Open Access Article
Open Access ArticleEfficient conversion of ethanol to 1-butanol and C5–C9 alcohols over calcium carbide†
Dong Wang,
Zhenyu Liu and
Qingya Liu *
*
State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing, 100029, China. E-mail: qyliu@mail.buct.edu.cn
First published on 17th June 2019
Abstract
Production of 1-butanol or alcohols with 4–9 carbon atoms (C4–C9 alcohols) from widely available bio-ethanol has attracted much interest in recent years in academia and industry of renewable chemicals and liquid fuels. This work discloses for the first time that calcium carbide (CaC2) has a superior catalytic activity in condensation of ethanol to C4–C9 alcohols at 275–300 °C. The 1-butanol yield reached up to 24.5% with ethanol conversion of 62.4% at the optimized conditions. The by-products are mainly alcohols with 5–9 carbons besides 2-butanol, and the total yield of all the alcohols reached up to 56.3%. The reaction route was investigated through controlled experiments and quantitative analysis of the products. Results indicated that two reaction routes, aldol-condensation and self-condensation, took place simultaneously. The aldol-condensation route involves coupling of ethanol with acetaldehyde (formed from ethanol dehydrogenation) to form 2-butenol, which is subsequently hydrogenated to 1-butanol. The alkynyl moiety in CaC2 plays an important role in the catalytic pathways of both routes and affords the good activity of CaC2. CaC2 is converted to acetylene [C2H2] and calcium hydroxide [Ca(OH)2] simultaneously by the H2O that was generated from the condensation of alcohols.
1. Introduction
With gradual depletion of fossil fuels, widespread concerns have been raised regarding production of fuels or chemicals from renewable biomass such as plants, agriculture wastes and forest residues.1,2 Ethanol has been produced on industrial scales through fermentation of lignocellulose3 and is one of the largest volume bio-fuels. It is used directly as a fuel or blended in gasoline as an additive;4,5 however, a relatively low energy density, high hygroscopicity and corrosion to metal parts restrict its application in the transportation sector.6 1-Butanol is considered to be an excellent alternative to ethanol because it possesses an energy density closer to gasoline, low solubility in water and less-corrosive nature.7,8 1-Butanol is also a bulk platform chemical for synthesis of 1-butyl esters plasticizer, general extraction agent of perfumes and drugs, and paint solvent.9 Although fermentation of biomass to 1-butanol has been developed, economical production on an industrial scale was reported to remain a challenge.10 Efficient conversion of ethanol to 1-butanol has been of great interest during the past decade.The Guerbet reaction is well-known and converts a primary aliphatic alcohol into its β-alkylated dimer alcohol by elimination of H2O without the need for hydrogen. Many catalysts have been developed for this transformation, including hybrid homogeneous-heterogeneous catalysts (alkali metal hydroxides or alkoxides in the presence of RANEY® nickel/copper),11,12 homogeneous Ru and Ir complexes13,14 and heterogeneous basic solids. The hybrid and homogeneous catalysts show high activities and excellent selectivity towards 1-butanol under mild reaction conditions but suffer from corrosion or separation problems.15 Heterogeneous catalysts overcome these problems and have been widely studied in both flow and batch reactors, such as hydroxyapatites (HAP),16–19 MgO,20,21 Mg–Al oxides or metal-doped ones,22–27 Cu/CeO2,28 Ni/Al2O3,29 Na/ZrO2 (ref. 30) and alkali-exchanged zeolites.31 The relatively optimal ethanol conversion and 1-butanol yield of these catalysts, along with the reaction conditions, are summarized in Table S1.† On the whole, studies with flow reactors were carried out at higher temperatures than those with batch reactors. The maximum 1-butanol yield was 30.0% over Cu/CeO2 catalyst at 330 °C in a flow reactor, and the maximum space-time yield was 705 gpro kgcat−1 h−1 over Cu10Ni10-porous metal oxides (hydrotalcites) at 320 °C with a batch reactor. Development of a highly efficient heterogeneous catalyst at mild reaction conditions is still a hot topic for synthesis of 1-butanol or higher alcohols from ethanol.
The Guerbet reaction mechanism over heterogeneous catalysts has been widely discussed and are overviewed herein in order to develop a novel catalyst. A number of authors have proposed aldol-condensation route which involves several consecutive steps as shown in Scheme 1.32,33 The primary alcohol is firstly oxidized to aldehyde by dehydrogenation. Then, the β-C in one aldehyde molecule attacks another aldehyde molecule to form hydroxyl-aldehyde, followed by dehydration to form olefin-aldehyde. The olefin-aldehyde is finally reduced to dimer alcohol by hydrogenation. In this route, the acid sites of heterogeneous catalysts adsorb ethanol and acetaldehyde, and the base sites catalyze the hydrogen transfer reaction.16,19,22,34 A direct condensation mechanism shown in Scheme 2 has also been reported on various basic solids,17,18,20 in which the β-H in ethanol is activated by a catalyst and the activated ethanol molecule subsequently condenses with another ethanol molecule through dehydration to form 1-butanol. In both routes, activation of β-H is considered to be a crucial step.20,35
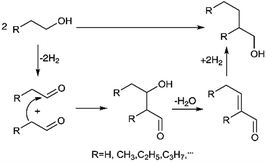 | ||
| Scheme 1 Conventional aldol-condensation route of primary alcohol to dimer one.32,33 | ||
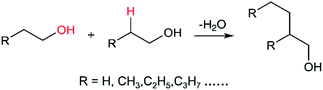 | ||
| Scheme 2 Direct condensation route of primary alcohol to dimer one.17,18,20 | ||
It is reported that the alkynyl moiety of calcium carbide (CaC2) is susceptible to nucleophilic attack of the acidic terminal hydrogen,36 which makes it possible to promote β-H activation and hydrogen transfer. CaC2 has been found to have a superior activity in activating C–H bond of acetone during synthesis of isophorone.37 Besides C–H activation, the Ca2+ in CaC2, similar to that in HAP, may serve as an acidic site to adsorb alcohols to initiate its condensation. These analyses indicate a potential catalytic application of CaC2 in ethanol condensation. If CaC2 takes a catalytic effect, it may convert in situ to C2H2 due to formation of H2O in ethanol condensation. That is, this protocol may couple the catalytic activity and reactivity of CaC2. CaC2 has been reported to be produced from bio-char at lower temperatures by auto-thermal heating,38,39 which renders the idea to be more attractive. In the present contribution, condensation of ethanol was evaluated over CaC2 in a batch reactor at different reaction conditions. The reaction pathway was investigated through detailed analysis of the main products and controlled experiments.
