Open Access Article
Open Access ArticlePreparation of terpolymer capsules containing Rosmarinus officinalis essential oil and evaluation of its antifungal activity
Juliete Silva Nevesa,
Zita Lopes-da-Silvab,
Maria de Sousa Brito Netac,
Sacha Braun Chavesc,
Yanna Karla de Medeiros Nóbregab,
Angelo Henrique de Lira Machado a and
Fabricio Machado
a and
Fabricio Machado *a
*a
aInstituto de Química, Universidade de Brasília, Campus Universitário Darcy Ribeiro, CEP 70910-900, Brazil. E-mail: fmachado@unb.br; Tel: +55-061-3107-3868
bFaculdade de Ciências da Saúde, Universidade de Brasília, Campus Universitário Darcy Ribeiro, CEP 70910-900, Brazil
cDepartamento de Genética e Morfologia, Instituto de Biologia, Universidade de Brasília, Campus Universitário Darcy Ribeiro, CEP 70910-900, Brazil
First published on 22nd July 2019
Abstract
The essential oil from Rosmarinus officinalis presents antifungal activity and is used in industry as a natural preserving agent. However, essential oils are unstable compounds. So, the encapsulation of essential oils is a technique used to protect it, minimizing degradation and reducing undesired interaction with the other formulation components. Thus, this work focuses on the synthesis of terpolymeric capsules containing essential oil from Rosmarinus officinalis, aiming to use it as an antifungal component in cosmetics. The capsules were obtained via terpolymerization of methyl methacrylate, styrene and methacrylic acid in a dispersed phase polymerization process. The properties of the polymers and the fungicide activity were evaluated. The studied essential oil presented a Minimum Inhibitory Concentration (MIC) ranging from 2.25 to 4.5 mg mL−1 and a Minimum Fungicidal Concentration (MFC) from 4.5 to 9.0 mg mL−1 for strains of Candida albicans, Candida glabrata and Candida parapsilosis, and after the encapsulation process, the antifungal activity of the oil was maintained. Additionally, cytotoxicity assays against fibroblast cell lines and human keratinocytes showed that the polymeric nanocapsules containing Rosmarinus officinalis essential oil can be regarded as a very promising material intended for cosmetics and drug delivery applications.
1. Introduction
Cutaneous mycoses are among the most common fungal infections, mainly caused by filamentous fungi such as Trichophyton, Microsporum and Epidermophyton, which feed on keratinized tissues such as hair, nails and skin, or other opportunistic species such as Candida. Onychomycosis is a superficial infection caused by these fungi and has been a worldwide public health problem, mainly because it causes invasive infections in immunodepressed patients.1–3Conventional treatment for these infections is usually done with the oral and topical use of antifungal agents. However, in addition to adverse drug effects, the use of antifungal agents may lead to the development of antifungal resistance. In this context, essential oils that have antifungal activity may be an alternative for the prevention of the growth of pathogenic fungi, and in the treatment of immunodepressed onychomycoses, because the development of resistance to essential oils is rare.1
Studies in the literature report the antifungal properties of Rosmarinus officinalis (rosemary). The major terpenes found in its essential oil are camphor, α-pinene, myrcene, γ-terpinene and 1,8-cineol.4 Pinene, a monoterpene, has antimicrobial activity, interferes with the chain of respiration reactions of the mitochondria and, in addition, influences the transport of potassium ions through the cell membrane, altering the operation of the Na+ and K+ pumps and also H+ altering the electrical potential of cell membranes, leading the cell to death.5–8
The antifungal activity of the essential oil of Rosmarinus officinalis is also reported in the literature, from which Bomfim et al. can be cited,9 who evaluated its activity against the fungi Fusarium verticillioides and reported a minimal inhibitory concentration of 150 μg mL−1. Bozin et al.10 tested the antimicrobial activity of this oil against Candida albicans, 5 dermatomycetes fungi and 13 bacterial strains, and a significant antimicrobial activity was observed.
However, essential oils are unstable compounds, which can be easily degraded by exposure to light or due to temperature variations. So, the encapsulating of essential oils is a technique used to minimize degradation and reduce undesired interactions between the other components of the formulation. The encapsulation, in addition to preserving the chemical properties as well as the biological activity, allows them to be released in a controlled way in the medium, avoiding the rapid loss of material, since the essential oils are volatile.2,11–14
The techniques presented in the literature for the encapsulation of essential oils are phase inversion precipitation, solvent evaporation, spray drying and in situ polymerization in the dispersed phase, for example mini-emulsion.
Pena et al.15 used polysulfone to prepare microcapsules of vanillin by a phase inversion precipitation technique, the maximum efficiency obtained by authors was 69.6%. The SEM images of this material showed capsules with a mean diameter of approximately 18 μm. This technique limits the control of capsules size and a polydisperse material was synthetized.
Hofmeister et al.16 prepared a terpolymer of methyl methacrylate, butyl methacrylate and methacrylic acid by miniemulsion polymerization to encapsulate α-pinene and evaluated the controlled release by pH variation. The method reported by the authors yielded 90% encapsulation efficiency where capsule sizes below 200 nm are obtained. By miniemulsion polymerization was possible to control the capsules size, however the use of co-stabilizers was required to the colloidal stabilization of the reaction medium.
In the suspension polymerization, the monomers and the essential oil (organic phase) are dispersed in a continuous phase, usually water, due to the combined action of agitation and suspending agents. In order to start the polymerization reaction, a radical initiator soluble in the organic phase is added to the medium. The polymerization reaction occurs in the monomer droplets, which behaves like mass polymerization micro-reactors. This characteristic enables the encapsulation of the essential oil by suspension polymerization.17
In this work, capsules containing the essential oil of the Rosmarinus officinalis were prepared by suspension polymerization using methyl methacrylate, styrene and methacrylic acid monomers and the influence of the composition of the polymer on the stability of the capsules was evaluated. By this polymerization method was possible to control the capsules size without the use of co-stabilizers.
