CrossRef CAS PubMed.
- C. Torborg and M. Beller, Adv. Synth. Catal., 2009, 351, 3027–3043 CrossRef CAS.
- F. Naso, F. Babudri and G. M. Farinola, Pure Appl. Chem., 1999, 71, 1485–1492 CAS.
- A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 2002, 41, 4176–4211 CrossRef CAS.
- C. A. Fleckenstein and H. Plenio, Chem. Soc. Rev., 2010, 39, 694–711 RSC.
- U. Christmann and R. Vilar, Angew. Chem., Int. Ed., 2005, 44, 366–374 CrossRef CAS.
- R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461–1473 CrossRef CAS.
- D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 6338–6361 CrossRef CAS PubMed.
- G. C. Fu, Acc. Chem. Res., 2008, 41, 1555–1564 CrossRef CAS PubMed.
- N. Marion and S. P. Nolan, Acc. Chem. Res., 2008, 41, 1440–1449 CrossRef CAS.
- W. A. Herrmann, Angew. Chem., Int. Ed., 2002, 41, 1290–1309 CrossRef CAS.
- G. C. Fortman and S. P. Nolan, Chem. Soc. Rev., 2011, 40, 5151–5169 RSC.
- J. F. Hartwig, Acc. Chem. Res., 2008, 41, 1534–1544 CrossRef CAS PubMed.
- A. Fihri, P. Meunier and J.-C. Hierso, Coord. Chem. Rev., 2007, 251, 2017–2055 CrossRef CAS.
- S. Klaus, H. Neumann, A. Zapf, D. Strubing, S. Hubner, J. Almena, T. Riermeier, P. Groß, M. Sarich, W.-R. Krahnert, K. Rossen and M. Beller, Angew. Chem., Int. Ed., 2006, 45, 154–158 CrossRef CAS PubMed.
- A. Zapf, A. Ehrentraut and M. Beller, Angew. Chem., Int. Ed., 2000, 39, 4153–4155 CrossRef CAS PubMed.
- S. Harkal, F. Rataboul, A. Zapf, C. Fuhrmann, T. Riermeier, A. Monsees and M. Beller, Adv. Synth. Catal., 2004, 346, 1742–1748 CrossRef CAS.
- A. Zapf, R. Jackstell, F. Rataboul, T. Riermeier, A. Monsees, C. Fuhrmann, N. Shaikh, U. Dingerdissen and M. Beller, Chem. Commun., 2004, 38–39 RSC.
- Q. Dai, W. Gao, D. Liu, L. M. Kapes and X. Zhang, J. Org. Chem., 2010, 71, 3928–3934 CrossRef PubMed.
- D. Liu, W. Gao, Q. Dai and X. Zhang, Org. Lett., 2005, 7, 4907–4910 CrossRef CAS PubMed.
- R. J. Lundgren, B. D. Peters, P. G. Alsabeh and M. Stradiotto, Angew. Chem., Int. Ed., 2010, 49, 4071–4074 CrossRef CAS.
- R. J. Lundgren, A. Sappong-Kumankumah and M. Stradiotto, Chem.–Eur. J., 2010, 16, 1983–1991 CrossRef CAS.
- H. Li, C. C. C. J. Seechurn and T. J. Colacot, ACS Catal., 2012, 2, 1147–1164 CrossRef CAS.
- M. G. Bock, R. M. DiPardo, B. E. Evans, K. E. Rittle, R. M. Freidinger, R. S. L. Chang and V. J. Lotti, J. Med. Chem., 1988, 31, 264 CrossRef CAS.
- L. Costantino and D. Barlocco, Curr. Med. Chem., 2006, 13, 65 CrossRef CAS.
- R. W. DeSimone, K. S. Currie, S. A. Mitchell, J. W. Darrow and D. A. Pippin, Comb. Chem. High Throughput Screening, 2004, 7, 473 CrossRef CAS.
- S. Bongarzone and M. L. Bolognesi, Expert Opin. Drug Discovery, 2011, 6, 251 CrossRef CAS PubMed.
- L. Yet, Privileged Structures in Drug Discovery-Medicinal Chemistry and Synthesis, Wiley & Sons, Hoboken, NJ, 2018 Search PubMed.
- C. Enguehard-Gueiffier and A. Gueiffier, Mini-Rev. Med. Chem., 2007, 7, 888 CrossRef CAS.
- D. J. Sanger and H. Depoortere, CNS Drug Rev., 1998, 4, 323–340 CrossRef CAS.
- T. Makoto, M. Hiroshi, K. Ryoji, O. Yasuo, K. Naoki, Y. Hiroyuki and K. Katsuya, Bone, 2008, 43, 894–900 CrossRef.
- D. Spina, Drugs, 2003, 63, 2575–2594 CrossRef CAS.
- D. Dheer, K. R. Reddy, S. K. Rath, P. L. Sangwan, P. Das and R. Shankar, RSC Adv., 2016, 6, 38033–38036 RSC.
- S. Samanta, S. Jana, S. Mondal, K. Monir, S. K. Chandra and A. Hajra, Org. Biomol. Chem., 2016, 14, 5073–5078 RSC.
- M. Murata and S. L. Buchwald, Tetrahedron, 2004, 60, 7397–7403 CrossRef CAS.
- S. Marhadour, M.-A. Bazin and P. Marchand, Tetrahedron Lett., 2012, 53, 297–300 CrossRef CAS.
- Heterocyclic derivatives as metabotropic glutamate receptor modulators, EP2650284, Merz Pharma GmbH & Co. KGaA, 2013, p. 30.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra02200g |
| This journal is © The Royal Society of Chemistry 2019 |