2. Experimental
2.1 Material
CaC2 with a purity of 97%+ (Acros Organics) was ground and screened to 80–100 mesh in a glove box under nitrogen atmosphere. The alcohols including methanol, ethanol, 1-propanol, isopropanol, 1-butanol and 2-butanol (Beijing Chemical Works) were dehydrated with 3A molecular sieves. The commercial reagents of calcium ethoxide (95%), calcium hydroxide, calcium oxide, magnesia, HAP and hydrotalcites (Mg/Al = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were used as received.
1) were used as received.
2.2 Reaction experiment
Typically, ethanol (10.0 g, 217 mmol) and CaC2 (1.5 g, 23 mmol) were added into a quartz tube in a stirred autoclave reactor (Parr 4597, 50 mL). Then, the reactor was sealed, purged with Ar of 2.0 MPa for 5 times to remove air and heated to a specified temperature under stirring at a speed of 200 rpm. After the indicated reaction time, the reactor was cooled to room temperature with ice water. The gas product was collected carefully with a gas collecting bag, and the solid and liquid products were quantitatively transferred to a centrifuge tube under nitrogen atmosphere for separation.2.3 Analysis of reaction products
The composition of gas product was analyzed by a gas chromatography (Agilent GC-7890B) equipped with a thermal conductivity detector (TCD) and two packed columns of Agilent Porapak Q (6 ft × 1/8 × 2.0 mm) and Mol Sieve 5A (6 ft × 1/8 × 2.0 mm). He of 30 mL min−1 was employed as the carrier gas. The temperatures of injection port, column and detector were 100, 70 and 200 °C, respectively. The average density of gas product was estimated according to its composition; and the mass of gas product was estimated according to the average density and the volume of gas product at the atmospheric pressure (detailed calculation can be found in ESI†).The solid product was dried at 120 °C under vacuum for 12 h and then weighed. X-ray diffraction (XRD) analysis of the solid product was performed on a D8FOCUS Powder diffractometer using Cu Kα radiation (λ = 1.5432 Å) at 40 kV and 40 mA. Step scans were taken over a range of 2θ from 5 to 80° at a speed of 6° min−1. The organic carbon content of the solid product was analyzed by a total organic carbon analyzer (SSM-5000A, Shimadzu, Japan).
The mass of liquid product was obtained by subtracting the mass of gas and solid products from the total mass of reactants. Qualitative analysis of the liquid product was performed on an Agilent GC-mass spectrometry (GC-MS) equipped with a HP-5 capillary column (30 m × 0.25 mm × 0.25 μm) and a quadrupole analyzer system (5977B) with high efficiency ion source (HES) GC/MSD operated at 300 °C. The carrier gas was 1.0 mL min−1 He, the sample dosage was 1.0 μL and the split ratio was 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The injection temperature was 250 °C, and the temperature program of column was 35 °C for 5 min, 10 °C min−1 to 230 °C and 230 °C for 1 min. The scan m/z of MS ranged from 30 to 350.
1. The injection temperature was 250 °C, and the temperature program of column was 35 °C for 5 min, 10 °C min−1 to 230 °C and 230 °C for 1 min. The scan m/z of MS ranged from 30 to 350.
Quantitative analysis of the liquid products of interest was performed on an Agilent GC-7890B equipped with a HP-5 capillary column (30 m × 0.32 mm × 0.25 μm) and a flame ionization detector (FID). Acetone was used as the internal standard and the mass ratio of acetone to sample was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. N2 was used as the carrier gas at a flow rate of 1.0 mL min−1 and the detector temperature was 250 °C. The other conditions were the same as those of GC-MS.
10. N2 was used as the carrier gas at a flow rate of 1.0 mL min−1 and the detector temperature was 250 °C. The other conditions were the same as those of GC-MS.
Based on the product analysis results, ethanol conversion, yields of various alcohols, carbon yields of gas products and solid residual, as well as carbon balance (% C) were determined (For more details, see formulas (6)–(10) in ESI†).
