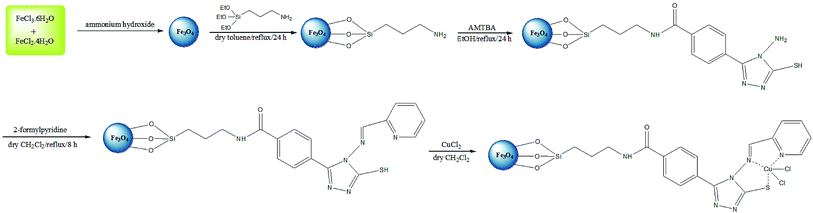
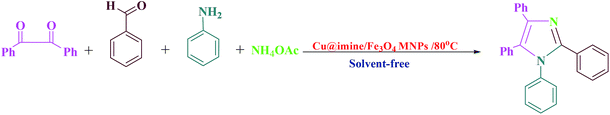
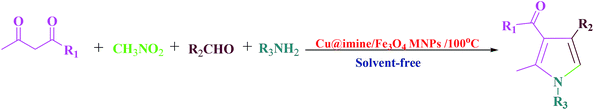Open Access Article
Open Access ArticleAn efficient and recyclable nanocatalyst for the green and rapid synthesis of biologically active polysubstituted pyrroles and 1,2,4,5-tetrasubstituted imidazole derivatives†
Myo Thwin a,
Boshra Mahmoudib,
Olga A. Ivaschukc and
Qahtan A. Yousif*d
a,
Boshra Mahmoudib,
Olga A. Ivaschukc and
Qahtan A. Yousif*d
aYangon Technological University, Myanmar
bResearch Center, Sulaimani Polytechnic University, Sulaimani 46001, Kurdistan Region, Iraq
cBelgorod National Research University, Belgorod, Russia
dUniversity of Al-Qadisiyah, College of Science, Department of Chemistry, Republic of Iraq. E-mail: Qahtan.Adnan@qu.edu.iq; Tel: +9647813112362
First published on 21st May 2019
Abstract
An effective process for the green and rapid synthesis of biologically active polysubstituted pyrroles and 1,2,4,5-tetrasubstituted imidazoles derivatives using Cu@imine/Fe3O4 MNPs catalyst under solvent-free conditions is explained. This catalyst showed high reactivity for the synthesis of a set of different derivatives of polysubstituted pyrroles and 1,2,4,5-tetrasubstituted imidazole derivatives under appropriate reaction conditions and short times. Moreover, the catalyst was also recycled and reused for six runs with no considerable reduction in reactivity and yields. Compared to the reported procedures, this method consistently demonstrates the advantages of low catalyst loading, short reaction times, easy separation and purification of the products, high yields, and high recoverability and recoverability of the catalyst.
Introduction
Recently, studies for finding separable and reusable nanocatalysts are seeing rapid progress in chemistry, especially in modern catalysis research. The immobilization of organic and inorganic functional groups on heterogeneous supports is in fact an effective process to achieve this goal.1 Nowadays, compared to the typical separation, magnetic separation is receiving more attention due to activity as a highly efficient and rapid separation procedure for products and catalysts.2 Magnetic nanoparticles (MNPs) have unique physical and chemical features such as large surface area per weight that causes these materials to act as supports of homogeneous catalysts or heterogeneous promoters for catalytic reactions. The functionalized magnetic nanoparticles can be simply isolated from the reaction solution by an appropriate external magnetic field, without any tedious workup procedures, which reduces the catalyst loss during separation.3,4 In addition, magnetic-supported catalysts can be recovered several times with no considerable reduction in their initial activity. High coercivity, greater selectivity, environmental friendliness, noncorrosive nature, operational simplicity, moisture insensitivity, and low toxicity are the other factors that have attracted more attention of scientists to these nanoscale materials.5–9Poly substituted imidazoles are one of the core structural skeleton in many key molecules that have attracted considerable interest because of their biological activities such as histidine, histamine, and biotin.10–12 These were firstly prepared by via cyclocondensation reaction between 1,2-dicarbonyl compounds, different aldehydes, and a nitrogen resource.13,14 A variety of catalysts in the papers following this method have been reported.15–22 Although most of these synthetic approaches provide an improvement in the production of the above mentioned heterocyclic compounds, many of these procedures suffer from one or more drawbacks like applying dangerous organic solvents and costly reagents, harsh reaction conditions, difficult and complex work-up and purification, considerable values of waste ingredients, use of toxic reagents and non-recoverability of the catalyst as well as long reaction time and low efficiencies.23,24 Consequently, the improvement of easy, effective, and mild procedures with easily separable and reusable novel catalysts to overcome these shortcomings.
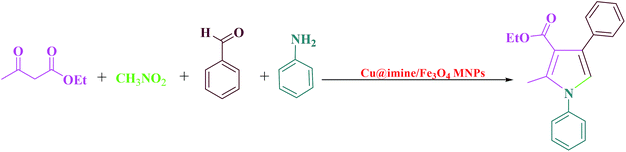
Pyrroles, as an important class of N-heterocyclic derivatives and significant building blocks, are one of the most important groups of heterocyclic compounds that have attracted considerable interest in organic production as well as in the drug exploration. They have been extensively utilized due to their promising pharmacological and therapeutic activities such as antianginal, antitumor antifungal, anti-oxidant, antibacterial, and anti-inflammatory.25 Moreover, polysubstituted pyrroles owing to their enormous importance exhibited other medicinal applications. Because of the aforementioned merits of the polysubstituted pyrroles, to date various approaches have been improved to synthesize these kinds of heterocyclic compounds. These were synthesized through Paal–Knorr condensation reaction, Knorr reaction and Hantzsch reaction.26 Herein we decided to study the preparation of polysubstituted pyrrole derivatives with various substituents from the reaction of aromatic aldehydes, ethyl acetoacetate, nitromethane and aniline under solvent-free conditions using Cu@imine/Fe3O4 MNPs as a new, eco-friendly, reusable and promising heterogeneous nanocatalyst. Although numerous methods have been utilized for the preparation of polysubstituted pyrrole derivatives, some of these strategies suffer from disadvantages such as use of high temperature, the need for excess amounts of the catalyst, long reaction times, and need for microwave or ultrasound irradiation. Therefore, it is necessary to develop an improved strategy for the preparation of polysubstituted pyrrole derivatives under mild reaction conditions.