The efficiency of the oil encapsulation was evaluated based in the essential oil free (unencapsulated) determined by gas chromatography and the antifungal activity of the oil and capsules was evaluated by the broth microdilution method adapted from CLSI M27-A2 protocol to evaluate if the activity of the oil would be maintained after encapsulation.
2. Experimental
2.1 Materials
Antifungal activity assays were performed using essential oils of Rosmarinus officinalis (EO, Mundo dos óleos, Brazil), Sterilized ultrapure water, Tween 80 (Merck; Germany), Agar Sabouraud Dextrose (Acumedia; Brazil), Agar Dextrose (Acumedia; Brazil) and fluconazole (Sigma-Aldrich; Brazil).
3. Methods
3.1 Suspension polymerization
To a glass reactor (nominal volume 400 mL) was added distilled water and poly(vinyl alcohol). The system was heated in a water bath at 75 °C under mechanical stirring at 500 rpm. The mixture of monomers, initiator and essential oil was added as indicated in Table 1. The mixture was kept under mechanical stirring at 2000 rpm for 60 minutes at 75 °C, the temperature was then raised to 85 °C and the system was kept under stirring for an additional 180 minutes. Then, the system was turned off and the polymeric material was stored in a glass vial.| Experiment | Water (g) | Monomer (g) | PVA (g) | PBO (g) | Essential oil (g) | ||
|---|---|---|---|---|---|---|---|
| MMA | MA | STY | |||||
| MAA/MMA_R.O. | 70 | 19.6 | 8.4 | 0.0 | 0.46 | 1.5 | 1.8 |
| MAA/MMA | 70 | 19.6 | 8.4 | 0.0 | 0.46 | 1.5 | 0.0 |
| MMA/STY | 70 | 19.6 | 0.0 | 8.4 | 0.62 | 1.5 | 1.8 |
| MAA/MMA/STY_R.O. | 70 | 19.6 | 1.4 | 7.0 | 0.62 | 2.1 | 1.8 |
| MAA/MMA/STY | 70 | 19.6 | 1.4 | 7.0 | 0.62 | 2.1 | 0.0 |
3.1.1.1 Suspension of essential oil. To evaluate the Minimum Inhibitory Concentration (MIC), 5 mL of a suspension of the essential oils in water with a concentration of 0.144 g mL−1 were initially prepared. 4.2 mL of sterile distilled water, 0.8 mL of the essential oil and 0.1 mL of Tween 80 were added in a sterile tube. Then, the mixture was kept under stirring for 3 min on Vortex shaker (Phoenix, Brazil).
3.1.1.2 Fungal suspension. From 24 hours Candida strains cultures on Sabouraud Dextrose Agar (Acumedia, Brazil), fungal suspensions were prepared in a final concentration of 2 × 103 colony forming units (CFU) mL−1.
3.1.1.3 Minimal inhibitory concentration (MIC). The Minimum Inhibitory Concentration (MIC) of essential oil and polymers was determinate by broth microdilution method performed according to CLSI M27-A2 protocol with some modifications (Table 2).
| Candida albicans ATCC SC5314 | Candida glabrata ATCC 2001 | Candida parapsilosis ATCC 22019 | |
|---|---|---|---|
| Rosmarinus officinalis | 18 a 1.13 mg mL−1 | 18 a 1.13 mg mL−1 | 18 a 1.13 mg mL−1 |
| Polymers | 18 a 0.28 mg mL−1 | 18 a 0.28 mg mL−1 | 18 a 0.28 mg mL−1 |
In the wells of the positive control were sequentially added 100 μL of the fungal suspension (2 × 103 CFU mL−1) and 100 μL of the fluconazole solution with a concentration of 2.0 mg mL−1. In the growth control wells were added 100 μL of the medium and 100 μL of the fungal suspension (2 × 103 CFU mL−1), and in the sterility control wells only 200 μL of the culture medium was added. The microplate was incubated at 37 ± 2 °C for 48 hours. The MIC was defined as the lowest product concentration that produced visible inhibition of yeast growth.
3.1.1.4 Minimal fungicidal concentration (MFC). Fungicidal Concentration (MFC) was obtained by subculture 10 μL of each well in Sabouraud Dextose Agar (Acumedia; Brazil) medium in which fungal growth and upper and lower concentrations were not observed.
After seeding, the plates were incubated at 37 ± 2 °C for 24 to 48 hours. At the end of this period, the plates were observed. The MFC was considered as the lowest product concentration where the growth of up to three CFU was observed. The tests were performed in triplicate for all species tested.
The cell lines were cultured in DMEM (Dulbecco's Modified Eagle's Medium). Both cells were buffered with sodium bicarbonate and supplemented with 10% fetal bovine serum (FBS) and antibiotic (100 Ul mL−1 penicillin and 100 μg mL−1 streptomycin) at 1%, pH 7.2. The cells were kept in cell culture flasks in an incubator at 37 °C, under atmosphere of 95% humidified air and 5% CO2. The experiments were performed with the cell in the logarithmic phase of growth. For the execution of the experiments after quantification, the cells were transferred to culture microplates, according to the experiment to be performed.
