Open Access Article
Open Access ArticleEffects of putrescine on gene expression in relation to physical barriers and antioxidant capacity in organs of weaning piglets†
Guangmang Liu *abc,
Weiwei Moabc,
Xiaomei Xuabc,
Xianjian Wuabc,
Gang Jiaabc,
Hua Zhaoabc,
Xiaoling Chenabc,
Caimei Wu*abc and
Jing Wangd
*abc,
Weiwei Moabc,
Xiaomei Xuabc,
Xianjian Wuabc,
Gang Jiaabc,
Hua Zhaoabc,
Xiaoling Chenabc,
Caimei Wu*abc and
Jing Wangd
aInstitute of Animal Nutrition, Sichuan Agricultural University, Chengdu 611130, Sichuan, China. E-mail: liugm@sicau.edu.cn; zhuomuniao278@163.com; Tel: +86-28-86290976
bKey Laboratory for Animal Disease-Resistance Nutrition of China, Ministry of Education, Chengdu 611130, Sichuan, China
cKey Laboratory of Animal Disease-resistant Nutrition and Feed, Ministry of Agriculture and Rural Affairs, Chengdu 611130, Sichuan, China
dMaize Research Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, China
First published on 24th June 2019
Abstract
Weaning stress can cause metabolic disorders, gastrointestinal dysfunction, physical barrier injury and disease susceptibility, thus leading to impaired growth and health of animals. Putrescine has the potential to reduce stress effects. However, the role of putrescine supplementation on barrier function and antioxidant capacity in animals' organs is largely unknown. This study evaluates the effects of putrescine on the physical barrier function, antioxidant status and related signalling molecule levels of weaning piglets' organs. A total of 24 weaning piglets were assigned to four treatment groups: (1) basal diet (control) and basal diets supplemented with (2) 0.05%, (3) 0.1% and (4) 0.15% putrescine. At the end of the 11 day experiment, ileum, liver, thymus and spleen samples were collected from the piglets. Compared with the control group, 0.15% putrescine can significantly increase anti-hydroxyl radical capacity (ileum and spleen), anti-superoxide anion capacity (liver, thymus and spleen), catalase (ileum, liver, thymus and spleen), total superoxide dismutase (ileum, thymus and spleen), glutathione peroxidase (ileum, liver and thymus), glutathione S-transferase activity (ileum, liver, thymus and spleen), glutathione content (liver and spleen) and total antioxidant capacity (ileum and thymus); decrease malondialdehyde (ileum, liver, thymus and spleen), protein carbonyl content (ileum, liver, thymus and spleen); enhance mRNA expression of zonula occludens (ZO)-1 (spleen), ZO-2 (liver, thymus and spleen), occludin (ileum, liver, thymus and spleen), claudin 1 (ileum, liver, thymus and spleen), claudin 2 (ileum, thymus and spleen), claudin 3 (ileum, liver, thymus and spleen), claudin 14 (ileum, liver and spleen), claudin 16 (ileum and liver), superoxide dismutase 1 (ileum, liver and thymus), glutathione peroxidase 1 (ileum, liver, thymus and spleen), catalase (ileum, liver, thymus and spleen), glutathione reductase (thymus and spleen), glutathione S-transferase (ileum, liver, thymus and spleen) and nuclear erythroid 2-related factor 2 (liver and thymus); decrease mRNA level of myosin light chain kinase (ileum, liver, thymus and spleen) and Kelch-like ECH-associated protein 1 (liver and spleen) (P < 0.05). 0.05% putrescine can significantly affect some of the above-mentioned parameters (P < 0.05). Collectively, putrescine supplementation improves organs' physical barrier function and antioxidant capacity in dose- and tissue-dependent and independent effects; such improvements are beneficial to the health of weaning piglets.
Introduction
While early weaning can effectively bring great economic profits to pig farms, the practice may also be detrimental to animal health. Weaning can decrease feed intake, enhance the expression of pro-inflammatory cytokines, impair the integrity of intestinal structures and lead to reduced antioxidant defence.1–3 Therefore, preventing the problems induced by weaning is essential in pig production. Nutritional interventions, such as arginine, glutamine and spermine administration, are helpful in mitigating the damage associated with weaning.4–6Putrescine (1,4-diaminobutane) can be synthesised from several amino acids, such as ornithine, arginine and glutamine, in mammals and is believed to be the precursor of spermidine and spermine.7–9 Putrescine is found in all eukaryotic cells and promotes cell proliferation.10,11 It helps facilitate the growth of neonatal animals, improves intestinal development, enhances the activity of digestive enzymes, up-regulates blood antibody levels and improves eggshell quality.12–15 The diamine also possesses anti-inflammatory activity.16 Spermine elevates the immune status by decreasing pro-inflammatory and anti-inflammatory cytokine levels in the spleen and thymus.17 Thus, putrescine may exert a positive role in the immune organs of the body. Body immunity depends on the structural integrity of immune organs, and this integrity is related to the antioxidant capacity of the related organs.18–21 Spermine improves the antioxidant capacity of the spleen and thymus in piglets and elevates the antioxidant status of intestines in weaning rats.17,22 Despite previous studies on other helpful compounds, however, the effect of putrescine on the antioxidant defence system of the immune organs of weaning animals has yet to be reported.
The functions of the antioxidant defence system are mainly attributed to enzymatic and non-enzymatic antioxidant activities, which are influenced by the gene expression of antioxidant enzymes.17 To date, whether putrescine intake regulates antioxidant enzyme gene expression in the immune organs of weaning animals remains poorly understood. The expression of antioxidant enzyme genes is usually influenced by the transcription factor called nuclear factor erythroid 2-related factor 2 (Nrf2).23 Nrf2 is restricted in the cytoplasm by Kelch-like ECH-associated protein 1 (Keap1), a negative regulator of Nrf2.23 The effects of putrescine on the signal molecules (Nrf2 and Keap1) of immune organs in animals have yet to be demonstrated. The structural integrity of immune organs is also affected by tight junction proteins.21,24 Putrescine administration during the suckling period plays an important role in increasing protein expression (e.g. occludin and claudin-3) in the jejunum of early-weaned piglets.25 Moreover, glutamine impedes acetaldehyde induction and decreases the permeability of the Caco-2 cell monolayer to endotoxins by alleviating the reduction of tight junctions, including zonula occludens-1 (ZO-1) and occludin.26 ZO-1, ZO-2 and polyamine synthesis are reduced in differentiated IEC-Cdx2L1 cells by treatment with α-difluoromethylornithine, a typical inhibitor of the syntheses of putrescine, spermidine and spermine.27 As such, we predict that putrescine intake after weaning may influence the tight junction proteins of immune organs in piglets and believe that this topic warrants further investigation.