3. Results and discussion
3.1 Qualitative analysis of the liquid product
Taking the reaction at 190 °C for 8 h as an example, the distribution of liquid product was determined by GC-MS. The total ion chromatogram shown in Fig. S1† indicates that there are twelve obvious peaks with some minor ones. MS spectra of the twelve peaks are shown in Table S2 in the ESI.† Besides ethanol, they are ethyl vinyl ether (termed EVE), 2-butanol, 1-butanol, 2-pentanol, 3-hexanol, 2-ethyl-1-butanol, 1-hexanol, 4-heptanol, 2-heptanol, 3-methylcyclohexanol and 4-nonanol. It is noted that acetaldehyde, 3-hydroxybutanal, 2-butenal and butyraldehyde frequently reported in literatures were not detected in our reaction; and tiny ethyl acetate and 2-butenol were observed (at retention time of 2.40 and 3.17 min in Fig. S1†).Obviously, all the main products except EVE are alcohols with carbon numbers from 4 to 9. Although this work focuses on the synthesis of 1-butanol, all the alcohols produced are ethanol-derived condensation products and in principle suitable as fuels or as blending agents in gasoline.40 Formation of 2-ethyl-1-butanol and 1-hexanol have been observed on many heterogeneous catalysts,19,41 which are formed by coupling of –OH in ethanol with β-H in 1-butanol and –OH in 1-butanol with β-H in ethanol, respectively. Formation of 2-butanol indicates coupling of –OH in ethanol with α-H in another ethanol; and 3-hexanol may be formed by coupling of –OH in ethanol with β-H in 2-butanol or α-H in 1-butanol. Formation of 2-pentanol is really surprising because it is odd carbon number alcohol (C5) and was seldom reported. Anyway, once the 2-pentanol is formed, it may couple with ethanol or butanol to form heptanols, 3-methylcyclohexanol and 4-nonanol (C7 and C9 alcohols).
3.2 Results of ethanol conversion and liquid product yield
The positive results were elaborated by quantifying ethanol conversion and yields of various alcohols. Herein, 1-butanol is discussed separately and the other nine alcohols are discussed as a group. The by-product ethyl acetate and intermediate 2-butenol are in trace amounts in all the runs and will not be discussed. The effect of reaction temperature was studied at 190–315 °C in 6 h and the results are summarized in entries 1–6 of Table 1. It is clear that the ethanol conversion increases from 26.4% to 50.0% with increasing the temperature. The yield of 1-butanol increases to the maximum of 19.5% with elevating temperature to 275 °C and then decrease at 300 and 315 °C, which results in the maximum selectivity of 42.4% at 275 °C. The yield of other alcohols increase in the whole temperature range, reaching up to 26.1% at 315 °C. The selectivity of total alcohols is as high as 84–85% at 275–300 °C. The selectivity of total alcohols at 190 °C is only 37.9% (10.1% plus 27.8%) due to consumption of ethanol by CaC2 to form EVE and calcium ethoxide as shown in Re (1).42 The carbon balance, including the carbon in the main liquid products, unreacted ethanol, gas products and solid residual, keeps at 95–96% at 190–300 °C and slightly decreases to 92.8% at 315 °C (Table S3 in the ESI†). Since the gas products and solid residual were quantified precisely, the decreased carbon balance at 315 °C suggests formation of more trace liquid products.
CaC2 + 3CH3CH2OH → Ca(OCH2CH3)2 + CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHOCH2CH3 CHOCH2CH3
| (1) |
| Entry | Temp. (°C) | Time (h) | Ethanol conv. (%) | 1-Butanol yield (%) | Other alcohols yield (%) | STY (gpro kgcat−1 h−1) | Carbon yieldb (%) | |
|---|---|---|---|---|---|---|---|---|
| Solid | Gas | |||||||
| a The amounts of ethanol and CaC2 are 217 and 23 mmol, respectively.b The ratio of the amount of organic carbon in gas product or solid residual to the total carbon fed into the reactor.c Number in the parentheses is the selectivity. | ||||||||
| 1 | 190 | 6 | 26.4 | 2.7 (10.1)c | 7.3 (27.8)c | 24 | 8.7 | 2.8 |
| 2 | 235 | 6 | 35.8 | 12.2 (34.1) | 12.6 (35.2) | 109 | 7.4 | 3.0 |
| 3 | 255 | 6 | 40.9 | 16.6 (40.6) | 15.8 (38.6) | 148 | 7.0 | 3.9 |
| 4 | 275 | 6 | 46.0 | 19.5 (42.4) | 19.3 (41.9) | 174 | 5.4 | 4.3 |
| 5 | 300 | 6 | 48.9 | 18.6 (38.0) | 22.9 (46.9) | 166 | 4.6 | 5.2 |
| 6 | 315 | 6 | 50.0 | 13.4 (26.8) | 26.1 (52.2) | 119 | 2.6 | 7.0 |
| 7 | 275 | 1 | 31.7 | 12.8 (40.4) | 9.9 (31.2) | 687 | 8.9 | 4.2 |
| 8 | 275 | 3 | 38.9 | 15.9 (40.9) | 14.7 (37.8) | 284 | 7.5 | 4.3 |
| 9 | 275 | 6 | 46.0 | 19.5 (42.4) | 19.3 (41.9) | 174 | 5.4 | 4.3 |
| 10 | 275 | 8 | 50.1 | 19.0 (37.9) | 24.1 (48.1) | 127 | 5.1 | 4.4 |
| 11 | 275 | 12 | 52.5 | 17.6 (33.5) | 25.0 (47.6) | 78 | 4.9 | 5.2 |
| 12 | 275 | 15 | 54.3 | 16.2 (29.8) | 25.5 (47.0) | 58 | 4.7 | 7.2 |
The effect of reaction time was investigated at 275 °C in 1–15 h and the results are summarized in entries 7–12 of Table 1. At 275 °C for 1 h, the ethanol conversion and 1-butanol yield have been as high as 31.7% and 12.8%, respectively. With increasing the reaction time, the ethanol conversion slightly increases to 54.3% in 15 h; the 1-butanol yield slightly increases to the maximum of 19.5% at 6 h and then decreases; the yield of other alcohols gradually increases in 8 h and then keeps at 24–26% in 8–12 h. The maximum yield of total alcohols of 43.1% (19.0% plus 24.1%) was achieved at 8 h with ethanol conversion of 50.1%. The carbon balance is higher than 95% in 8 h and slightly decreases after then (Table S3†), which is similar to the observation on the effect of temperature and suggests conversion of major liquid products to minor ones after 8 h. To obtain a higher 1-butanol yield, the optimal reaction conditions are 275–300 °C for 6–8 h, at which the yield of other alcohols also reaches a higher value.