Experimental
General
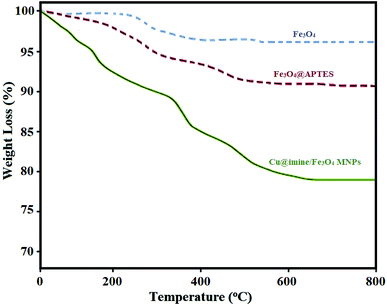
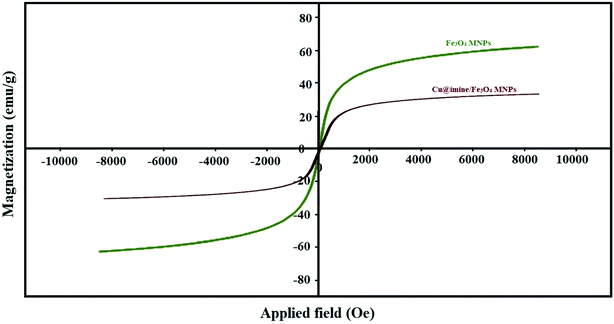
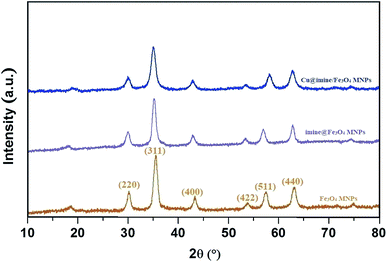
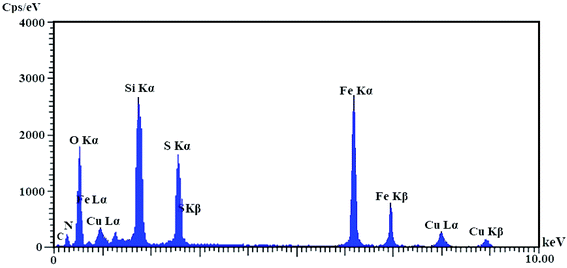
All the pure chemical substances were prepared from Fluka, Aldrich, and Merck chemical companies and applied with no further purification. Melting points of the substrate were measured with Electrothermal-9100 apparatus and uncorrected. Fourier transform infrared (FT-IR) spectra were recorded with a PerkinElmer PXI spectrometer in KBr wafers. X-ray diffraction (XRD) patterns of samples were taken on a Siemens D-5000 Xray diffractometer (Germany) with Cu Kα radiation. Magnetic susceptibility measurements were measured by a vibrating sample magnetometry (VSM; Lake Shore 7200 at 300 kVsm). Thermogravimetric analysis was examined by a PerkinElmer instrument under nitrogen atmosphere at a heating rate of 10 °C min−1. Scanning electron microscope (SEM) images were recorded with a SEM-LEO 1430VP instrument. The chemical composition of synthesized nanoparticles was obtained with an energy dispersive X-ray spectroscopy (EDX) (ESEM, Philips, and XL30).Catalyst synthesis
Results and discussion
Catalyst characterization
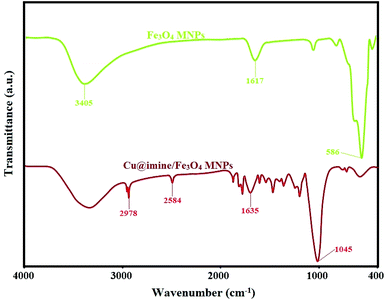
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and Si–O groups. The bands at 1635, 2584 and 2987 cm−1 have been attributed to C
C and Si–O groups. The bands at 1635, 2584 and 2987 cm−1 have been attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, S–H and C–H stretching vibrations, respectively.
N, S–H and C–H stretching vibrations, respectively.