The present work was performed to determine whether putrescine supplementation could affect physical barrier function, antioxidant status, and expression of related signalling molecules of several immune organs. The findings could provide partial theoretical evidence for the effects of putrescine on animal health.
Materials and methods
Animals and diets
A total of 24 male castrated piglets (Duroc × [Landrace × Yorkshire]) weaned at 21 days of age, weighting approximately 6 kg, were randomly allocated to the metabolism cage. During the feeding experiment, piglets were housed under conditions of constant humidity (50–60%) and temperature (25 °C), and piglets obtained ad libitum access to feedstuff and clean water. The piglets were fed 4 times daily (08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00, 12
00, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00, 16
00, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 and 20
00 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00). The basal diet in this experiment was able to meet the NRC (2012) recommendation for the nutrient requirements of weaning piglets. Table 1 has shown the basal diet formulation. All piglets fed basic diets were acclimated to the experimental environment for 3 day. After the acclimatization period, all piglets were randomly divided to 4 groups with 6 piglets per group. In an 11 day experiment, the piglets were offered one of the following four diets: basal diet (control group), basal diet supplemented with 0.05% putrescine (P7505 MSDS, Sigma Chemical Co., St. Louis, MO, USA), basal diet supplemented with 0.1% putrescine and basal diet supplemented with 0.15% putrescine. All animal procedures were performed according to the Guidelines for Care and Use of Laboratory Animals of National Research Council and approved by the Animal Ethics Committee of Sichuan Agricultural University.
00). The basal diet in this experiment was able to meet the NRC (2012) recommendation for the nutrient requirements of weaning piglets. Table 1 has shown the basal diet formulation. All piglets fed basic diets were acclimated to the experimental environment for 3 day. After the acclimatization period, all piglets were randomly divided to 4 groups with 6 piglets per group. In an 11 day experiment, the piglets were offered one of the following four diets: basal diet (control group), basal diet supplemented with 0.05% putrescine (P7505 MSDS, Sigma Chemical Co., St. Louis, MO, USA), basal diet supplemented with 0.1% putrescine and basal diet supplemented with 0.15% putrescine. All animal procedures were performed according to the Guidelines for Care and Use of Laboratory Animals of National Research Council and approved by the Animal Ethics Committee of Sichuan Agricultural University.
| Ingredients (g kg−1) | |
|---|---|
| a Supplied per kilogram of diet: vitamin A, 6000 IU; vitamin D3, 600 IU; vitamin E, 50 IU; vitamin K3, 1.5 mg; thiamine, 2.0 mg; riboflavin, 8.0 mg; pantothenic acid, 20 mg; niacin, 30 mg; pyridoxine, 3.0 mg; choline, 800 mg; folic acid, 0.6 mg; biotin, 0.10 mg; vitamin B12, 0.04 mg; Zn, 100 mg (ZnSO4); Cu, 16 mg (CuSO4·5H2O); Fe, 125 mg (FeSO4); Mn, 15 mg (MnSO4·H2O); Se, 0.3 mg (Na2SeO3); I, 0.2 mg (KI).b Digestible energy was calculated from data provide by Feed Database in China. | |
| Corn | 579.5 |
| Soybean meal | 250 |
| Fish meal | 50 |
| Dried whey | 45 |
| Spray-dried plasma protein | 25 |
| Soybean oil | 20 |
| Limestone meal | 5 |
| Dicalcium phosphate | 11 |
| Sodium chloride | 3 |
| L-Lysine HCl | 1 |
| DL-Methionine | 0.5 |
| Vitamin–mineral premixa | 10 |
| Analyzed composition (g kg−1) | |
| Digestible energy (MJ kg−1)b | 14.35 |
| Crude protein | 229.5 |
| Lysine | 14.3 |
| Methionine | 3.7 |
| Calcium | 9.1 |
| Total phosphorus | 7.2 |
Sample collection
At the end of the 11 day feeding experiment, 24 piglets were slaughtered to collect samples. The ileum, liver, thymus and spleen samples were frozen in liquid nitrogen and then stored at −80 °C before analysis. The putrescine dosage followed a previous experiment.28Biochemical analysis
We measured the malondialdehyde (MDA), protein carbonyl (PC), and glutathione (GSH) contents, and the anti-superoxide anion (ASA), anti-hydroxyl radical (AHR), total superoxide dismutase (T-SOD), catalase (CAT), the glutathione peroxidase (GPx), glutathione S-transferase (GST) activities, and total antioxidant capacity (T-AOC) in the ileum, liver, thymus and spleen. Antioxidant indicators were determined in accordance with a previous study.17 Briefly, these indicators were tested employing a microplate reader (SpectraMax M2, Molecular Devices, USA). The method for detecting the MDA content followed the process of Cao et al. (2015).6 The PC content was analysed according to a previous study and calculated from the peak absorbance at 370 nm. ASA and AHR capacity were surveyed as previously described.29 SOD and GPx activities were tested following a previous study protocol. CAT activity was measured employing the colourimetric method from a previous experiment.30 GST activity was evaluated as described previously,31 and the GSH content was tested as described by the previous study. T-AOC was measured spectrophotometrically at 520 nm in terms of developing stable and coloured chelates as described by the previous study.32 The protein content of tissue samples was used to calculate the foregoing antioxidant parameter activities and detected according to the method described by Bradford (1976) employing a protein analysis kit (Coomassie Brilliant Blue) and bovine serum albumin as the protein standard.Real-time quantitative PCR analysis