The space-time yield (STY) is usually used to evaluate activities of different catalysts or change/deactivation of a catalyst. The STY in this work significantly decreases from 687 to 58 gpro kgcat−1 h−1 with increasing the reaction time from 1 to 15 h, suggesting gradual change of catalytic component. To obtain an optimal STY, the reaction time should be shorten to 1 h at the expense of 1-butonal yield.
The catalysts frequently used in literatures, Mg–Al oxides, HAP and MgO were evaluated in our batch reactor at 275 °C for 8 h and compared with CaC2 at the same conditions. As seen in Table 2, the blank test in the absence of a catalyst shows a lower ethanol conversion and little formation of 1-butanol and other alcohols. Compared to the blank test, all the catalysts improve the ethanol conversion and 1-butanol yield as expected while the efficiency of CaC2 is more obvious than those of other catalysts. Furthermore, the reaction over CaC2 yields the largest amount of other alcohols. These results indicate that CaC2 is superior to Mg–Al oxide, HAP and MgO in converting ethanol to 1-butanol and other medium-chain alcohols at 275 °C in the batch reactor. It is noted that 2-butenal was observed over HAP and Mg–Al oxide catalysts while 2-butenol was observed over CaC2 and MgO, suggesting occurrence of different aldol-condensation reactions. Formation of 2-butenol over MgO catalyst was also reported in literature.21
| Entry | Catalysta | Ethanol conv. (%) | 1-Butanol yield (%) | Other alcohols yield (%) | Yield of main by-product (%) |
|---|---|---|---|---|---|
| a The amounts of all the catalysts are 23 mmol.b Not detected. | |||||
| 1 | None | 6.4 | NDb | ND | ND |
| 2 | CaC2 | 50.1 | 19.0 | 24.1 | 1.0 (2-butenol) |
| 3 | HAP | 28.4 | 11.8 | 6.2 | 2.0 (2-butenal) |
| 4 | Mg–Al oxide (Mg/Al = 3) | 24.4 | 10.4 | 2.1 | 1.2 (2-butenal) |
| 5 | MgO | 32.9 | 9.9 | 3.6 | 1.4 (2-butenol) |
3.3 Evolution of CaC2 during ethanol reaction
The above discussion has shown the conversion of CaC2 to calcium ethoxide [Ca(OCH2CH3)2]. To further understand evolution of CaC2 at the optimized temperatures of 275–300 °C, XRD results of the solid residuals are illustrated in Fig. 1, along with those of the raw material CaC2 and Ca(OCH2CH3)2 for comparison. The typical diffraction peaks of CaC2 are located at 2θ of 28.1 and 32.7° and those of Ca(OCH2CH3)2 are located at 2θ of 10.7, 30.8 and 49.7°. As seen in Fig. 1, the raw material CaC2 contains impurities of CaO and calcium hydroxide [Ca(OH)2] (2θ = 34.1, 18.2, 50.2°). The solid residual obtained at 275 °C for 1 h shows obvious diffraction peaks of CaC2 and Ca(OCH2CH3)2 and a very weak diffraction peak of Ca(OH)2. With increasing the reaction time to 3 and 6 h, diffraction peaks of CaC2 disappear, those of Ca(OCH2CH3)2 are still obvious and weak diffraction peaks of Ca(OH)2 are discernible. The solid residuals obtained at 275 °C for 8 h and 300 °C for 6 h are mainly Ca(OH)2 with little Ca(OCH2CH3)2. It should be pointed out although little C-containing compounds were observed on the last two residuals by XRD, a certain amount of organic carbon was detected by TOC (entries 5 and 10 in Table 1) and the residuals are black; this phenomenon is attributed to EVE polymerization since it is recognized to be unstable,43 which is confirmed by the carbon balance calculation on the carbon in CaC2 (Table S4 in ESI†).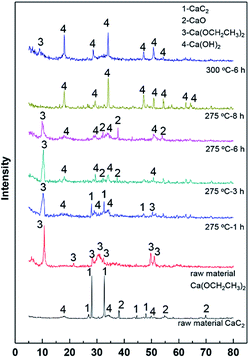 | ||
| Fig. 1 XRD patterns of the solid residuals after ethanol reaction over CaC2, along with those of the raw material CaC2 and calcium ethoxide. | ||
The XRD results clearly indicate that the main calcium compound is CaC2 initially, CaC2 and Ca(OCH2CH3)2 in 1–3 h, Ca(OCH2CH3)2 in 3–6 h and Ca(OH)2 after 6–8 h. To identify the catalytic activities of Ca(OCH2CH3)2 and Ca(OH)2, ethanol conversion over them and the impurity CaO were evaluated at 275 °C and the results are summarized in Table 3. In comparison with the blank test shown in Table 2, Ca(OCH2CH3)2, Ca(OH)2 and CaO do promote ethanol conversion and alcohols formation. However, the 1-butanol yields over Ca(OCH2CH3)2 and Ca(OH)2 for 6 h are only 6.0% and 3.8%, respectively, which are far smaller than the 1-butanol yield over CaC2 (19.5%, entry 3 in Table 3); the yields of other alcohols over Ca(OCH2CH3)2 and Ca(OH)2 are also far smaller than that of CaC2. This result accounts for the decreasing STY with increasing the reaction time (Table 1). The catalytic activity of CaO is higher than those of Ca(OCH2CH3)2 and Ca(OH)2 but obviously lower than that of CaC2. Since all the compounds are calcium-containing, the far difference in their catalytic activities indicates an important role of alkynyl group in efficient conversion of ethanol into higher alcohols.