The cell viability assay is aimed at analyzing the cell (FIBRO and HaCat) death by the synthesized materials in assays carried out during 24 h at concentrations of 0.01 mg mL−1, 0.05 mg mL−1, 0.1 mg mL−1, 0.25 mg mL−1 and 0.5 mg mL−1. The cell lines (FIBRO and HaCat) were seeded in 96-well plates with concentrations of 5 × 103 and 3 × 103 cells per well in DMEM medium.
After 24 h, the initial culture medium was replaced with 200 μL of culture medium containing different samples at concentrations (0.01 mg mL−1, 0.05 mg mL−1, 0.1 mg mL−1, 0.25 mg mL−1 and 0.5 mg mL−1). Cell viability was evaluated by using a colorimetric method based on 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide), MTT. The quantification was performed by spectrophotometry, using the wavelength of 595 nm. The results were obtained as an average of three independent experiments performed in triplicates.
3.2 Characterization
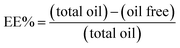 | (1) |
The essential oil free was determined by gas chromatography. The samples were prepared by mixing 2 mL of hexane with 2 g of the polymer suspension. The mixture was centrifuged and the supernatant containing the essential oil free was analyzed in triplicate and the amount was determined by comparing the results with a calibration curve prepared from defined essential oil concentrations.
4. Results and discussion
4.1 Antifungal activity of essential oil
During the analysis, the control wells containing the fluconazole positive control, sterility control and growth control were used to validate the technique used. Growth of the fungi studied in the wells of positive control and sterility control was not observed, as expected, and, in the control of growth, the fungi grew normally.The MIC and CFM of R. officinalis essential oil for the fungi Candida albicans, Candida glabrata and Candida parapsilosis are shown in Table 3.
| Species | Minimum inhibitory concentration | Minimum fungicidal concentration | ||
|---|---|---|---|---|
| (mg mL−1) | (%) | (mg mL−1) | (%) | |
| Candida albicans SC5314 | 2.25 | 0.25 | 4.50 | 0.50 |
| Candida glabrata ATCC 2001 | 2.25 | 0.25 | 4.50 | 0.50 |
| Candida parapsilosis ATCC 22019 | 4.50 | 0.50 | 9.00 | 1.00 |
The results of the broth microdilution method show antifungal activity of R. officinalis against the Candida species tested, and a higher sensitivity of the strains of C. albicans and C. glabrata in relation to C. parapsilosis in the presence of the R. officinalis essential oil.
The biological activity of the studied essential oil is consequence of its chemical composition, which was assessed by gas chromatography (GC/MS) and a representative chromatogram is shown in Fig. 1. Table 4 shows the major components with the respective retention times.
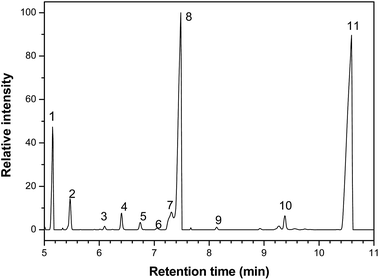 | ||
| Fig. 1 Chromatogram obtained by gas chromatography-mass spectrometry analysis of Rosmarinus officinalis essential oil, where the numbers from 1 to 11 correspond to the majority components, whose names are shown in the Table 4. | ||
| Number | Component | Retention time (min) |
|---|---|---|
| 1 | α-Pinene | 5.1 |
| 2 | Canfene | 5.5 |
| 3 | α-Fenchene | 6.1 |
| 4 | Myrcene | 6.4 |
| 5 | α-Felandrene | 6.7 |
| 6 | α-terpinene | 7.0 |
| 7 | p-Cimene | 7.3 |
| 8 | 1,8-Cineole | 7.5 |
| 9 | γ-Terpinene | 8.1 |
| 10 | α-Pinene oxide | 9.4 |
| 11 | Camphor | 10.5 |
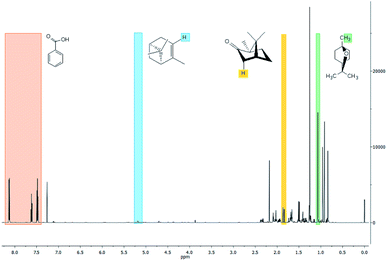
The three major compounds were α-pinene, 1,8-cineole and camphor, which correspond respectively to 8%, 34% and 52% (w/w) of the essential oil. The fraction of these compounds was determined by the quantitative NMR (qNMR) analysis of the 1H NMR spectrum (Fig. 2). From the integration of the benzoic acid signals (used as internal standard, observed at 8.17, 7.65 and 7.51 ppm), α-pinene (5.22 ppm), camphor (1.8 ppm) and 1,8-cineol (1.07 ppm), it was possible to quantify the concentration of each compound in the essential oil.
Hammer et al.19 investigated the antifungal activity of α-pinene, 1,8-cineol, α-terpinene and β-myrcene compounds and the last one had no antifungal activity. The other compounds showed activity for both yeast and dermatophyte fungi.
Some studies have shown that, in yeast cells, α-pinene and γ-terpinene interact with the cell membrane increasing permeability, inhibit cellular respiration and ionic transport processes, causing changes in the electrical potential of the cell membrane and consequent death of the cell.8,20
4.2 Conversion
In dispersed phase polymerization systems, the instability of the medium is undesirable, as it leads to the formation of agglomerates, which deposit on the walls of the reactor, making it difficult to exchange heat during the polymerization process and consequent temperature control.The data presented by Vijayendran21 showed that the solubility of monomer in the aqueous phase influences the area per molecule of surfactant, when the solubility increases, the area per molecule decreased. This effect resulted in poor stability of the particles and the flocculation was observed. However, the author observed that the interfacial tension of polymer–water interface decreases with increasing of the polymer polarity. The increasing of the monomer polarity may favor the self-stabilization of polymer particles. The organization of polar monomer molecules on the particles surface decreases the interfacial tension and promotes the stabilization.