This survey is aimed to report the efficient and rapid production of polysubstituted pyrrole and 1,2,4,5-tetrasubstituted imidazoles derivatives using lower loading of the very recently introduced nanocatalyst, i.e., Cu@imine/Fe3O4 MNPs, under solvent-free conditions. The catalytic activity of Cu@imine/Fe3O4 MNPs was investigating to report the efficient and rapid production of polysubstituted pyrrole derivatives under solvent-free conditions. To achieve the optimum conditions especially in agreement with green chemistry, the reaction between ethyl acetoacetate, nitromethane, benzaldehyde, and aniline with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios were selected as the model reactions for synthesizing polysubstituted pyrrole to examine the impacts of the amount of used catalyst, solvent, and temperature. The results of the optimization reactions were presented in Table 1. The outcomes were evaluated qualitatively using thin layer chromatography (TLC). As observed, in polar organic solvents such as ethanol and acetonitrile, under reflux conditions, the reaction yield was good in long reaction times (Table 1, Entries 1–2). In order to improve the eco-friendly of the reaction conditions, the reaction was occurred in water, but this solvent wasn't observed to be effective and only 37% of product was achieved after 70 min (Table 1, Entry 3). Finally, the reaction was performed in non-polar organic solvents such as dichloromethane, chloroform and tetrahydrofuran under reflux conditions, but none of these solvents were observed to be impressive (Table 1, Entries 4–6). The outcomes presented that when the reaction was performed under solvent-free conditions at 100 °C, the target product afforded in best optimal conditions in terms of rate and yield (Table 1, Entry 7). Due to the key role of the catalyst amount as a significant factor for a reaction, we studied the catalytic performance of the Cu@imine/Fe3O4 MNPs using 0.24–0.48 mol% of the catalyst under solvent-free conditions (Table 2, Entries 7–9), and the yield has increased remarkably in the existence of 0.36 mol% of catalyst, whereas the reaction time has decreased (Table 2, Entry 7). By reducing the value of catalyst to 0.24 mol%, the performance of the product was dropped (Table 1, Entry 8). It was observed that the reaction yield was remained unchanged even in quantities more than the optimum catalyst amount, and 0.48 mol% of the catalyst could not cause the obvious increase in yield of the product (Table 1, Entry 9). When the reaction was surveyed without using the catalyst, no formation of product was found in this case (Table 1, Entry 10). To complete the reaction, effect of temperature on the rate of model reaction was investigated by diverse temperatures from room temperature to 120 °C under solvent-free conditions (Table 1, Entries 11–17). It was observed that the yield of the product increases significantly by increasing the temperature from room temperature to 100 °C (Table 1, Entries 7 and 11–15), but a further enhance in temperature did not demonstrate any significant impact on the product yield (Table 1, Entries 16–17). It should be noted that when the reaction was carried out in the presence of metal halides (Table 3, Entries 18–20), low to moderate yields of the desired product was obtained.
1 molar ratios were selected as the model reactions for synthesizing polysubstituted pyrrole to examine the impacts of the amount of used catalyst, solvent, and temperature. The results of the optimization reactions were presented in Table 1. The outcomes were evaluated qualitatively using thin layer chromatography (TLC). As observed, in polar organic solvents such as ethanol and acetonitrile, under reflux conditions, the reaction yield was good in long reaction times (Table 1, Entries 1–2). In order to improve the eco-friendly of the reaction conditions, the reaction was occurred in water, but this solvent wasn't observed to be effective and only 37% of product was achieved after 70 min (Table 1, Entry 3). Finally, the reaction was performed in non-polar organic solvents such as dichloromethane, chloroform and tetrahydrofuran under reflux conditions, but none of these solvents were observed to be impressive (Table 1, Entries 4–6). The outcomes presented that when the reaction was performed under solvent-free conditions at 100 °C, the target product afforded in best optimal conditions in terms of rate and yield (Table 1, Entry 7). Due to the key role of the catalyst amount as a significant factor for a reaction, we studied the catalytic performance of the Cu@imine/Fe3O4 MNPs using 0.24–0.48 mol% of the catalyst under solvent-free conditions (Table 2, Entries 7–9), and the yield has increased remarkably in the existence of 0.36 mol% of catalyst, whereas the reaction time has decreased (Table 2, Entry 7). By reducing the value of catalyst to 0.24 mol%, the performance of the product was dropped (Table 1, Entry 8). It was observed that the reaction yield was remained unchanged even in quantities more than the optimum catalyst amount, and 0.48 mol% of the catalyst could not cause the obvious increase in yield of the product (Table 1, Entry 9). When the reaction was surveyed without using the catalyst, no formation of product was found in this case (Table 1, Entry 10). To complete the reaction, effect of temperature on the rate of model reaction was investigated by diverse temperatures from room temperature to 120 °C under solvent-free conditions (Table 1, Entries 11–17). It was observed that the yield of the product increases significantly by increasing the temperature from room temperature to 100 °C (Table 1, Entries 7 and 11–15), but a further enhance in temperature did not demonstrate any significant impact on the product yield (Table 1, Entries 16–17). It should be noted that when the reaction was carried out in the presence of metal halides (Table 3, Entries 18–20), low to moderate yields of the desired product was obtained.