| Entry | Ca-compound | Dosage (mmol) | Ethanol conv. (%) | 1-Butanol yield (%) | Other alcohols' yield (%) |
|---|---|---|---|---|---|
| a The amounts of ethanol in all the cases are 217 mmol. | |||||
| 1 | Ca(OCH2CH3)2 | 23 | 14.3 | 6.0 | 5.4 |
| 2 | Ca(OH)2 | 23 | 12.6 | 3.8 | 3.0 |
| 3 | CaC2 | 23 | 46.0 | 19.5 | 19.3 |
| 4 | CaO | 23 | 32.9 | 10.3 | 8.1 |
| 5 | CaC2 | 12 | 30.6 | 12.2 | 11.2 |
| 6 | CaC2 | 34 | 62.4 | 24.5 | 31.8 |
Since CaC2 is gradually converted, the effects of CaC2 loading on ethanol conversion and yields of alcohol products were studied at 275 °C. The results shown in Table 3 indicate that the yields of 1-butanol and other alcohols increase with the CaC2 loading (entries 3, 5 and 6) and they are roughly in proportion (Fig. S2 in ESI†). The STYs of 1-butanol at CaC2 loadings of 12, 23 and 34 mmol are 203, 174 and 144 gpro kgcat−1 h−1, respectively, indicating a decreasing catalytic efficiency of CaC2 with increasing the loading.
3.4 Reaction pathway of ethanol to 1-butanol over CaC2 at 275–300 °C
As said above, formation of 2-butenol suggests that the ethanol reaction over CaC2 may involve the aldol-condensation route with 2-butenol as intermediate. This route starts from the formation of acetaldehyde by ethanol dehydrogenation, but little acetaldehyde was detected in this work. To find evidence for the formation of acetaldehyde, the amounts of various gas products are shown in Table 4. It is clear that a certain amount of H2 was produced, which confirms occurrence of ethanol dehydrogenation to form acetaldehyde. Little acetaldehyde in the product suggests that its aldol reaction is very fast, although aldol reaction is considered to the limiting step in many reports.16,32,33,44 It is noted that C2H2, C2H4, CH4 and CO are also observed in the order of C2H2 > C2H4 > CH4 ≈ CO. Minor CH4 and CO indicates decomposition of acetaldehyde as reported in literatures45 and C2H4 indicates dehydration of ethanol;21–23 C2H2 undoubtedly results from CaC2 conversion.| Entry | Temp. (°C) | Time (h) | Amount of products (mmol) | |||||
|---|---|---|---|---|---|---|---|---|
| C2H2 | C2H4 | CH4 | CO | H2 | EVE | |||
| a The amounts of ethanol and CaC2 are 217 and 23 mmol, respectively. | ||||||||
| 1 | 275 | 1 | 9.0 | 0.9 | 0.2 | 0.1 | 5.9 | 0.9 |
| 2 | 275 | 3 | 8.0 | 1.9 | 0.5 | 0.3 | 7.2 | 1.1 |
| 3 | 275 | 6 | 7.4 | 2.5 | 0.6 | 0.5 | 10.5 | 1.2 |
| 4 | 275 | 8 | 6.9 | 2.8 | 0.9 | 0.7 | 14.9 | 1.3 |
| 5 | 275 | 12 | 6.6 | 4.0 | 1.9 | 1.8 | 16.8 | 1.4 |
| 6 | 275 | 15 | 6.2 | 6.7 | 4.7 | 4.1 | 20.2 | 1.4 |
| 7 | 300 | 6 | 8.0 | 2.8 | 1.7 | 1.5 | 16.0 | 0.8 |
To further confirm the aldol-condensation reaction over CaC2, reactions of ethanol with all the possible intermediates, acetaldehyde, 2-butenal and 2-butenol, were carried out at 275 °C for 6 h. For comparison, the total amount of carbon in all the runs are the same. Results shown in Table 5 indicate that CaC2 promotes reaction of ethanol with all the intermediates to form 1-butanol and the 1-butanol yield follows the order: 2-butenol > 2-butenal > acetaldehyde. Furthermore, trace amounts of 2-butenal and 2-butenol are detected for ethanol + acetaldehyde reaction and a quantity of 2-butenol is detected for ethanol + 2-butenal reaction. These observations indicate that aldol-condensation via 2-butenal intermediate may also occur but that via 2-butenol intermediate proceeds more easily. Hydrogenation of 2-butenol to form 1-butanol requires hydrogen source. Although the hydrogen source is described as H2 in Scheme 1, it is really activated hydrogen atoms derived from ethanol dehydrogenation.16 These hydrogen atoms are consumed completely by olefin-aldehyde or enol species according to the stoichiometry. However, a certain amount of H2 was released in this work, as observed over HAP at 350–410 °C,18 suggesting that other hydrogen sources are involved in the hydrogenation reaction. Ethanol was reported to be an alternative and the reaction was said to be Re (2).18,32 In fact, the Re (2) is really hydrogenation of 2-butenol by activated hydrogen of ethanol, which also results in the by-product acetaldehyde. The absence of acetaldehyde in this work suggests that hydrogenation of 2-butenol may not be as Re (2) even if ethanol provides hydrogen or there are other undiscovered hydrogen sources.