For this reason, when the water-soluble monomers are used, as methacrylic acid and methyl methacrylate, a lower concentration of surfactant is required to stabilize the suspension. In this way, when the solubility in water of the monomer is low or the monomer is totally insoluble in water, as styrene, higher concentration of surfactant is required to stabilize the suspension and avoid the flocculation of particles.
Therefore, for these polymerization reactions, the concentration of the suspending agent (PVA) was reduced by 25% compared to the initial one, thus, an improvement in the stability of the reaction medium was observed, without the formation of agglomerates in the reactor.
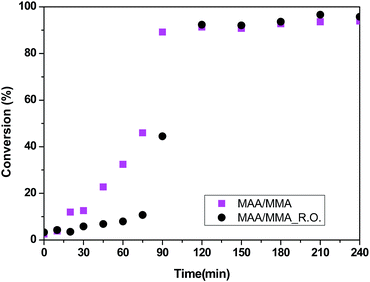
The conversion curves of methacrylic acid/methyl methacrylate copolymers (samples MAA/MMA and MAA/MMA_R.O.) are shown in Fig. 3, where is possible to observe the difference between the conversions of the two materials in the initial minutes of the reactions. The material that contains essential oil has a lower conversion in the initial 80 minutes, which can be explained by the auto-oxidation of γ-terpinene and α-pinene present in the essential oil of R. officinalis. These compounds react with the free radical of initiator, benzoyl peroxide, present in the reaction medium, reducing the rate of polymerization. Despite that, monomer conversion around 100% is observed. Additionally, it can be observed that both reactions present a strong nonlinear behavior that takes place due to the presence of viscosity effects related to important kinetic phenomena such as autoacceleration of the polymerization and, diffusion and heat-transfer limitations.
In order to evaluate the variation of the properties of the material with the use of hydrophobic monomer, the methacrylic acid was replaced by styrene and a new copolymer was synthesized. Maintaining the previous experimental procedure, the system remained unstable with the formation of agglomerates in the reactor. As the styrene present a lower polarity and solubility then methacrylic acid, the PVA concentration was increased by 25% to try to stabilize the suspension. However, the attempt to increase the PVA concentration was unsuccessful, although the formation of agglomerates was observed. The conversion curve is shown in Fig. 4.
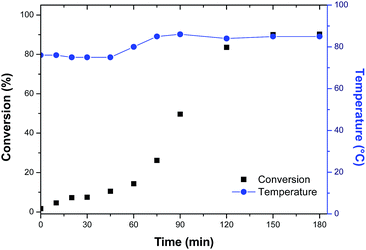 | ||
| Fig. 4 Polymerization conversion profiles of capsules of Rosmarinus officinalis essential oil with methyl methacrylate/styrene (MMA/STY_R.O.). | ||
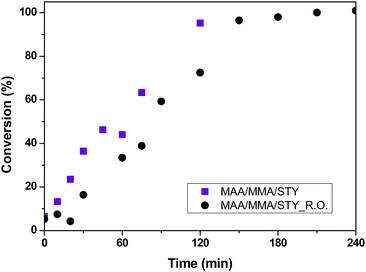
To stabilize the medium, methacrylic acid was then added to the composition of the polymeric material, where a terpolymer was obtained with 70% methyl methacrylate, 25% styrene and 5% methacrylic acid feed. This was the most stable composition for this system. However, it was observed the formation of larger polymer particles, which difficult the sampling of the material after 120 minutes of reaction, and as can be observed in the conversion curve, shown in Fig. 5, an irregularity of the points was detected after 120 minutes. The conversion curve of the terpolymer also shows the influence of the essential oil on conversion in the early beginning of the reaction.
4.3 Morphology and particle size
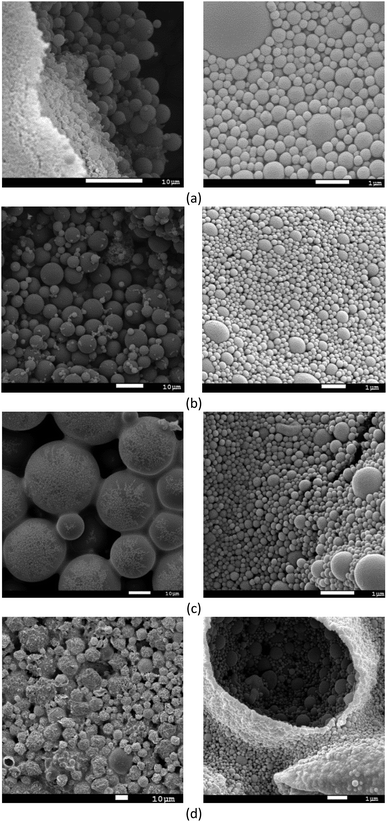
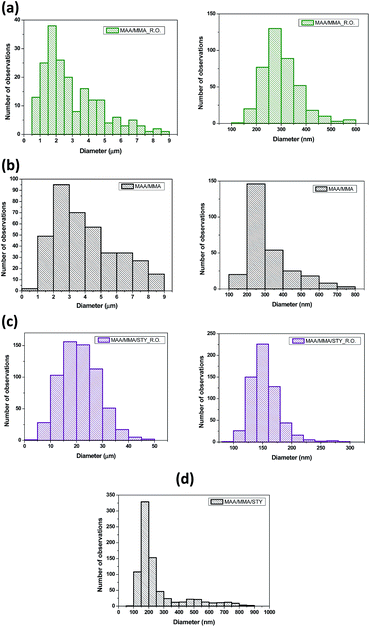
The SEM images of the MAA/MMA_R.O. and MAA/MMA materials are shown in Fig. 6a and b, where it is possible to observe the formation of particles with spherical morphology and unimodal particle size distribution for all analyzed samples, except for MAA/MMA/STY sample, which exhibited an average particle size equal to 192 nm. The average diameters of these materials are shown in Table 5. As depicted in Fig. 6 and 7, nanosized polymer particles were also formed, whose average particle diameter was determined to be equal 285, 266, and 150 nm for polymer samples MAA/MMA_R.O., MAA/MMA and MAA/MMA/STY_R.O., respectively (Table 5). Considering that the nanoencapsulation may increase the contact efficiency of the essential oil with fungi, which may improve the release of essential oil from the polymer particles, increasing the efficient of antifungal activity. It is also reasonable to assume that antifungal activity may significantly depend on the particle size distribution of the encapsulated material.| Polymers | Average size | |
|---|---|---|
| MAA/MMA_R.O. | 2.4 μm | 285 nm |
| MAA/MMA | 3.5 μm | 266 nm |
| MAA/MMA/STY_R.O. | 20 μm | 150 nm |
| MAA/MMA/STY | 192 nm | |
In the SEM images of the styrene-containing materials in the composition, Fig. 6c and d, it is possible to note that the composition of the material influences the size of the polymer particles. The material MAA/MMA/STY_R.O. presents particles with spherical morphology, unimodal size distribution (Fig. 6c) and different mean diameters of the methacrylic acid/methyl methacrylate copolymers (Table 5). Fig. 7 illustrates the particle size distribution of polymer samples indicated in Table 5.