The method separating total RNA from the ileum, liver, thymus and spleen was in accord with our previous study.17 Briefly, the quality of total RNA was able to be evaluated by using 1.0% agarose gel electrophoresis (Beckman DU-800; CA, USA). The purity of total RNA was able to be detected by spectrophotometric analysis (A260: 280 nm ratio) in the ileum, liver, thymus and spleen samples. Subsequently, total RNA (1 μg) in tissues could be used to obtain cDNA using the PrimeScript™ RT reagent (Takara, Dalian, China). Specific primers of the genes in the experiment were designed with Primer Express Software (version 3.0; Applied Biosystems, Foster City, CA, USA) and synthesized by TaKaRa Biotechnology Company (Takara, Dalian, China) as presented in Table S1.† Real-time quantitative PCR analysis were carried out by using the SYBR® Green I PCR Reagent Kit and a real-time PCR instrument (ABI 7900HT, Applied Biosystems, USA). The PCR mixture (10 μL) was composed of 5 μL SYBR Premix Ex Taq II with the ROX reference dye, 1 μL cDNA, 1 μL each of forward and reverse primers, and 2 μL ddH2O. The thermal cycling conditions for PCR initiated with a pre-run step at 95 °C for 10 s, 42 cycles of denaturation at 95 °C for 10 s, annealing at 58 °C for 35 s, and extension at 72 °C for 15 s.17Statistical analysis
The results were expressed as mean ± standard error of the mean (SEM). All data in the study were analyzed by one-way analysis of variance (ANOVA) of SPSS 22.0 (SPSS Inc., Chicago, IL, USA). When ANOVA exhibited differences between groups, multiple comparisons were done with Tukeys' multiple-range test at the level of P < 0.05. The homogeneity of variances for data was assessed by Levene's tests. Correlations analysis were done using Pearson's correlation analysis. Statistical differences were considered as significant when p-values were P < 0.05.Results
Antioxidant indicators
Table 2 presents the antioxidant indicators in the ileum. T-AOC activity was higher by 17.83%, but PC content was lower by 27.98% in 0.05% putrescine group compared with the control group (P < 0.05). Moreover, 0.10% putrescine increased CAT activity (by 32.24%), T-AOC (by 27.99%), but decreased MDA (by 28.68%) and PC contents (by 28.92%) compared with the control group (P < 0.05). Supplementation with 0.15% putrescine increased CAT activities (by 82.09%) and T-AOC (by 31.48%) compared with the 0.10% putrescine-supplemented group (P < 0.05). Supplementation with 0.15% putrescine increased AHR (by 27.20%), CAT (by 140.79%), T-SOD (by 101.13%), GPx (by 16.58%), GST activities (by 28.13%) and T-AOC (by 68.27%), but decreased MDA (by 33.25%) and PC contents (by 49.34%) compared with the control group (P < 0.05).| Parameters | Treatments | SEM | P-Value | |||
|---|---|---|---|---|---|---|
| Control | 0.05% putrescine | 0.10% putrescine | 0.15% putrescine | |||
| a AHR, anti-hydroxyl radical; ASA, anti-superoxide anion; CAT, catalase; GSH, glutathione; MDA, malondialdehyde; T-AOC, total antioxidant capacity; T-SOD, total superoxide dismutase; PC, protein carbonyl; GPx, glutathione peroxidase; GST glutathione S-transferase. Data are presented as means ± SEM, n = 6. a–c Mean values within a row with different superscript letters were significantly different (P < 0.05). | ||||||
| AHR, U mg−1 protein | 64.93b | 66.30b | 77.54ab | 82.59a | 2.179 | 0.002 |
| ASA, U g−1 protein | 178.07 | 180.63 | 192.61 | 198.20 | 3.269 | 0.080 |
| CAT, U mg−1 protein | 4.56c | 4.94c | 6.03b | 10.98a | 0.551 | 0.000 |
| GSH, mg g−1 protein | 8.14 | 8.36 | 8.47 | 8.81 | 0.126 | 0.316 |
| MDA, nmol mg−1 protein | 3.94a | 3.08ab | 2.81b | 2.63b | 0.150 | 0.003 |
| T-AOC, U mg−1 protein | 5.61c | 6.61b | 7.18b | 9.44a | 0.314 | 0.000 |
| T-SOD, U mg−1 protein | 20.30b | 22.64b | 27.07b | 40.83a | 1.878 | 0.000 |
| PC, nmol mg−1 protein | 5.29a | 3.81b | 3.76b | 2.68b | 0.235 | 0.000 |
| GPx, U mg−1 protein | 229.39b | 240.01ab | 248.44ab | 267.43a | 4.870 | 0.029 |
| GST, U mg−1 protein | 248.71b | 265.01b | 292.05ab | 318.67a | 7.546 | 0.001 |
Table 3 presents the antioxidant indicators in the liver. Supplementation with 0.05% putrescine increased ASA (by 9.95%) and CAT activities (by 20.16%), but decreased MDA content (by 28.70%) compared with the control group (P < 0.05). Moreover, supplementation with 0.15% putrescine increased CAT (by 16.30%), but decreased MDA content (by 20.78%) compared with the 0.05% putrescine group (P < 0.05). Supplementation with 0.10% putrescine increased ASA (by13.56%), CAT (by 35.62%) and GST activities (by 27.27%), but decreased MDA content (by 37.04%) compared with the control group (P < 0.05). Supplementation with 0.15% putrescine increased GSH content (by 28.36%) and increased ASA capacity (by 14.06%), CAT (by 39.75%), GPx (by 25.23%) and GST activities (by 36.01%), but decreased MDA (by 43.52%) and PC contents (by 42.64%) compared with the control group (P < 0.05).