CH3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2OH + C2H5OH → CH3CH2CH2CH2OH + CH3CHO CHCH2OH + C2H5OH → CH3CH2CH2CH2OH + CH3CHO
| (2) |
| Entry | Reactants | Ethanol conversion (%) | Intermediate conversion (%) | Yield (%) | |||
|---|---|---|---|---|---|---|---|
| 1-Butanol | 2-Butenal | 2-Butenol | Other alcohols | ||||
| a Reaction condition: 275 °C, 6 h, 23 mmol CaC2; the total amounts of reactants in all the runs are 217 mmol. | |||||||
| 1 | Ethanol/acetaldehyde (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
33.2 | 64.8 | 10.7 | 1.2 | 1.9 | 7.3 |
| 2 | Ethanol/2-butenal (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
40.9 | 66.2 | 11.9 | — | 6.4 | 8.8 |
| 3 | Ethanol/2-butenol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
37.8 | 70.4 | 14.1 | 0.3 | — | 9.7 |
| 4 | Ethanol | 46.0 | — | 19.5 | 0.0 | 0.6 | 19.3 |
It should be noted that the 1-butanol yield of ethanol itself reaction is obviously higher than those of ethanol with 2-butenol, 2-butenal or acetaldehyde (Table 5). That is to say, substitution of a certain amount of ethanol with intermediates is unbeneficial to the formation of higher alcohols. This information suggests that in addition to the aldol-condensation, self-condensation of ethanol over CaC2 may also take place. However, it is difficult to quantify the proportions of these two routes.
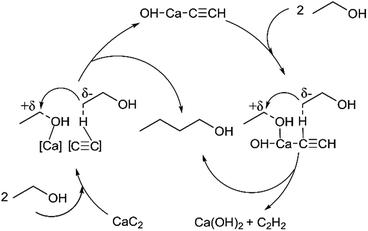
Although the hydrogen source is still vague, aldol-condensation does take place during ethanol coupling reaction over CaC2 at 275–300 °C. As addressed above, the alkynyl moiety plays an important role in the catalytic activity of CaC2. By taking this into account and referencing the catalytic mechanism of the heterogeneous catalysts, adsorption and activation of ethanol as well as condensation of acetaldehyde with ethanol over CaC2 are speculated and illustrated in Scheme 3. Firstly, ethanol is hydrogen-dissociatively adsorbed as aldehyde or enol species (a) where the H in the hydroxyl group and β-H of ethanol are adsorbed on the alkynyl moiety of CaC2 (base sites) and the O in the hydroxyl group and α-H of ethanol are adsorbed on the Ca atom (Lewis acid sites). Next, the aldehyde species react with the neighboring adsorbed ethanol molecule via nucleophilic addition to form unsaturated C4 alcohol species like 2-butenol (b). Finally, the adsorbed 2-butenol is hydrogenated to form 1-butanol by the proton-like hydrogen (c). In this process, one CaC2 molecule is converted to calcium acetylide [HO–Ca–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH]. The [HO–Ca–C
CH]. The [HO–Ca–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH] could continue to catalyze ethanol condensation while itself is finally converted to Ca(OH)2 and C2H2.
CH] could continue to catalyze ethanol condensation while itself is finally converted to Ca(OH)2 and C2H2.
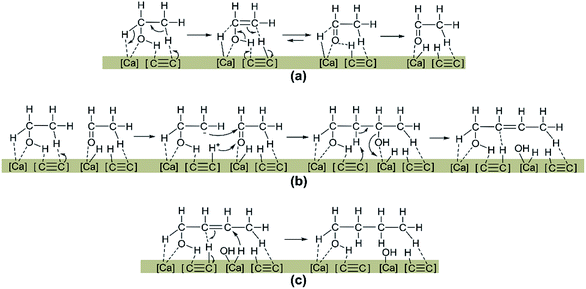 | ||
| Scheme 3 A plausible CaC2-catalyzed aldol-condensation of ethanol to 1-butanol. (a) dehydrogenation of ethanol; (b) aldol condensation; (c) hydrogenation. | ||
Self-condensation of ethanol should also involve β-H activation and a plausible catalytic pathway is shown in Scheme 4. The β-H in one ethanol molecule is adsorbed on the alkynyl moiety of CaC2 and the hydroxyl group of another ethanol molecule is adsorbed on the Ca atom of the same CaC2 molecule. Then the corresponding β-C combine with the α-C to form 1-butanol. In this process, CaC2 is converted to [HO–Ca–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH], which continues to activate β-H and hydroxyl group in different ethanol molecules, resulting in formation of another 1-butanol molecule. In comparison to the above aldol-condensation route, two ethanol molecules are activated simultaneously in the self-condensation route, which seems simpler and more feasible. Anyway, the strong hydrogen abstraction ability of alkynyl moiety is of importance in both routes.
CH], which continues to activate β-H and hydroxyl group in different ethanol molecules, resulting in formation of another 1-butanol molecule. In comparison to the above aldol-condensation route, two ethanol molecules are activated simultaneously in the self-condensation route, which seems simpler and more feasible. Anyway, the strong hydrogen abstraction ability of alkynyl moiety is of importance in both routes.