The material MAA/MMA/STY_R.O., terpolymer containing no essential oil of R. officinalis, we observed in Fig. 6d the formation of agglomerates with irregular morphology. However, upon closer inspection of the material, these agglomerates were formed as spherical particles and have a large size distribution.
4.4 Glass transition temperatures
The glass transition temperatures (Tg) of the materials determined by DSC are given in Table 6. It was observed that the polymers containing higher methacrylic acid had higher Tg. This effect is related to the chemical nature of the monomers used. The Tg of a polymeric material depends, among other factors, on the interaction force between the chains, the larger the interaction force the greater the Tg of the material.| Polymers | Mn (kg mol−1) | Mw (kg mol−1) | ĐM | Tg (°C) |
|---|---|---|---|---|
| MAA/MMA | 61 | 188 | 3.0 | 148 |
| MAA/MMA_R.O. | 52 | 134 | 2.5 | 135 |
| MAA/MMA/STY | 30 | 61 | 2.0 | 100 |
| MAA/MMA/STY_R.O. | 29 | 57 | 1.9 | 90 |
Methacrylic acid presents in its chemical structure (Fig. 8) hydroxyl group that promotes the interaction between the polymer chains through hydrogen bonds, strong interactions that increase the Tg of the polymer.
Analyzing the Tg values presented in Table 6, the decrease in the glass transition temperature of the materials due to the addition of the essential oil was observed. In addition to the chemical nature of the monomers, Tg is also influenced by the molar mass of the polymer. Materials with low molar mass have more mobile polymer chains, therefore, the decrease in molar mass results in a decrease in Tg.
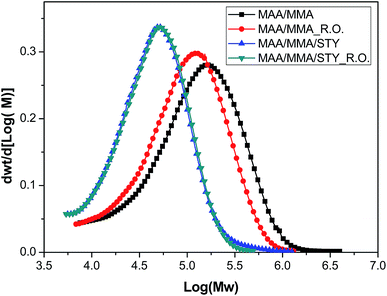
The average molar masses of these polymers were determined by gel permeation chromatography (GPC) and the molar mass distribution of the materials is shown in Fig. 9 and the mass-average molar mass (Mw), number-average molar mass (Mn) and molar-mass dispersity (ĐM) are presented in Table 6. No significant variations in the molar mass distribution between the polymers with and without the essential oil were observed, indicating that the growth of the polymer chains was not influenced by the presence of the essential oil. However, as the glass transition temperature is changed, it is understood that the essential oil acts as a plasticizer, increasing the mobility of the polymer chains and reducing Tg.
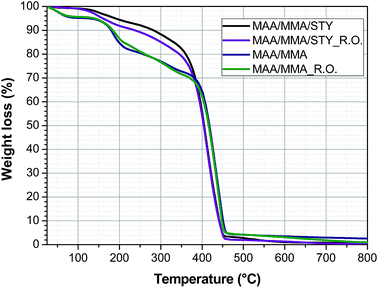
4.5 Thermal stability
The thermal stability of the materials was determined by thermogravimetric analysis and the thermogravimetric curves were presented in Fig. 10. This suggests that addition of the essential oil does not significantly change the thermal stability of the polymer.The copolymers MAA/MMA and the capsules MAA/MMA_R.O. have four stages of mass loss. In the temperature range between 25 °C and 100 °C the loss of mass of the residual water in the sample is observed. Between 120 °C and 210 °C the observed mass loss is associated with the loss of water and methanol produced by reactions between the polymer chains.22
Mansur et al.22 state that these reactions occur due to the presence of carboxylic acid groups in the polymer chain, which is closed related to the loss of mass observed in this temperature range. This may justify that the terpolymers of MAA/MMA/STY did not show a marked loss in this temperature range.
The copolymers further exhibit a mass loss between 210 °C and 360 °C, associated with the degradation of unsaturated and low molar mass chains and a final loss between 360 °C and 460 °C relative to degradation of the polymer chain.