| Parameters | Treatments | SEM | P-Value | |||
|---|---|---|---|---|---|---|
| Control | 0.05% putrescine | 0.10% putrescine | 0.15% putrescine | |||
| a AHR, anti-hydroxyl radical; ASA, anti-superoxide anion; CAT, catalase; GSH, glutathione; MDA, malondialdehyde; T-AOC, total antioxidant capacity; T-SOD, total superoxide dismutase; PC, protein carbonyl; GPx, glutathione peroxidase; GST, glutathione S-transferase. Data are presented as means with SEM, n = 6. a–c Mean values within a row with different superscript letters were significantly different (P < 0.05). | ||||||
| AHR, U mg−1 protein | 312.42 | 317.46 | 332.50 | 352.40 | 6.865 | 0.160 |
| ASA, U g−1 protein | 173.28b | 190.53a | 196.77a | 197.64a | 2.215 | 0.000 |
| CAT, U mg−1 protein | 8.73c | 10.49b | 11.84ab | 12.20a | 0.334 | 0.000 |
| GSH, mg g−1 protein | 6.17b | 6.81b | 6.57b | 7.92a | 0.173 | 0.000 |
| MDA, nmol mg−1 protein | 2.16a | 1.54b | 1.36bc | 1.22c | 0.080 | 0.000 |
| T-AOC, U mg−1 protein | 4.44 | 4.47 | 4.76 | 5.02 | 0.124 | 0.344 |
| T-SOD, U mg−1 protein | 64.90 | 68.34 | 69.49 | 76.10 | 1.540 | 0.061 |
| PC, nmol mg−1 protein | 3.87a | 3.34ab | 2.69ab | 2.22b | 0.195 | 0.007 |
| GPx, U mg−1 protein | 260.52b | 290.89b | 292.30b | 326.26a | 6.150 | 0.000 |
| GST, U mg−1 protein | 157.11c | 179.26bc | 199.96ab | 213.68a | 5.724 | 0.000 |
Table 4 presents the antioxidant indicators in the thymus. Supplementation with 0.05% putrescine increased T-SOD (by 6.70%) and CAT activities (by 22.12%), but decreased MDA content (by 33.21%) relative to the control group (P < 0.05). Moreover, supplementation with 0.15% putrescine increased T-SOD activity (by 32.83%) compared with the 0.05% putrescine-supplemented group (P < 0.05). Supplementation with 0.10% putrescine increased ASA capacity (by15.73%), and GST activity (by 34.90%), but decreased MDA content (by 39.42%) compared with the control group (P < 0.05). Supplementation with 0.15% putrescine increased ASA capacity (by 19.80%), CAT (by 25.35%), T-SOD (by 41.73%), GPx (by 49.23%), GST activities (by 38.03%) and T-AOC (by 49.66%), but decreased MDA (by 36.86%) and PC contents (by 51.30%) compared with the control group (P < 0.05).
| Parameters | Treatments | SEM | P-Value | |||
|---|---|---|---|---|---|---|
| Control | 0.05% putrescine | 0.10% putrescine | 0.15% putrescine | |||
| a AHR, anti-hydroxyl radical; ASA, anti-superoxide anion; CAT, catalase; GSH, glutathione; MDA, malondialdehyde; T-AOC, total antioxidant capacity; T-SOD, total superoxide dismutase; PC, protein carbonyl; GPx, glutathione peroxidase; GST, glutathione S-transferase. Data are presented as means with SEM, n = 6. a–c Mean values within a row with different superscript letters were significantly different (P < 0.05). | ||||||
| AHR, U mg−1 protein | 127.69 | 129.27 | 128.44 | 137.66 | 2.157 | 0.340 |
| ASA, U g−1 protein | 101.30c | 107.20bc | 117.23ab | 121.36a | 2.188 | 0.001 |
| CAT, U mg−1 protein | 20.12b | 24.57a | 23.23ab | 25.22a | 0.608 | 0.006 |
| GSH, mg g−1 protein | 4.94 | 5.67 | 5.23 | 5.66 | 0.152 | 0.258 |
| MDA, nmol mg−1 protein | 2.74a | 1.83b | 1.66b | 1.73b | 0.122 | 0.001 |
| T-AOC, U mg−1 protein | 2.94b | 3.74ab | 3.16b | 4.40a | 0.170 | 0.004 |
| T-SOD, U mg−1 protein | 88.69c | 94.63b | 96.14bc | 125.70a | 3.201 | 0.000 |
| PC, nmol mg−1 protein | 3.08a | 2.51a | 2.89a | 1.50b | 0.163 | 0.000 |
| GPx, U mg−1 protein | 62.85b | 75.07b | 74.95b | 93.79a | 2.846 | 0.000 |
| GST, U mg−1 protein | 43.70b | 52.10ab | 58.95a | 60.32a | 2.043 | 0.006 |
Table 5 presents the antioxidant indicators in the spleen. Supplementation with 0.05% putrescine increased GSH content (by 23.10%) and T-SOD (by 10.60%) and GST activities (by 44.07%), but decreased MDA (by 16.81%) and PC content (by 20.00%) compared with the control group (P < 0.05). Supplementation with 0.10% putrescine increased GST activity (by 16.64%), but decreased MDA (by 18.58%) and PC content (by 26.40%) compared with the 0.05% putrescine-supplemented group (P < 0.05). Moreover, 0.15% putrescine increased GSH content (by 14.81%), but decreased MDA (by 20.35%) and PC contents (by 30.61%) compared with the 0.05% putrescine-supplemented group (P < 0.05). Supplementation with 0.10% putrescine increased GSH content (by 31.05%) and ASA capacity (by5.72%), T-SOD (by 13.46%) and GST activities (by 68.05%), but decreased MDA (by 32.27%) and PC content (by 41.12%) compared with the control group (P < 0.05). 0.15% putrescine increased GSH content (by 41.34%) and AHR (by 35.43%), ASA capacity (by 5.77%), CAT (by 29.44%), T-SOD (by 19.67%), and GST activities (by 63.40%), but decreased MDA (by 33.74%) and PC content (by 44.49%) compared with the control group (P < 0.05).