| Entry | Solvent | Catalyst (mol%) | Temp. | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: ethyl acetoacetate (1 mmol), nitromethane (1 mmol), benzaldehyde (1 mmol), aniline (1 mmol), and the required amount of catalyst.b The yields represent the separated product. | |||||
| 1 | Ethanol | 0.36 | Reflux | 50 | 79 |
| 2 | Acetonitrile | 0.36 | Reflux | 50 | 71 |
| 3 | Water | 0.36 | Reflux | 70 | 37 |
| 4 | Dichloromethane | 0.36 | Reflux | 70 | 52 |
| 5 | Chloroform | 0.36 | Reflux | 70 | 56 |
| 6 | Tetrahydrofuran | 0.36 | Reflux | 70 | 49 |
| 7 | Solvent-free | 0.36 | 100 °C | 25 | 95 |
| 8 | Solvent-free | 0.24 | 100 °C | 45 | 76 |
| 9 | Solvent-free | 0.48 | 100 °C | 30 | 94 |
| 10 | Solvent-free | — | 100 °C | 70 | Trace |
| 11 | Solvent-free | 0.36 | 25 °C | 70 | 31 |
| 12 | Solvent-free | 0.36 | 60 °C | 40 | 82 |
| 13 | Solvent-free | 0.36 | 70 °C | 35 | 85 |
| 14 | Solvent-free | 0.36 | 80 °C | 35 | 88 |
| 15 | Solvent-free | 0.36 | 90 °C | 30 | 92 |
| 16 | Solvent-free | 0.36 | 110 °C | 25 | 93 |
| 17 | Solvent-free | 0.36 | 120 °C | 25 | 91 |
| 18 | Solvent-free | BF3–SiO2/0.36 | 100 °C | 120 | 71 |
| 19 | Solvent-free | MgCl2/0.36 | 100 °C | 90 | 59 |
| 20 | Solvent-free | SbCl5–SiO2/0.36 | 100 °C | 100 | 62 |
| Entry | R2CHO (3) | R1 | R3 | Product | Time (min) | Yield (%) | TONb | TOFc (h−1) | Mp (obsd) (°C) | Mp (lit) (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: ethyl acetoacetate (1 mmol), nitromethane (1 mmol), benzaldehyde (1 mmol), and aniline (1 mmol)) and Cu@imine/Fe3O4 MNPs (0.36 mol%) at 100 °C.b Number of moles of product produced from 1 mole of catalyst.c TON per unit of time. | ||||||||||
| 1 | C6H5– | OMe | Ph–CH2– | 5a | 20 | 93 | 258 | 774 | Oil | Oil27 |
| 2 | 4-Cl–C6H4– | OMe | Ph–CH2– | 5b | 15 | 95 | 264 | 1056 | Oil | Oil28 |
| 3 | 4-NO2–C6H4– | OMe | Ph–CH2– | 5c | 15 | 97 | 269 | 1076 | Oil | Oil28 |
| 4 | 4-CH3–C6H4– | OMe | Ph–CH2– | 5d | 20 | 91 | 253 | 759 | Oil | Oil28 |
| 5 | 4-OCH3–C6H4– | OMe | Ph–CH2– | 5e | 15 | 92 | 256 | 1024 | Oil | Oil27 |
| 6 | 2-Furyl– | OMe | Ph–CH2– | 5f | 20 | 94 | 261 | 783 | 128–131 | 130–132 (ref. 30) |
| 7 | 2-Thienyl– | OMe | Ph–CH2– | 5g | 15 | 96 | 266 | 1064 | 120–123 | 122–124 (ref. 30) |
| 8 | 4-Cl–C6H4– | OEt | Ph–CH2– | 5h | 15 | 93 | 258 | 1032 | Oil | Oil28 |
| 9 | 4-NO2–C6H4– | OEt | Ph–CH2– | 5i | 20 | 95 | 264 | 792 | Oil | Oil29 |
| 10 | 4-CH3–C6H4– | OEt | Ph–CH2– | 5j | 20 | 91 | 253 | 759 | Oil | Oil27 |
| 11 | 4-OCH3–C6H4– | OEt | Ph–CH2– | 5k | 20 | 89 | 247 | 741 | Oil | Oil29 |
| 12 | C6H5– | OEt | C6H5– | 5l | 25 | 95 | 264 | 634 | Oil | Oil31 |
| 13 | 4-Cl–C6H4– | OEt | C6H5– | 5m | 20 | 90 | 250 | 750 | 126–128 | 127–129 (ref. 32) |
| 14 | 4-CH3–C6H4– | OEt | 4-Cl–C6H4– | 5n | 20 | 91 | 253 | 759 | 119–122 | 120–122 (ref. 32) |
| 15 | 4-Cl–C6H4– | OEt | 4-OCH3–C6H4– | 5o | 20 | 93 | 258 | 774 | 128–131 | 130–132 (ref. 28) |
| 16 | 4-OCH3–C6H4– | OEt | C6H5– | 5p | 20 | 91 | 253 | 759 | 106–109 | 108–110 (ref. 32) |
| Entry | Solvent | Catalyst (mol%) | Temp. | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: benzil (1 mmol), benzaldehyde (1 mmol), benzylamine (1 mmol), ammonium acetate (4 mmol), and the required amount of catalyst.b The yields refer to the separated product. | |||||
| 1 | Ethanol | 0.36 | Reflux | 55 | 69 |
| 2 | Acetonitrile | 0.36 | Reflux | 55 | 61 |
| 3 | Water | 0.36 | Reflux | 80 | 78 |
| 4 | Dichloromethane | 0.36 | Reflux | 70 | 45 |
| 5 | Chloroform | 0.36 | Reflux | 70 | 49 |
| 6 | Tetrahydrofuran | 0.36 | Reflux | 70 | 43 |
| 7 | Solvent-free | 0.36 | 80 °C | 35 | 95 |
| 8 | Solvent-free | 0.24 | 80 °C | 55 | 79 |
| 9 | Solvent-free | 0.48 | 80 °C | 35 | 92 |
| 10 | Solvent-free | 0.36 | 25 °C | 120 | 35 |
| 11 | Solvent-free | 0.36 | 60 °C | 65 | 69 |
| 12 | Solvent-free | 0.36 | 70 °C | 45 | 75 |
| 13 | Solvent-free | 0.36 | 90 °C | 35 | 93 |
| 14 | Solvent-free | 0.36 | 100 °C | 35 | 91 |
| 15w | Solvent-free | BF3–SiO2/0.36 | 80 °C | 150 | 68 |
| 16 | Solvent-free | MgCl2/0.36 | 80 °C | 80 | 56 |
| 17 | Solvent-free | SbCl5–SiO2/0.36 | 80 °C | 150 | 65 |
After optimization of the reaction conditions and to establish the effectiveness and the acceptability of the technique, we explored the scope and generalization of the reaction with a wide range of aldehydes and amines including benzylamine, aniline and substituted anilines under the optimal conditions. The outcomes were listed in Table 2. It was observed that under similar conditions, a variety of benzaldehydes containing electron-withdrawing and electron-donating groups, such as H, CH3, CH3O, NO2 and Cl in the para positions of the benzene ring as well as heteroaromatic aldehydes in the existence of methyl acetoacetate, nitromethane, benzylamine, and the required amount of the Cu@imine/Fe3O4 MNPs, simply converted to the resulted products in short reaction times with good to excellent isolated yields (Table 2, Entries 1–7). Also, using above aromatic aldehydes, ethyl acetoacetate, nitromethane and aniline the target products were achieved in good to high separated yields (Table 2, Entries 8–11). Various aromatic aldehydes containing electron-withdrawing and electron-donating groups (H, CH3, CH3O, and Cl) and anilines with the above-mentioned substituted electron-withdrawing and electron-donating groups in the para positions of the benzene ring were efficiently reacted with ethyl acetoacetate and nitromethane in excellent yields and times (Table 2, Entries 12–16).