The terpolymer MAA/MMA/STY and the capsule MAA/MMA/STY_R.O. present two stages of mass loss, the first between 120 °C and 300 °C associated with loss of unsaturated chains and the second between 300 °C and 450 °C associated with degradation of the polymer chain.
4.6 Encapsulation efficiency
The efficiency of the encapsulation was quantitatively investigated by gas chromatography. The amount of the unencapsulated oil was calculated using the eqn (1) based on the area of the chromatographic signals assigned to the two major compounds of the essential oil: 1,8-cineol (Fig. 11a) and camphor (Fig. 11b).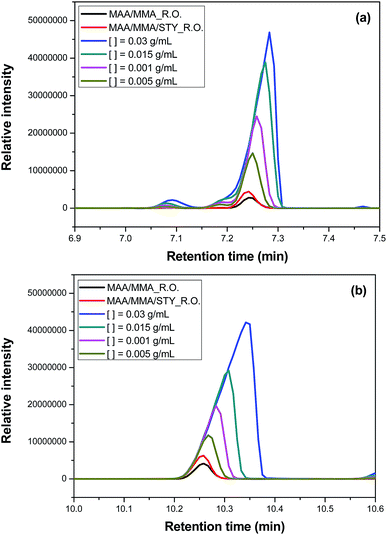 | ||
| Fig. 11 Chromatogram from gas chromatography-mass spectrometry analyses to evaluate the efficiency of the encapsulation in (a) 1,8-cineol and (b) camphor. | ||
It has been observed that the composition of the polymer influences the encapsulation efficiency. For the terpolymer containing MAA/MMA/STY the efficiency was higher than for the copolymer containing MAA/MMA. For the terpolymer, the encapsulation efficiency was 92% for camphor and 95% for 1,8-cineol. For the copolymer, was observed an efficiency of 84% for camphor and 91% for 1,8-cineol.
The encapsulation efficiency may be linked to the difference in hydrophobicity of the monomers used. As discussed above, other than MAA and MMA, STY is not soluble in the aqueous phase. When added to the composition, it improves the solubility of the essential oil in the monomer droplets. Since the suspension polymerization reaction occurs in the monomer drops, the increasing solubility of the essential oil in the monomer drop increases the efficiency of the encapsulation of the essential oil.
4.7 Antifungal activity of polymers
In this stage of the work, are presented the results of the evaluation of the antifungal activity of the polymers and the capsules.Due to the broth microdilution method is performed in aqueous medium, the solubility of some components in water may directly influences the results of the assay. Furthermore, in literature, some works showed that the components of the oils interact with the polystyrene of the plate where the tests are carried out, influencing the final results.8,23
Therefore, it would be expected that the polymers containing essential oil would have lower antifungal activity when compared to free essential oil activity, but this is not observed when analyzing the test results.
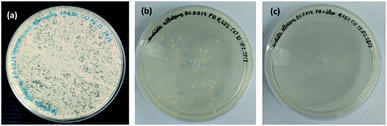
In Fig. 12, when comparing the Candida albicans culture plates with the same concentration (0.25%) of essential oil (Fig. 12a), terpolymer (MAA/MMA/STY) (Fig. 12b) and encapsulated essential oil MAA/MMA/STY_R.O (Fig. 12c), a significant difference in the fungus growth evidencing that the encapsulated material presents better antifungal activity than the other materials. When the encapsulated material was used, the fungal growth was not observed.
It was also observed that even the polymer that does not contain essential oil showed antifungal activity. This observation suggests that, besides the oil, there is some other component in the formulation of the polymer with antifungal activity (Table 7).
To facilitate the formation of the oil-water suspension, Tween 80 was added to the medium, a surfactant able to assist in the formation of the suspension. Griffin et al.8 observed that the results of microdilution tests are influenced by the concentration of Tween used.
| Candida albicans ATCC SC5324 | Candida glabrata ATCC 2001 | Candida parapsilosis ATCC 22019 | ||||
|---|---|---|---|---|---|---|
| MIC (%) | MFC (%) | MIC (%) | MFC (%) | MIC (%) | MFC (%) | |
| MAA/MMA | 0.03 | 0.06 | 0.06 | 0.13 | <0.03 | 0.03 |
| MAA/MMA_R.O. | 0.03 | 0.06 | 0.06 | 0.13 | <0.03 | 0.03 |
| MAA/MMA/STY | 0.25 | 0.50 | 0.13 | 0.25 | 0.06 | 0.13 |
| MAA/MMA/STY_R.O. | 0.13 | 0.25 | 0.13 | 0.25 | 0.03 | 0.06 |
| Essential oil | 0.25 | 0.50 | 0.25 | 0.50 | 0.50 | 1.00 |
By increasing the Tween concentration, they found smaller MICs and CFMs. Tween is a surfactant that can interact with fungal cell membranes. As well as Tween, PVA is a suspension agent used in the polymerization reactions and wasn't removed at the end of the process. The presence of this material in the polymer may influence the results of the antifungal activity justifying the MIC and CFM values found even for the polymers without the essential oil.
Another factor that can influence the results, would be the presence of residual PBO in the polymer, this compound has antibacterial action, being used in the pharmaceutical industry in the formulation of medications for the treatment of acne.24–26
In Fig. 13, it is possible to observe the variation of the peroxide index throughout the reaction for the terpolymer MAA/MMA/STY. As expected, the index is higher at the beginning of the reaction, when the initiator begins to be consumed due the formation of the primary radicals. Along the reaction course, the initiator is consumed and the peroxide index decays. However, even at the end of the reaction, after 240 minutes, the presence of peroxide in the material could be observed, which may influence the result of fungicidal activity for the evaluated polymers. However, when the material MAA/MMA/STY is compared with the material MAA/MMA/STY_R.O. a small improvement of antifungal activity is observed when the essential oil is present.