| Parameters | Treatments | SEM | P-Value | |||
|---|---|---|---|---|---|---|
| Control | 0.05% putrescine | 0.10% putrescine | 0.15% putrescine | |||
| a AHR, anti-hydroxyl radical; ASA, anti-superoxide anion; CAT, catalase; GSH, glutathione; MDA, malondialdehyde; T-AOC, total antioxidant capacity; T-SOD, total superoxide dismutase; PC, protein carbonyl; GPx, glutathione peroxidase; GST, glutathione S-transferase. Data are presented as means with SEM, n = 6. a–c Mean values within a row with different superscript letters were significantly different (P < 0.05). | ||||||
| AHR, U mg−1 protein | 59.89b | 68.19b | 68.85b | 81.11a | 1.933 | 0.000 |
| ASA, U g−1 protein | 99.67b | 102.88ab | 105.37a | 105.42a | 0.857 | 0.044 |
| CAT, U mg−1 protein | 11.89b | 12.23b | 13.41b | 15.39a | 0.359 | 0.000 |
| GSH, mg g−1 protein | 5.54c | 6.82b | 7.26ab | 7.83a | 0.201 | 0.000 |
| MDA, nmol mg−1 protein | 8.15a | 6.78b | 5.52c | 5.40c | 0.275 | 0.000 |
| T-AOC, U mg−1 protein | 7.93 | 8.57 | 8.69 | 8.96 | 0.158 | 0.116 |
| T-SOD, U mg−1 protein | 9.81b | 10.85a | 11.13a | 11.74a | 0.188 | 0.000 |
| PC, nmol mg−1 protein | 5.35a | 4.28b | 3.15c | 2.97c | 0.232 | 0.000 |
| GPx, U mg−1 protein | 126.59 | 137.65 | 138.58 | 142.75 | 2.240 | 0.056 |
| GST, U mg−1 protein | 46.83c | 67.47b | 78.70a | 76.52ab | 2.867 | 0.000 |
mRNA levels of antioxidant-related parameters
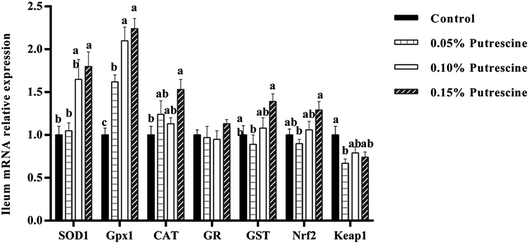
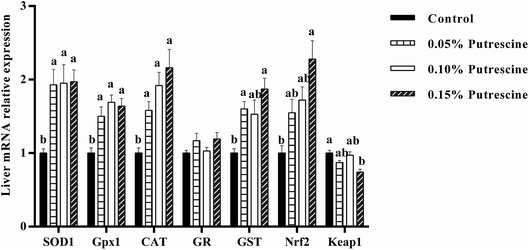
The mRNA levels of antioxidant-related genes in the ileum of piglet are presented in Fig. 1. 0.05% putrescine increased GPX1, but decreased Keap1 mRNA expression compared with the control group (P < 0.05). Supplementation with 0.10% putrescine increased GPx1 compared with the 0.05% putrescine-supplemented group (P < 0.05). Supplementation with 0.15% putrescine increased SOD1, GST, Nrf2 and GPx1 mRNA expression compared with the 0.05% putrescine-supplemented group (P < 0.05). Supplementation with 0.10% putrescine increased GPx1 mRNA expression compared with the control group (P < 0.05). Supplementation with 0.15% putrescine increased SOD1, GPx1 and CAT mRNA expression compared with the control group (P < 0.05).The mRNA levels of antioxidant-related genes in the liver of piglet are presented in Fig. 2. 0.05% putrescine group increased SOD1, GPX1, CAT and GST mRNA expression compared with the control group (P < 0.05). Supplementation with 0.10% putrescine increased SOD1, GPX1 and CAT mRNA expression compared with the control group (P < 0.05). Supplementation with 0.15% putrescine increased SOD1, GPx1, CAT, GST and Nrf2 mRNA expression, but decreased Keap1 mRNA expression compared with the control group (P < 0.05).
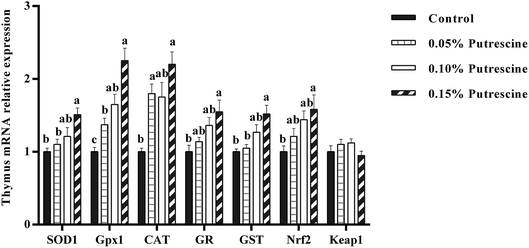
The mRNA levels of antioxidant-related genes in the thymus of piglet are presented in Fig. 3. Supplementation with 0.05% putrescine increased SOD1, GPX1, CAT and GST mRNA expression relative to the control group (P < 0.05). Supplementation with 0.10% putrescine increased GPX1 mRNA expression compared with the control group (P < 0.05). Supplementation with 0.15% putrescine increased SOD1, GPx1, CAT, GR, GST and Nrf2 mRNA expression compared with the control group (P < 0.05).
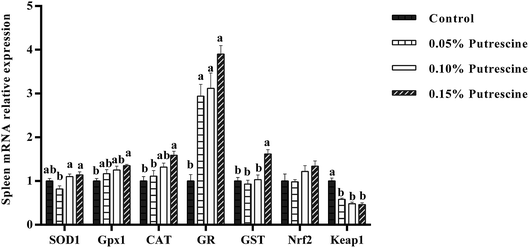
The mRNA levels of antioxidant-related genes in the spleen of piglet are presented in Fig. 4. Supplementation with 0.05% putrescine increased GR mRNA expression, but decreased Keap1 mRNA expression compared with the control group (P < 0.05). Supplementation with 0.10% putrescine increased GR mRNA expression compared with the control group (P < 0.05). Supplementation with 0.15% putrescine increased GPx1, CAT, GR, and GST mRNA expression and decreased Keap1 expression compared with the control group (P < 0.05).