3.5 Exploration of substrate scope
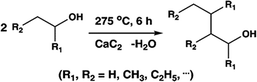
Reactions of aliphatic alcohols other than ethanol were tested in the presence of CaC2 at 275 °C in order to understand tolerance of this protocol. The yields of main alcohol products are shown in Table 6. It is clear that methanol fails to yield ethanol while other alcohols, either straight-chain or branched-chain, succeed in generating β-alkylated dimer alcohols. The yield of 2-ethyl-1-hexanol coupled by 1-butanol is as high as 32.6%. These results indicate the importance of β-H in condensation of alcohols over CaC2. The yield of dimer alcohols generated from straight-chain primary alcohols is much higher than that of generated from the branched-chain due to a lower steric hindrance.46 For example, 1-propanol and 1-butanol yield more dimer alcohols than isopropanol and 2-butanol (entries 3–6 in Table 6). If an alcohol contains more than one β-C, the condensation reaction occurs at the β-C with the most hydrogen atoms. For example, the condensation product of 2-butanol is mainly 5-methyl-l,3-heptanol (entry 6 in Table 6) rather than 3,4-dimethyl-l,2-hexanol. In short, CaC2 is effective in catalyzing condensation of a wide range of aliphatic alcohols with β-C, affording the products in moderate to good yields.| Entry | Substrate | Alcohols conversion (%) | Main product | Yield (%) |
|---|---|---|---|---|
| a The amounts of alcohol and CaC2 are 217 and 23 mmol, respectively. | ||||
| 1 |  |
15.7 |  |
0.0 |
| 2 | 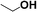 |
46.0 |  |
19.5 |
| 3 |  |
24.7 | 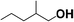 |
11.4 |
| 4 | 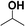 |
20.1 | 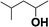 |
9.6 |
| 5 |  |
49.8 | 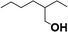 |
32.6 |
| 6 | 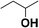 |
17.5 | 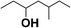 |
8.8 |
4. Conclusions
This work firstly indicates that the commercial CaC2 could be used directly to catalyze conversion of widely available ethanol to 1-butanol and other C5–C9 alcohols (liquid fuels) with satisfactory yields. The yield of 1-butanol reached up to 20–25% and that of total alcohols reached up to 39–56% with ethanol conversions of 46–64% at 275 °C and molar ratios of ethanol to CaC2 of 9.4–6.4. Both aldol-condensation route and self-condensation route are involved in the ethanol coupling over CaC2. The alkynyl moiety in CaC2 plays an important role in the catalytic pathways of both routes, which could be attributed to its strong hydrogen abstraction ability. CaC2 is also effective in catalyzing condensation of a wide range of aliphatic alcohols with β-C, featuring excellent substrate tolerance. This finding opens up the catalytic application of CaC2 in organic synthesis involving activation of C–H bond. In this process, CaC2 is gradually converted to C2H2 and Ca(OH)2. Development of a novel reactor for efficient in situ conversion of C2H2 to ethyl vinyl ether is prospective.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Major State Basic Research Project (No. 2011CB201306).References
- B. R. Caes, R. E. Teixeira, K. G. Knapp and R. T. Raines, ACS Sustainable Chem. Eng., 2015, 3, 2591–2605 CrossRef CAS.
- O. D. Mante, T. A. Butcher, G. Wei, R. Trojanowski and V. Sanchez, Energy Fuels, 2015, 29, 6536–6543 CrossRef CAS.
- Q. Zhu, C. Shen, J. Wang and T. Tan, ACS Sustainable Chem. Eng., 2017, 5, 8181–8191 CrossRef CAS.
- M. A. Qubeissi, N. A. Esawi, S. S. Sazhin and M. Ghaleeh, Energy Fuels, 2018, 32, 6498–6506 CrossRef.
- R. M. Balabin, R. Z. Syunyaev and S. A. Karpov, Energy Fuels, 2007, 21, 2460–2465 CrossRef CAS.
- B. Zhang and S. M. Sarathy, Appl. Energy, 2016, 181, 38–53 CrossRef CAS.
- Y. Xu and C. T. Avedisian, Energy Fuels, 2015, 29, 3467–3475 CrossRef CAS.
- B. G. Harvey and H. A. Meylemans, J. Chem. Technol. Biotechnol., 2011, 86, 2–9 CrossRef CAS.
- M. Uyttebroek, W. V. Hecke and K. Vanbroekhoven, Catal. Today, 2015, 239, 7–10 CrossRef CAS.
- L. A. Hazelwood, J. M. Daran, A. J. V. Maris, J. T. Pronk and J. R. Dickinson, Appl. Environ. Microbiol., 2008, 74, 2259–2266 CrossRef CAS PubMed.
- C. Carlini, M. D. Girolamo, A. Macinai, M. Marchionna, M. Noviello, A. M. R. Galletti and G. Sbrana, J. Mol. Catal. A: Chem., 2003, 200, 137–146 CrossRef CAS.
- R. Cano, M. Yus and D. J. Ramon, Chem. Commun., 2012, 48, 7628–7630 RSC.
- K. N. T. Tseng, S. Lin, J. W. Kampf and N. K. Szymczak, Chem. Commun., 2016, 52, 2901–2904 RSC.
- G. Xu, T. Lammens, Q. Liu, X. Wang, L. Dong, A. Caiazzo, N. Ashraf, J. Guan and X. Mu, Green Chem., 2014, 16, 3971–3977 RSC.
- F. Su and Y. Guo, Green Chem., 2014, 16, 2934–2957 RSC.