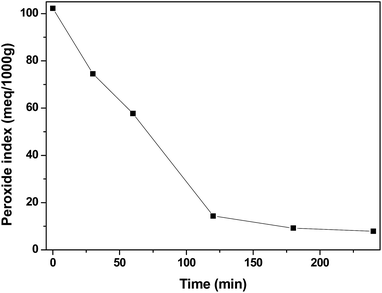 | ||
| Fig. 13 Peroxide index profile for methacrylic acid/methyl methacrylate/styrene (MAA/MMA/STY) terpolymer. | ||
4.8 Cell viability
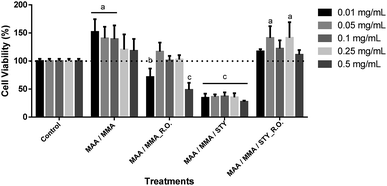
For the evaluation of the cell viability for human fibroblast (FIBRO) and keratinocyte (HaCat) lines, a 24 hour treatment was carried out for the above-mentioned strains by using concentrations of 0.01 mg mL−1, 0.05 mg mL−1, 0.1 mg mL−1, 0.25 mg mL−1 and 0.5 mg mL−1 in triplicates, as well as, triplicate of the independent experiment. Ultrapure water was used as control in order to compare the results, representing 100% of cell viability for each lineage as shown in Fig. 14 and Fig. 15.According to the obtained experimental data concerning the fibroblast line (FIBRO), MAA/MMA and MAA/MMA/STY_R.O. samples do not exhibit cytotoxicity against FIBRO. However, a decrease in cell viability after 24 hours of exposure (p < 0.05) was observed for the concentrations equal to 0.01 mg mL−1 and 0.05 mg mL−1 containing MAA/MMA_R.O., leading to a viability reduction of approximately of 30% and 40%, respectively. For MAA/MMA/STY sample, it was noticed a decrease in the cell viability in almost 50% for all concentrations, as can be observed in Fig. 14.
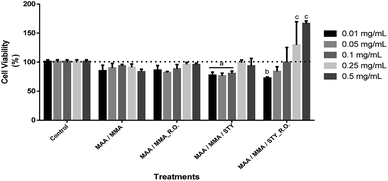
Cytotoxicity assays were also carried out with HaCat line (Fig. 15). After 24 hours of exposure of the treatments, it was observed a decrease in the cell viability between 10 and 20% in comparison with the control (p < 0.05). However, this decrease is not considered biologically relevant (Fig. 15) as the MAA/MMA/STY sample presented a cell viability of 20 to 30% with concentrations of 0,01 mg mL−1, 0,05 mg mL−1, 0,1 mg mL−1 when compared with the control. In comparison with the control, MAA/MMA/STY_R.O. sample showed a decrease in cellular viability of approximately 30% in the concentration of 0.01 mg mL−1. The results presented in Fig. 15 is very promising, indicating that the synthesized polymeric materials may be employed, as for instance, in cosmetics formulations and drug delivery applications.
Considering the results obtained only MAA/MMA/STY sample exhibited considerable toxicity for the cells tested. However, the toxicity exhibited by MAA/MMA/STY terpolymer may be associated to the presence of residual styrene into the polymer matrix. As shown in Fig. 5, the monomers conversion was not complete, and the residual styrene may have influenced the cytotoxicity results. The toxicity of styrene27 has been previously explored in studies published in the open literature, which consider that a toxicity effect caused by covalent binding to essential cellular macromolecules.28 Parkki et al.29 and Sumner et al.30 have observed during in vivo studies that the inhalation exposure of styrene is responsible for the degeneration or changes in organs such as liver.
The cell assays are essential for obtaining experimental data that may designate toxicity against non-pathological cell lines of a compound or component under study in order to be able to assess its biocompatibility.31
5. Conclusions
In this work we investigate the antifungal activity of the essential oil of Rosmarinus officinalis before and after the encapsulation process. The oil was encapsulated via suspension polymerization using as monomers methyl methacrylate, styrene and methacrylic acid. The methodology adopted allowed the control of the size of the capsules and the SEM images show the material with spherical morphology.The characterization studies of the polymer material show that the presence of the essential oil does not have a great influence on the properties of the material such as glass transition temperature and thermal stability, showing that the influence of the oil in the conversion in the initial times is due to the antioxidant activity of the oil.
The encapsulation efficiency test shows the influence of the polymer composition on the encapsulation of the essential oil, obtaining efficiency above 90% for the terpolymer containing methacrylic acid/methyl methacrylate/styrene and above 84% for the copolymer containing MAA/MMA.
The study of the antifungal activity of the oil shows that, before and after the encapsulation process, the materials present antifungal activity for the tested species.
Considering to the results from cytotoxicity assays, only the MAA/MMA/STY terpolymer showed considerable toxicity, whose behavior may be associated to the presence of residual styrene into the polymer matrix. On the other hand, the other polymeric materials tested, exhibited a non-toxic feature and may be used for other technological purposes such as cosmetic applications, as carriers of drugs, fungicides or bactericides, as the polymeric materials synthesized here present desirable chemical, physical and biological properties.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES – Finance Code 001) and Fundação de Apoio à Pesquisa do Distrito Federal (FAPDF). The authors thank Prof. Daniela Mara Oliveira for kindly donating fibroblast cell lines.References
- T. C. Vlahovic, Clin. Podiatr. Med. Surg., 2016, 33, 305 CrossRef PubMed.