To obtain the optimize reaction conditions of the production of 1,2,4,5-tetrasubstituted imidazoles derivatives, the reaction between benzil, benzylamine, benzaldehyde and ammonium acetate with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 molar ratios using 0.36 mol% of catalyst was examined in the existence of diverse solvents, the concentration of used catalyst, and temperatures. To find the solvent effect, the reaction was carried out in the existence of various polar and non-polar solvents such as ethanol, acetonitrile, water, dichloromethane, chloroform and tetrahydrofuran. When water was applied as a solvent, the reaction proceeded smoothly for producing the desired product (5a) in good yield (Table 3, Entry 3). The model reaction in the rest of the solvents mentioned above demanded long reaction times, and provided the product 5a in poor to moderate yields (Table 1, Entries 1–2 and 4–6). Interestingly, none of these solvents were impressive and the model reaction proceeded rapidly to obtain the desired product in excellent yield 95% under solvent-free conditions (Table 3, Entry 7). Over optimizing the reaction conditions, the impact of catalytic activity of the Cu@imine/Fe3O4 MNPs on the model reaction was also studied at 0.24, 0.36, and 0.48 mol% to test their efficiency under solvent-free conditions (Table 3, Entries 7–9). It was observed that the product yield has increased in the existence of 0.36 mol% of catalyst (Table 3, Entry 7). Hence, 0.36 mol% was chosen as optimum catalyst amount for the reaction. Following choice of the optimal concentration of catalyst, the effect of the temperature on yield of the model reaction was evaluated by diverse temperatures from room temperature to 100 °C in the existence of required amount of catalysts under solvent-free conditions (Table 3, Entries 7 and 10–14). It was found that by raising the reaction temperature, yield of the product increases. Maximum yield (95%) was obtained when the temperature of 80 °C was applied to the reaction system (Table 3, Entry 7). Further rise in the temperature had no considerable impact on the product yield (Table 3, Entries 13–14). It should be mentioned that the product yield has decreased remarkably at temperatures below 80 °C, whereas the reaction time has increased (Table 3, Entries 10–12). Therefore, as shown in Table 3, using 0.36 mol% of Cu@imine/Fe3O4 MNPs under solvent-free and 80 °C gave the maximum yield of the target product in the shortest time. It is noteworthy that when the reaction was carried out in the presence of metal halides (Table 3, Entries 15–17), low to moderate yields of the desired product was obtained.
4 molar ratios using 0.36 mol% of catalyst was examined in the existence of diverse solvents, the concentration of used catalyst, and temperatures. To find the solvent effect, the reaction was carried out in the existence of various polar and non-polar solvents such as ethanol, acetonitrile, water, dichloromethane, chloroform and tetrahydrofuran. When water was applied as a solvent, the reaction proceeded smoothly for producing the desired product (5a) in good yield (Table 3, Entry 3). The model reaction in the rest of the solvents mentioned above demanded long reaction times, and provided the product 5a in poor to moderate yields (Table 1, Entries 1–2 and 4–6). Interestingly, none of these solvents were impressive and the model reaction proceeded rapidly to obtain the desired product in excellent yield 95% under solvent-free conditions (Table 3, Entry 7). Over optimizing the reaction conditions, the impact of catalytic activity of the Cu@imine/Fe3O4 MNPs on the model reaction was also studied at 0.24, 0.36, and 0.48 mol% to test their efficiency under solvent-free conditions (Table 3, Entries 7–9). It was observed that the product yield has increased in the existence of 0.36 mol% of catalyst (Table 3, Entry 7). Hence, 0.36 mol% was chosen as optimum catalyst amount for the reaction. Following choice of the optimal concentration of catalyst, the effect of the temperature on yield of the model reaction was evaluated by diverse temperatures from room temperature to 100 °C in the existence of required amount of catalysts under solvent-free conditions (Table 3, Entries 7 and 10–14). It was found that by raising the reaction temperature, yield of the product increases. Maximum yield (95%) was obtained when the temperature of 80 °C was applied to the reaction system (Table 3, Entry 7). Further rise in the temperature had no considerable impact on the product yield (Table 3, Entries 13–14). It should be mentioned that the product yield has decreased remarkably at temperatures below 80 °C, whereas the reaction time has increased (Table 3, Entries 10–12). Therefore, as shown in Table 3, using 0.36 mol% of Cu@imine/Fe3O4 MNPs under solvent-free and 80 °C gave the maximum yield of the target product in the shortest time. It is noteworthy that when the reaction was carried out in the presence of metal halides (Table 3, Entries 15–17), low to moderate yields of the desired product was obtained.
With the optimized condition in hand, we explored the scope and generalization of the catalyst over a variety of aldehydes and amines as substrates for synthesizing 1,2,4,5-tetrasubstituted imidazoles. The outcomes were presented in Table 4. These results illustrates that the reactions are similarly facile with both electron-withdrawing and electron-donating groups, such as H, CH3, CH3O, NO2 and Cl in the meta and para positions of the benzene ring and amines, causing good to high yields of the relating imidazoles.