The expression of tissue barrier-related genes
The mRNA expression of barrier-related genes in ileum is shown in Table 6. Supplementation with 0.10% putrescine increased claudin 1 mRNA expression compared with the control group (P < 0.05). Moreover, supplementation with 0.15% putrescine increased occludin, claudin 1, claudin 2, claudin 3, claudin 14, claudin 15 and claudin 16 mRNA expression compared with the control group (P < 0.05). Moreover, putrescine intake decreased MLCK mRNA level compared with the control group (P < 0.05). 0.05% or 0.15% putrescine decreased claudin 12 gene expression (P < 0.05).| Parameters | Treatments | SEM | P-Value | |||
|---|---|---|---|---|---|---|
| Control | 0.05% putrescine | 0.10% putrescine | 0.15% putrescine | |||
| a ZO-1, zonula occludens 1; ZO-2, zonula occludens 2; MLCK, myosin light chain kinase. Data are presented as means with SEM, n = 6. a,b Mean values within a row with different superscript letters were significantly different (P < 0.05). | ||||||
| ZO-1 | 1.00 | 1.23 | 1.17 | 1.48 | 0.066 | 0.067 |
| ZO-2 | 1.00ab | 0.94b | 1.21a | 1.12ab | 0.038 | 0.043 |
| Occludin | 1.00b | 1.13ab | 1.06ab | 1.38a | 0.050 | 0.026 |
| Claudin 1 | 1.00b | 1.16ab | 1.48a | 1.46a | 0.064 | 0.008 |
| Claudin 2 | 1.00b | 1.16b | 1.37ab | 1.60a | 0.067 | 0.004 |
| Claudin 3 | 1.00b | 0.98b | 1.02b | 1.47a | 0.063 | 0.008 |
| Claudin 12 | 1.00a | 0.74b | 0.79ab | 0.67b | 0.039 | 0.013 |
| Claudin 14 | 1.00b | 1.21ab | 1.28ab | 1.64a | 0.075 | 0.012 |
| Claudin 15 | 1.00b | 1.15ab | 1.16ab | 1.45a | 0.055 | 0.018 |
| Claudin 16 | 1.00b | 1.24ab | 1.26ab | 1.40a | 0.050 | 0.029 |
| MLCK | 1.00a | 0.76b | 0.79b | 0.70b | 0.0334 | 0.006 |
The mRNA expression of barrier-related genes in liver is shown in Table 7. Supplementation with 0.05% putrescine increased claudin 1 and claudin 14 mRNA levels, but reduced MLCK gene expression compared with the control group (P < 0.05). Supplementation with 0.10% putrescine increased ZO-1, claudin 1, claudin 12, claudin 14, claudin 15 and claudin 16 mRNA expression compared with the control group (P < 0.05). Supplementation with 0.15% putrescine increased ZO-1, ZO-2, occludin, claudin 1, claudin 3, claudin 12, claudin 14, claudin 15 and claudin 16 mRNA levels, but reduced MLCK gene expression compared with the control group (P < 0.05).
| Parameters | Treatments | SEM | P-Value | |||
|---|---|---|---|---|---|---|
| Control | 0.05% putrescine | 0.10% putrescine | 0.15% putrescine | |||
| a ZO-1, zonula occludens 1; ZO-2, zonula occludens 2; MLCK, myosin light chain kinase. Data are presented as means with SEM, n = 6. a,b Mean values within a row with different superscript letters were significantly different (P < 0.05). | ||||||
| ZO-1 | 1.00b | 1.25ab | 1.58a | 1.65a | 0.079 | 0.004 |
| ZO-2 | 1.00b | 1.11b | 1.38ab | 1.72a | 0.077 | 0.001 |
| Occludin | 1.00b | 1.13b | 1.46ab | 1.62a | 0.076 | 0.005 |
| Claudin 1 | 1.00b | 1.72a | 1.87a | 2.17a | 0.122 | 0.001 |
| Claudin 2 | 1.00 | 0.82 | 1.04 | 1.16 | 0.067 | 0.346 |
| Claudin 3 | 1.00b | 1.14ab | 1.42ab | 1.63a | 0.077 | 0.009 |
| Claudin 12 | 1.00b | 1.23b | 1.86a | 2.01a | 0.105 | 0.000 |
| Claudin 14 | 1.00b | 1.59a | 1.62a | 1.91a | 0.093 | 0.001 |
| Claudin 15 | 1.00b | 1.17b | 1.99a | 1.81a | 0.106 | 0.000 |
| Claudin 16 | 1.00b | 1.33ab | 1.78a | 1.90a | 0.102 | 0.001 |
| MLCK | 1.00a | 0.68b | 0.72ab | 0.47b | 0.053 | 0.001 |
The mRNA expression of barrier-related genes in thymus is shown in Table 8. Supplementation with 0.05% putrescine increased claudin 1 mRNA expression compared with the control group (P < 0.05). Supplementation with 0.15% putrescine increased occludin, claudin 1, claudin 2, claudin 3, claudin 15, claudin 16 mRNA levels, but reduced MLCK mRNA expression compared with the control group (P < 0.05).
| Parameters | Treatments | SEM | P-Value | |||
|---|---|---|---|---|---|---|
| Control | 0.05% putrescine | 0.10% putrescine | 0.15% putrescine | |||
| a ZO-1, zonula occludens 1; ZO-2, zonula occludens 2; MLCK, myosin light chain kinase. Data are presented as means with SEM, n = 6. a,b Mean values within a row with different superscript letters were significantly different (P < 0.05). | ||||||
| ZO-1 | 1.00 | 1.15 | 1.12 | 1.37 | 0.054 | 0.090 |
| ZO-2 | 1.00b | 1.07b | 1.05b | 1.48a | 0.055 | 0.002 |
| Occludin | 1.00b | 1.07ab | 1.12ab | 1.41a | 0.054 | 0.029 |
| Claudin 1 | 1.00b | 1.43a | 1.39ab | 1.56a | 0.067 | 0.009 |
| Claudin 2 | 1.00b | 1.09b | 1.34b | 2.32a | 0.123 | 0.000 |
| Claudin 3 | 1.00b | 1.24b | 1.29ab | 1.73a | 0.077 | 0.002 |
| Claudin 12 | 1.00 | 0.88 | 0.96 | 1.29 | 0.062 | 0.073 |
| Claudin 14 | 1.00 | 0.96 | 0.97 | 1.26 | 0.048 | 0.064 |
| Claudin 15 | 1.00b | 1.09b | 1.22ab | 1.41a | 0.047 | 0.006 |
| Claudin 16 | 1.00b | 1.00b | 1.20b | 1.57a | 0.061 | 0.000 |
| MLCK | 1.00a | 0.82a | 0.90a | 0.50b | 0.047 | 0.000 |
The mRNA expression of barrier-related genes in spleen is shown in Table 9. Supplementation with 0.10% putrescine increased ZO-1 and claudin 3 mRNA expression compared with the control group (P < 0.05). Supplementation with 0.15% putrescine increased ZO-1, ZO-2, occludin, claudin 1, claudin 2, claudin 3, claudin 12 and claudin 14 mRNA expression compared with the control group (P < 0.05). Moreover, 0.05%, 0.10% and 0.15% putrescine reduced claudin 15 and MLCK mRNA expression compared with the control group (P < 0.05).