- S. Ogo, A. Onda, Y. Iwasa, K. Hara, A. Fukuoka and K. Yanagisawa, J. Catal., 2012, 296, 24–30 CrossRef CAS.
- T. Tsuchida, S. Sakuma, T. Takeguchi and W. Ueda, Ind. Eng. Chem. Res., 2006, 45, 8634–8642 CrossRef CAS.
- J. Scalbert, F. Thibault-Starzyk, R. Jacquot, D. Morvan and F. Meunier, J. Catal., 2014, 311, 28–32 CrossRef CAS.
- L. Silvester, J. F. Lamonier, J. Faye, M. Capron, R. N. Vannier, C. Lamonier, J. L. Dubois, J. L. Couturier, C. Calais and F. Dumeignil, Catal. Sci. Technol., 2015, 5, 2994–3006 RSC.
- A. S. Ndou, N. Plint and N. J. Coville, Appl. Catal., A, 2003, 251, 337–345 CrossRef CAS.
- T. W. Birky, J. T. Kozlowski and R. J. Davis, J. Catal., 2013, 298, 130–137 CrossRef CAS.
- D. L. Carvalho, R. R. D. Avillez, M. T. Rodrigues, L. E. P. Borges and L. G. Appel, Appl. Catal., A, 2012, 415–416, 96–100 CrossRef CAS.
- M. León, E. Díaz and S. Ordónez, Catal. Today, 2011, 164, 436–442 CrossRef.
- M. León, E. Díaz, A. Vega, S. Ordónez and A. Auroux, Appl. Catal., B, 2011, 102, 590–599 CrossRef.
- O. M. Perrone, F. Lobefaro, M. Aresta, F. Nocito, M. Boscolo and A. Dibenedetto, Fuel Process. Technol., 2018, 177, 353–357 CrossRef CAS.
- I. C. Marcu, D. Tichit, F. Fajula and N. Tanchoux, Catal. Today, 2009, 147, 231–238 CrossRef CAS.
- Z. Sun, A. C. Vasconcelos, G. Bottari, M. C. A. Stuart, G. Bonura, C. Cannilla, F. Frusteri and K. Barta, ACS Sustainable Chem. Eng., 2017, 5, 1738–1746 CrossRef CAS.
- J. H. Earley, R. A. Bourne, M. J. Watson and M. Poliakoff, Green Chem., 2015, 17, 3018–3025 RSC.
- T. L. Jordison, C. T. Lira and D. J. Miller, Ind. Eng. Chem. Res., 2015, 54, 10991–11000 CrossRef CAS.
- J. T. Kozlowski and R. J. Davis, J. Energy Chem., 2013, 22, 58–64 CrossRef CAS.
- C. Yang and Z. Meng, J. Catal., 1993, 142, 37–44 CrossRef CAS.
- C. R. Ho, S. Shylesh and A. T. Bell, ACS Catal., 2016, 6, 939–948 CrossRef CAS.
- S. Hanspal, Z. D. Young, H. Shou and R. J. Davis, ACS Catal., 2015, 5, 1737–1746 CrossRef CAS.
- X. Wu, G. Fang, Y. Tong, D. Jiang, Z. Liang, W. Leng, L. Liu, P. Tu, H. Wang, J. Ni and X. Li, ChemSusChem, 2018, 11, 71–85 CrossRef CAS PubMed.
- A. Galadima and O. Muraza, Ind. Eng. Chem. Res., 2015, 54, 7181–7194 CrossRef CAS.
- R. Matake, Y. Niwa and H. Matsubara, Org. Lett., 2015, 17, 2354–2357 CrossRef CAS PubMed.
- Y. Li, H. Meng, Y. Lu and C. Li, Ind. Eng. Chem. Res., 2016, 55, 5257–5262 CrossRef CAS.
- G. Li, Q. Liu, Z. Liu, Z. Zhang, C. Li and W. Wu, Angew. Chem., Int. Ed., 2010, 122, 8480–8483 CrossRef PubMed.
- Z. Li, Z. Liu, R. Wang, X. Guo and Q. Liu, Chem. Eng. Sci., 2018, 192, 516–525 CrossRef CAS.
- M. Al-Hasan, Energy Convers. Manage., 2003, 44, 1547–1561 CrossRef CAS.
- T. Tsuchida, J. Kubo, T. Yoshioka, S. Sakuma, T. Takeguchi and W. Ueda, J. Catal., 2008, 259, 183–189 CrossRef CAS.
- Q. Liu, Q. Liu, R. Wang, T. Xu and Z. Liu, CIESC J., 2013, 64, 2573–2579 CAS.
- Q. Michaudel, T. Chauviré, V. Kottisch, M. J. Supej, K. J. Stawiasz, L. Shen, W. R. Zipfel, H. D. Abruña, J. H. Freed and B. P. Fors, J. Am. Chem. Soc., 2017, 139, 15530–15538 CrossRef CAS PubMed.
- J. I. D. Cosimo, C. R. Apesteguia, M. J. L. Ginés and E. Iglesia, J. Catal., 2000, 190, 261–275 CrossRef.
- D. Gabriëls, W. Y. Hernández, B. Sels, P. V. D. Voort and A. Verberckmoes, Catal. Sci. Technol., 2015, 5, 3876–3902 RSC.
- T. Matsu-ura, S. Sakaguchi, Y. Obora and Y. Ishii, J. Org. Chem., 2006, 71, 8306–8308 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra02568e |
| This journal is © The Royal Society of Chemistry 2019 |