| Entry | RCHO (2) | R1 | Product | Time (min) | Yield (%) | TONb | TOFc (h−1) | Mp (obsd) (°C) | Mp (lit) (°C) |
|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: benzil (1 mmol), benzaldehyde (1 mmol), benzylamine (1 mmol), ammonium acetate (4 mmol) and Cu@imine/Fe3O4 MNPs (0.36 mol%) at 80 °C.b Number of moles of product produced from 1 mole of catalyst.c TON per unit of time. | |||||||||
| 1 | C6H5– | C6H5– | 5a | 35 | 95 | 264 | 453 | 215–217 | 216–218 (ref. 33) |
| 2 | 4-Cl–C6H4– | C6H5– | 5b | 30 | 95 | 264 | 528 | 150–152 | 149–151 (ref. 33) |
| 3 | 3-NO2–C6H4– | C6H5– | 5c | 30 | 97 | 269 | 538 | 254–257 | 255 (ref. 34) |
| 4 | 4-CH3–C6H4– | C6H5– | 5d | 35 | 93 | 258 | 442 | 186–188 | 185–188 (ref. 33) |
| 5 | 4-OCH3–C6H4– | C6H5– | 5e | 35 | 92 | 256 | 439 | 170–172 | 171–173 (ref. 35) |
| 6 | 3,4-(OCH3)2–C6H4– | C6H5– | 5f | 35 | 94 | 261 | 447 | 173–176 | 175–178 (ref. 35) |
| 7 | C6H5– | n-Pr–NH2 | 5g | 30 | 96 | 266 | 532 | 88–91 | 87–89 (ref. 36) |
| 8 | 4-Cl–C6H4– | n-Pr–NH2 | 5h | 20 | 97 | 269 | 807 | 84–86 | 87–85 (ref. 36) |
| 9 | 4-NO2-C6H4- | n-Pr–NH2 | 5i | 20 | 98 | 272 | 816 | 159–162 | 161–163 (ref. 37) |
| 10 | 3-NO2–C6H4– | n-Pr–NH2 | 5j | 20 | 98 | 272 | 816 | 139–141 | 141–142 (ref. 37) |
| 11 | 4-CH3–C6H4– | n-Pr–NH2 | 5k | 25 | 93 | 258 | 619 | 80–83 | 78–83 (ref. 36) |
| 12 | C6H5– | 4-EtC6H4 | 5l | 35 | 96 | 266 | 456 | 141–143 | 142–144 (ref. 37) |
| 13 | 3-NO2–C6H4– | 4-EtC6H4 | 5m | 25 | 98 | 272 | 653 | 130–133 | 131–132 (ref. 37) |
| 14 | 4-CH3–C6H4– | 4-EtC6H4 | 5n | 35 | 94 | 261 | 447 | 157–160 | 159–161 (ref. 37) |
A proposed mechanism of the synthesis of replaced imidazoles catalyzed by Cu@imine/Fe3O4 MNPs is depicted in Scheme 2. In the beginning, carbonyl oxygen of aldehyde is coordinated to copper center on the nanocomposites surface to generate a better electrophilic species which more readily reacts with amine to give diamine intermediate 6. The next step involves formation of imino intermediate 7 by condensing diamine with 1,2-diketone, followed by dehydrating the relating products 5.
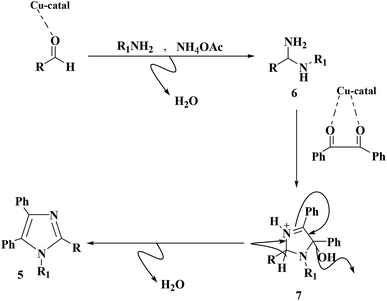 | ||
| Scheme 2 A plausible mechanism of the creation of 1,2,4,5-tetrasubstituted imidazoles derivatives in the existence of Cu@imine/Fe3O4 MNPs under solvent-free conditions. | ||
The mechanism corresponding to production of greatly substituted pyrrole mediated with the Cu@imine/Fe3O4 MNPs catalyst was presented in Scheme 3. The reaction occurs via initial production of the enamine 6 by nucleophilic addition of amine to b-ketoester followed by dehydration. Then, nitromethane reacts with aldehyde to generate intermediate 7. Subsequently, the enamine 6 reacts with intermediate 7 to afford Michael adduct 8. Following intramolecular nucleophilic cyclization by dehydration delivers the desired pyrrole.
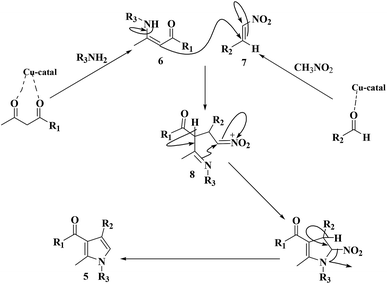 | ||
| Scheme 3 A plausible mechanisms for the creation of polysubstituted pyrrole derivatives in the existence of Cu@imine/Fe3O4 MNPs under solvent-free conditions. | ||
Recycling and recoverability of the Cu@imine/Fe3O4 MNPs were evaluated in the reaction between ethylacetoacetate, nitromethane, benzaldehyde, and aniline. At the end of the reaction, 10 mL hot mixture of ethyl acetate and ethanol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio) was poured in to the reaction solution to dissolve the raw product. Then, the catalyst was isolated from the reaction solution by a permanent magnet, washed with chloroform, dried and recovered for a minimum of six runs (Fig. 6).
10 ratio) was poured in to the reaction solution to dissolve the raw product. Then, the catalyst was isolated from the reaction solution by a permanent magnet, washed with chloroform, dried and recovered for a minimum of six runs (Fig. 6).
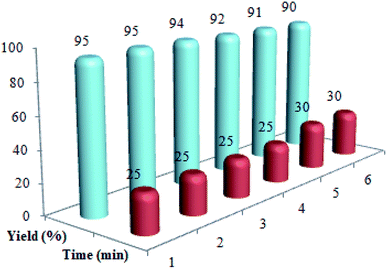 | ||
| Fig. 6 The recycling of Cu@imine/Fe3O4 MNPs in the preparation of polysubstituted pyrrole derivatives. | ||
To evaluate recycling and recoverability of the Cu@imine/Fe3O4 MNPs, after six periods of recycling, this catalyst was reused in the reaction of benzil, benzaldehyde, benzylamine and ammonium acetate under the optimal reaction conditions (Fig. 7). To achieve this purpose, at the end of the reaction, a hot mixture of ethyl acetate and ethanol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio) was added to the reaction solution and then the catalyst was removed simply by means of a permanent external magnet. The recovered Cu@imine/Fe3O4 MNPs was rinsed with chloroform, dried, and recovered for alternative reaction.