| Parameters | Treatments | SEM | P-Value | |||
|---|---|---|---|---|---|---|
| Control | 0.05% putrescine | 0.10% putrescine | 0.15% putrescine | |||
| a ZO-1, zonula occludens 1; ZO-2, zonula occludens 2; MLCK, myosin light chain kinase. Data are presented as means with SEM, n = 6. a–c Mean values within a row with different superscript letters were significantly different (P < 0.05). | ||||||
| ZO-1 | 1.00c | 1.15bc | 1.56ab | 1.80a | 0.086 | 0.000 |
| ZO-2 | 1.00b | 0.89b | 1.09b | 1.47a | 0.057 | 0.000 |
| Occludin | 1.00b | 1.15b | 1.40ab | 1.81a | 0.099 | 0.011 |
| Claudin 1 | 1.00b | 1.38ab | 1.35ab | 1.42a | 0.060 | 0.025 |
| Claudin 2 | 1.00b | 1.58ab | 1.29ab | 1.45a | 0.074 | 0.032 |
| Claudin 3 | 1.00c | 1.26bc | 2.06ab | 2.71a | 0.168 | 0.000 |
| Claudin 12 | 1.00b | 1.05ab | 1.35ab | 1.55a | 0.075 | 0.020 |
| Claudin 14 | 1.00b | 1.20ab | 1.25ab | 1.51a | 0.059 | 0.011 |
| Claudin 15 | 1.00a | 0.51c | 0.65bc | 0.68b | 0.044 | 0.000 |
| Claudin 16 | 1.00 | 1.42 | 1.46 | 1.33 | 0.071 | 0.072 |
| MLCK | 1.00a | 0.56b | 0.71b | 0.71b | 0.041 | 0.000 |
Discussion
Reducing the problems associated by early weaning is highly important for animal health, which depends on the structural integrity of organs such as the intestine, liver, thymus and spleen.33–35 The structural integrity of immune organs is associated with organ cellular membrane integrity, which can be disrupted by weaning, but is believed to be preserved by increasing the antioxidant capacity of immune organs.21,36 Spermine improves the antioxidant capacity of the spleen and thymus in pigs.17 The structural integrity of immune organs is related to tight junction proteins.21,24 This study explores whether putrescine can influence the antioxidant status of the ileum, spleen, liver and thymus and tight junction protein expression in these organs.MDA and PC contents are indices that reflect the degree of lipid peroxidation and protein oxidation.17 In the present study, the PC content of the liver and thymus was remarkably decreased after only 0.15% putrescine supplementation. Putrescine administration also reduced PC content in the ileum and spleen regardless of dose. Moreover, putrescine remarkably reduced MDA contents in the thymus, liver and spleen regardless of dose, although only 0.10% or 0.15% putrescine supplementation exerted such effects in the ileum. Thus, putrescine can decrease MDA content in weaned piglets. This result is consistent with those of previous studies demonstrating that spermine supplementation reduced MDA contents in the jejunum of rats and the spleen and thymus of piglets.17,22 And spermine supplementation could reduce MDA contents in the spleen of weaning rats regardless of dose.37 Based on these data, putrescine supplementation appears to be able to reduce oxidative damage to lipids and proteins in a dose- and tissue-dependent and independent manner.
Oxidative damage to lipids and proteins is induced by the presence of excess superoxide anions and hydroxyl radicals.38 AHR and ASA capacity reflect the scavenging activity of hydroxyl radicals and superoxide anions.17 In the present study, the AHR capacity of the spleen and ileum remarkably increased after putrescine administration but only at a dose of 0.15%; no substantial difference in this index was found in the liver and thymus. The results obtained are consistent with those of previous studies illustrating higher AHR capacity in the jejunum and improved AHR capacity in the spleen of weaning rats after administration of high spermine doses. Thus, putrescine can effectively decrease hydroxyl radicals in the spleen and ileum. ASA capacity in the spleen and thymus was remarkably enhanced after putrescine administration but only at doses of 0.10% and 0.15%. While ASA capacity in the liver was also remarkably enhanced by putrescine supplementation regardless of dose; by contrast, no substantial difference in dose-based effect was found in the ileum. This result is consistent with previous studies demonstrating increased ASA capacity in the liver, spleen and thymus in piglets after spermine supplementation and effective scavenging of superoxide anion free radicals in an in vitro model by putrescine.17,39,40 Taken together, findings thus far reveal that putrescine supplementation can modulate AHR and ASA capacity in a dose- and tissue-dependent and independent manner. Correlation analysis further indicated that ASA capacity was negatively associated with PC and MDA contents in the liver and spleen (Table 10), thereby indicating that putrescine can alleviate oxidative damage to proteins and lipids, which may be partly due to enhanced ASA capacity in these organs.