- R. Kumar, S. K. Shukla, A. Pandey, H. Pandey, A. Pathak and A. Dikshit, Int. J. Pharm. Sci. Res., 2016, 7, 3218 Search PubMed.
- W. P. Silva-Rocha, M. F. de Azevedo and G. M. Chaves, J. Med. Mycol., 2017, 27, 57 CrossRef CAS PubMed.
- A. M. Ojeda-Sana, C. M. van Baren, M. A. Elechosa, M. A. Juarez and S. Moreno, Food Control, 2013, 31, 189 CrossRef CAS.
- J. Sikkema, J. A. M. Debont and B. Poolman, Microbiol. Rev., 1995, 59, 201 CAS.
- S. Uribe, J. Ramirez and A. Pena, J. Bacteriol., 1985, 161, 1195 CAS.
- H. J. D. Dorman and S. G. Deans, J. Appl. Microbiol., 2000, 88, 308 CrossRef CAS PubMed.
- S. G. Griffin, S. G. Wyllie, J. L. Markham and D. N. Leach, Flavour Fragrance J., 1999, 14, 322 CrossRef CAS.
- N. D. Bomfim, L. P. Nakassugi, J. F. P. Oliveira, C. Y. Kohiyama, S. A. G. Mossini, R. Grespan, S. B. Nerilo, C. A. Mallmann, B. A. Abreu and M. Machinski, Food Chem., 2015, 166, 330 CrossRef PubMed.
- B. Bozin, N. Mlmica-Dukic, I. Samojlik and E. Jovin, J. Agric. Food Chem., 2007, 55, 7879 CrossRef CAS PubMed.
- A. V. Podshivalov, S. Bronnikov, V. V. Zuev, T. Jiamrungraksa and S. Charuchinda, J. Microencapsul., 2013, 30, 198 CrossRef CAS PubMed.
- J. C. Ribeiro, W. L. C. Ribeiro, A. L. F. Camurca-Vasconcelos, I. T. F. Macedo, J. M. L. Santos, H. C. B. Paula, J. V. Araujo, R. D. Magalhaes and C. M. L. Bevilaqua, Vet. Parasitol., 2014, 204, 243 CrossRef CAS PubMed.
- V. Sanna, G. Lubinu, P. Madau, N. Pala, S. Nurra, A. Mariani and M. Sechi, J. Agric. Food Chem., 2015, 63, 2026 CrossRef CAS PubMed.
- C. X. Wang, A. L. Tian, C. X. Wang and S. H. Fu, Polym. Bull., 2016, 73, 1267 CrossRef CAS.
- B. Pena, C. Panisello, G. Areste, R. Garcia-Valls and T. Gumi, Chem. Eng. J., 2012, 179, 394 CrossRef CAS.
- I. Hofmeister, K. Landfester and A. Taden, Macromolecules, 2014, 47, 5768 CrossRef CAS.
- L. Xie and Z.-H. Luo, Macromol. React. Eng., 2016, 10, 479 CrossRef CAS.
- N. S. Almeida, L. E. C. Benedito, A. O. Maldaner and A. L. de Oliveira, J. Braz. Chem. Soc., 2018, 29, 1944 CAS.
- K. A. Hammer, C. F. Carson and T. V. Riley, J. Appl. Microbiol., 2003, 95, 853 CrossRef CAS.
- S. D. Cox, C. M. Mann, J. L. Markham, H. C. Bell, J. E. Gustafson, J. R. Warmington and S. G. Wyllie, J. Appl. Microbiol., 2000, 88, 170 CrossRef CAS PubMed.
- B. R. Vijayendran, J. Appl. Polym. Sci., 1979, 23, 733 CrossRef CAS.
- C. R. E. Mansur, M. I. B. Tavares and E. E. C. Monteiro, J. Appl. Polym. Sci., 2000, 75, 495 CrossRef CAS.
- C. F. Carson, K. A. Hammer and T. V. Riley, Clin. Microbiol. Rev., 2006, 19, 50 CrossRef CAS PubMed.
- M. Kawashima, H. Hashimoto, A. B. Alio Saenz, M. Ono and M. Yamada, J. Dermatol., 2014, 41, 795 CrossRef CAS PubMed.
- R. M. Mays, R. A. Gordon, J. M. Wilson and S. Silapunt, Dermatol. Ther., 2012, 25, 23 CrossRef PubMed.
- L. H. Kircik, J. Drugs Dermatol. JDD, 2014, 13, S57 CAS.
- M. E. Andersen, G. Cruzan, M. B. Black, S. N. Pendse, D. E. Dodd, J. S. Bus, S. S. Sarang, M. I. Banton, R. Waites, D. B. Layko and P. D. McMullen, Regul. Toxicol. Pharmacol., 2018, 96, 153 CrossRef CAS PubMed.
- M. L. Scapellato, G. Marcuzzo, G. Mastrangelo, G. Sessa, M. Cellini, E. D. Rosa, B. Saia and G. B. Bartolucci, J. Occup. Health, 1998, 40, 313 CrossRef CAS.
- M. G. Parkki, J. Marniemi and H. Vainio, Toxicol. Appl. Pharmacol., 1976, 38, 59 CrossRef CAS PubMed.
- S. C. J. Sumner, R. C. Cattley, B. Asgharian, D. B. Janszen and T. R. Fennell, Chem. Biol. Interact., 1997, 106, 47 CrossRef CAS PubMed.
- L. A. V. de Luna, A. C. M. de Moraes, S. R. Consonni, C. D. Pereira, S. Cadore, S. Giorgio and O. L. Alves, J. Nanobiotechnol., 2016, 14, 12 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2019 |