10 ratio) was added to the reaction solution and then the catalyst was removed simply by means of a permanent external magnet. The recovered Cu@imine/Fe3O4 MNPs was rinsed with chloroform, dried, and recovered for alternative reaction.
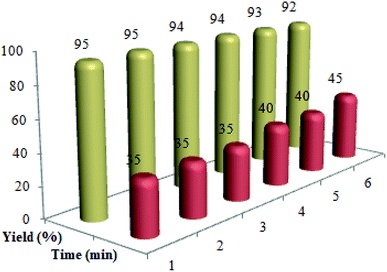 | ||
| Fig. 7 The recycling of Cu@imine/Fe3O4 MNPs in the synthesis of 1,2,4,5-tetrasubstituted imidazoles derivatives. | ||
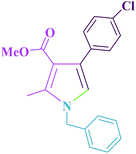
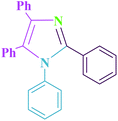
Table 5 shows the efficiency of Cu@imine/Fe3O4 MNPs as the novel heterogeneous magnetic nanocatalyst in preparing 1-benzyl-4-(4-chloro-phenyl)-2-methyl-1H-pyrrole-3-carboxylic acid methyl ester and 1,2,4,5-tetraphenyl-1H-imidazole compared with those obtained by some of the other reported homogeneous and heterogeneous catalysts. As clearly shown in Table 5, although all the reported catalysts can catalyze the reactions, most of them suffer from one or more disadvantages, such as long reaction times, low yields of the products, use of toxic catalyst, tedious work-up procedures, and high catalyst loading. For these reasons, the catalytic behaviour of our catalytic system is significant in terms of easy work-up procedures, low catalyst loading, low reaction times, and simple recovery of the catalyst.
| Entry | Catalyst | Temp (°C) | Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|---|
 |
|||||
| 1 | Nickel(II) chloride hexahydrate/solvent-free | rt | 480 | 78 | 30 |
| 2 | Nickel ferrite nanoparticles/solvent-free | 100 | 240 | 96 | 28 |
| 3 | Nano copper oxide/solvent-free | 100 | 720 | 78 | 29 |
| 4 | Heterogenized tungsten complex/solvent-free | 100 | 270 | 85 | 27 |
| 5 | Amberlyst-15/solvent-free | 110 | 30 | 91 | 25 |
| 6 | SbCu@silica-Fe3O4/solvent-free | rt | 25 | 88 | 32 |
| 7 | Cu@imine/Fe3O4 MNPs/solvent-free | 100 | 25 | 95 | This work |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
 |
|||||
| 1 | BF3/SiO2/solvent-free | 140 | 120 | 92 | 39 |
| 2 | TiCl4/SiO2/solvent-free | 110 | 190 | 75 | 40 |
| 3 | Glycerol as solvent | 90 | 180 | 96 | 41 |
| 4 | PEG-400 as a solvent | 110 | 360 | 86 | 38 |
| 5 | NaH2PO4/solvent-free | 120 | 35 | 90 | 42 |
| 6 | Fe3O4–PEG–Cu/solvent-free | 110 | 55 | 96 | 43 |
| 7 | γ-Fe2O3@TiO2–EG–Cu(II)/solvent-free | 100 | 20 | 98 | 44 |
| 8 | Cu@imine/Fe3O4 MNPs/solvent-free | 80 | 35 | 95 | This work |
Conclusion
We have described a simple, facile, and effective process for the eco-friendly and fast production of biologically active polysubstituted pyrroles and 1,2,4,5-tetrasubstituted imidazoles derivatives. The reaction system was significantly affected by catalyst loading and reaction temperature. The catalyst could be recovered and reused at least six times without any significant decrease in its activity. The attractive properties of this procedure are short reaction times, simple workup, high yields, reusability and re-activity of the catalyst as well as simple purification of the products.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The results were obtained in the framework of the implementation of the initiative scientific project No. 40.5084.2017/BCH “Study of methods and modeling processes in biotechnology and plant systematics”.References
- K. Niknam and D. Saberi, Tetrahedron Lett., 2009, 50, 5210 CrossRef CAS
.
- X. Liu, X. Zhao and M. Lu, Catal. Lett., 2015, 145, 1549 CrossRef CAS
.
- M. Hosseini-Sarvari, A. Khanivar and F. Moeini, J. Iran. Chem. Soc., 2016, 13, 45 CrossRef CAS
.
- L. Shiri, A. Ghorbani-Choghamarani and M. Kazemi, Aust. J. Chem., 2016, 69, 585 CrossRef CAS
.
- B. Karami, S. J. Hoseini, K. Eskandari, A. Ghasemi and H. Nasrabadi, Catal. Sci. Technol., 2012, 2, 331 RSC
.
- A. L. Morel, S. I. Nikitenko, K. Gionnet, A. Wattiaux, J. Lai-Kee-Him, C. Labrugere, B. Chevalier, G. Deleris, C. Petibois, A. Brisson and M. Simonoff, J. Am. Chem. Soc., 2008, 2, 847 CAS
.
- Y. Weia, B. Hanb, X. Hua, Y. Linc, X. Wangd and X. Denga, Procedia Eng, 2012, 27, 632 CrossRef
.
- Y. S. Kim and Y. H. Kim, J. Magn. Magn. Mater., 2003, 267, 105 CrossRef CAS
.
- M. Nasr-Esfahani, S. J. Hoseyni and F. Mohammadi, Chin. J. Catal., 2011, 23, 1484 CrossRef
.
- H. Koike, T. Konse, T. Sada, T. Ikeda, A. Hyogo, D. Hinman, H. Saito and H. Yanagisawa, Annu. Rep. Sankyo Res. Lab., 2003, 55, 1 CAS
.
- S. L. Abrahams, R. J. Hazen, A. G. Batson and A. P. Phillips, J. Pharmacol. Exp. Ther., 1989, 249, 359 CAS
.
- C. Leister, Y. Wang, Z. Zhao and C. W. Lindsley, Org. Lett., 2004, 6, 1453 CrossRef PubMed
.
- H. Debus, Ann. Chem. Pharm., 1858, 107, 199 CrossRef
.
- B. Radziszewski, Chem. Ber., 1882, 15, 1493 CrossRef
.
- A. Chawla, A. Sharma and A. Kumar, Pharma Chem., 2012, 4, 116 CAS
.
- M. Rahman, A. K. Bagdi, D. Kundu, A. Majee and A. Hajra, J. Heterocycl. Chem., 2012, 49, 1224 CrossRef CAS
.
- K. Ramesh, S. N. Murthy, K. Karnakar, Y. V. D. Nageswar, K. Vijayalakhshmi, B. L. A. Prabhavathi Devi and R. B. N. Prasad, Tetrahedron Lett., 2012, 53, 1126 CrossRef CAS
.
- K. Niknam, A. Deris, F. Naeimi and F. Majleci, Tetrahedron Lett., 2011, 52, 4642 CrossRef CAS
.
- A. Hasaninejad, A. Zare, M. Shekouhy and J. A. Rad, J. Comb. Chem., 2010, 12, 844 CrossRef CAS
.
- N. S. Murthy, B. Madhav and Y. V. D. Nageswar, Tetrahedron Lett., 2010, 51, 5252 CrossRef
.
- C. Mukhopadhyay, P. K. Tapaswi and M. G. B. Drew, Tetrahedron Lett., 2010, 51, 3944 CrossRef CAS
.
- P. F. Zhang and Z. C. Z. Chen, Synthesis, 2001, 2075 CrossRef CAS
.
- D. I. Ma Gee, M. Bahramnejad and M. Dabiri, Tetrahedron Lett., 2013, 54, 2591 CrossRef CAS
.
- A. Keivanloo1, M. Bakherad, E. Imanifar and M. Mirzaee, Appl. Catal., A, 2013, 467, 291 CrossRef
.
- P. R. K. Murthi, D. Rambabu, M. V. B. Rao and M. Pal, Tetrahedron Lett., 2014, 55, 507 CrossRef
.
- K. Konkala, R. Chowrasia, P. S. Manjari, N. L. C. Domingues and R. Katla, RSC Adv., 2016, 6, 43339 RSC
.
- A. B. Atar and Y. T. Jeong, Tetrahedron Lett., 2013, 54, 5624 CrossRef CAS
.
- F. M. Moghaddam, B. K. Foroushani and H. R. Rezvani, RSC Adv., 2015, 5, 18092 RSC
.
- H. Saeidian, M. Abdoli and R. Salimi, C. R. Chim., 2013, 16, 1063 CrossRef CAS
.
- A. T. Khan, M. Lal, P. R. Bagdi, R. S. Basha, P. Saravanan and S. Patra, Tetrahedron Lett., 2012, 53, 4145 CrossRef CAS
.
- N. Gupta, K. N. Singh and J. Singh, J. Mol. Liq., 2014, 199, 470 CrossRef CAS
.
- S. A. Hamrahian, J. Rakhtshah, S. M. Mousavi-Davijani and S. Salehzadeh, Appl. Organomet. Chem., 2018, 35, e4501 CrossRef
.
- B. Sadeghi, B. B. F. Mirjalili, S. Bidaki and M. Ghasemkhani, J. Iran. Chem. Soc., 2011, 8, 648 CrossRef CAS
.
- V. Kannan and K. Sreekumar, J. Mol. Catal. A: Chem., 2013, 376, 34 CrossRef CAS
.
- A. V. Borhade, D. R. Tope and S. G. Gite, Arabian J. Chem., 2017, 10, S559–S567 CrossRef CAS
.
- J. Safari and Z. Zarnegar, C. R. Chim., 2013, 16, 920 CrossRef CAS
.
- Z. Zarnegar and J. Safari, New J. Chem., 2014, 38, 4555 RSC
.
- X. C. Wang, H. P. Gong, Z. J. Quan, L. Li and H. L. Ye, Chin. Chem. Lett., 2009, 20, 44 CrossRef CAS
.
- B. Sadeghi, B. B. F. Mirjalili and M. M. Hashemi, Tetrahedron Lett., 2008, 49, 2575 CrossRef CAS
.
- B. F. Mirjalili, A. H. Bamoniri and L. Zamani, Sci. Iran., 2012, 19, 565 CrossRef CAS
.
- F. Nemati, M. M. Hosseini and H. Kiani, J. Saudi Chem. Soc., 2016, 20, S503–S508 CrossRef CAS
.
- Z. Karimi-Jaberi and M. Barekat, Chin. Chem. Lett., 2010, 21, 1183 CrossRef CAS
.
- Z. Zarnegar and J. Safari, New J. Chem., 2014, 38, 4555 RSC
.
- M. Nejatianfar, B. Akhlaghinia and R. Jahanshahi, Appl. Organomet. Chem., 2018, 32, 4095 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra02325a |
| This journal is © The Royal Society of Chemistry 2019 |