| Tissues | Independent parameters | Dependent parameters | Correlation coefficients | P-Value |
|---|---|---|---|---|
| a ASA, anti-superoxide anion; CAT, catalase; MDA, malondialdehyde; T-SOD, total superoxide dismutase; GSH, glutathione; GPx, glutathione peroxidase; GST, glutathione S-transferase; SOD1, superoxide dismutase 1; GR, glutathione reductase; Nrf2, nuclear erythroid 2-related factor 2. ZO-1, zonula occludens 1; ZO-2, zonula occludens 2; MLCK, myosin light chain kinase. | ||||
| Ileum | GPX activity | GPX1 mRNA level | 0.909 | 0.091 |
| CAT activity | CAT mRNA level | 0.909 | 0.091 | |
| Nrf2 | GST mRNA level | 0.999 | 0.001 | |
| MLCK | Claudin 16 | −0.978 | 0.022 | |
| Liver | PC content | ASA capacity | −0.912 | 0.088 |
| PC content | CAT activity | −0.972 | 0.028 | |
| PC content | T-SOD activity | −0.940 | 0.060 | |
| MDA content | ASA activity | −0.994 | 0.006 | |
| MDA content | CAT activity | −0.982 | 0.018 | |
| CAT activity | CAT mRNA level | 0.995 | 0.005 | |
| GST activity | GST mRNA level | 0.903 | 0.097 | |
| Nrf2 | CAT mRNA level | 0.969 | 0.031 | |
| Nrf2 | GST mRNA level | 0.954 | 0.046 | |
| MLCK | Claudin 1 | −0.967 | 0.033 | |
| Thymus | PC content | T-SOD activity | −0.963 | 0.037 |
| GPX activity | GPX1 mRNA level | 0.976 | 0.024 | |
| CAT activity | CAT mRNA level | 0.971 | 0.029 | |
| T-SOD activity | SOD1 mRNA level | 0.973 | 0.027 | |
| Nrf2 | GPX1 mRNA level | 0.966 | 0.034 | |
| Nrf2 | CAT mRNA level | 0.906 | 0.094 | |
| Nrf2 | SOD1 mRNA level | 0.930 | 0.070 | |
| Nrf2 | GST mRNA level | 0.948 | 0.052 | |
| MLCK | ZO-1 | −0.993 | 0.007 | |
| MLCK | ZO-2 | −0.978 | 0.022 | |
| MLCK | Occludin | −0.962 | 0.038 | |
| MLCK | Claudin 2 | −0.938 | 0.062 | |
| MLCK | Claudin 3 | −0.971 | 0.029 | |
| Spleen | PC content | ASA capacity | −0.993 | 0.007 |
| PC content | T-SOD activity | −0.954 | 0.046 | |
| MDA content | ASA capacity | −0.998 | 0.002 | |
| MDA content | T-SOD activity | −0.954 | 0.046 | |
| GPX activity | GPX1 mRNA level | 0.978 | 0.022 | |
| CAT activity | CAT mRNA level | 0.992 | 0.008 | |
| GSH content | GR mRNA level | 0.991 | 0.009 | |
| Nrf2 | CAT mRNA level | 0.958 | 0.042 | |
| MLCK | Claudin 1 | −0.902 | 0.098 | |
| MLCK | Claudin 2 | −0.968 | 0.032 | |
| MLCK | Claudin 15 | −0.997 | 0.003 | |
| MLCK | Claudin 16 | −0.914 | 0.086 | |
Free-radical scavenging properties partly depend on the enzymatic antioxidants in antioxidant defence systems. Thus, we investigated the effect of putrescine on antioxidant enzymes activities. CAT results in a decrease in hydroxyl radicals because of its capacity to change H2O2 into oxygen and water.41 SOD can effectively prevent the potential toxicity of superoxide anions.2 In the present study, treatment with 0.15% putrescine remarkably increased CAT and T-SOD activities in the ileum, thymus and spleen and increased CAT activity in the liver. Interestingly, putrescine remarkably increased CAT activity in the liver and increased T-SOD activity in the spleen regardless of dose. This result is consistent with the previous studies showing increased CAT and T-SOD activities in the jejunum of weaning rats, improved CAT and T-SOD activities in the thymus and spleen of piglets and enhanced CAT activity in the liver of piglets by spermine supplementation.17,22,37 Correlation analysis further indicated that CAT activity was negatively associated with PC and MDA contents in liver (Table 10), thus suggesting that putrescine alleviates oxidative damage by increasing CAT activity in this organ. SOD activity was also negatively associated with PC and MDA contents in the spleen (Table 10), also suggesting that putrescine alleviates oxidative damage by increasing T-SOD activity in this organ.
GPx and GST are useful for inhibiting oxidative damage. In particular, GPx can eliminate the excess H2O2 generated from SOD dismutation.22 In the present study, GPx activity in the ileum, liver and thymus remarkably increased after putrescine administration but only at a dose of 0.15%; no substantial difference in dose-based putrescine effect was found in the spleen. Moreover, GST is a glutathione-dependent enzyme that protects cells against peroxidative damage.17 Putrescine intake markedly increased GST activity in the spleen regardless of dose, and putrescine doses of 0.10% and 0.15% markedly increased GST activities in the liver and thymus. GPx activity in the ileum was also remarkably increased after putrescine administration but only at a dose of 0.15%. Thus, putrescine supplementation exerts dose- and tissue-dependent and independent effects on enzymatic antioxidants activities in weaning piglets.
Besides enzymatic antioxidants, non-enzymatic antioxidants also play an important role in scavenging free radicals. GSH, a non-enzymatic antioxidant, is an important component of the antioxidant defence system because it can detoxify H2O2 and lipid hydroxides, thereby reducing oxidative damage.42 In the present study, the GSH content of the liver remarkably increased after putrescine administration but only at a dose of 0.15% putrescine. While the GSH content of the spleen remarkably increased after putrescine administration regardless of dose, no substantial difference was found in the thymus and ileum; this difference in putrescine effect may be ascribed to tissue-specific differences. The findings are consistent with our previous studies demonstrating improved GSH contents in rat liver and spleen after spermine treatment.43 Taken together, putrescine intake exerts dose- and tissue-dependent and independent effects on non-enzymatic antioxidants activities in weaning piglets. T-AOC is a marker of total endogenous antioxidative capability.44 And T-AOC levels in the thymus remarkably increased after putrescine administration but only at a dose of 0.15%. T-AOC levels in the ileum also remarkably increased after putrescine administration regardless of dose. We noted no substantial difference in putrescine effect between the liver and spleen, which may be ascribed to tissue differences. Once again, the results are consistent with our previous study showing increased T-AOC in rat jejunum and liver after spermine treatment.22,43
Enzymatic antioxidant activity may be modulated by modifications to the corresponding mRNA gene expression.17 Thus, we also sought to determine whether putrescine administration exerts a positive role in the gene expression of antioxidant enzymes in the immune organs of weaning piglets. In this study, putrescine remarkably enhanced GPX1 gene expression in the ileum, liver and thymus regardless of dose, and increased GPX1 gene expression in the spleen was observed after 0.15% putrescine intake; variations in the effects of putrescine on these organs may be ascribed to tissue differences. Correlation analysis indicated that the mRNA levels of GPX1 were positively correlated with GPX activity in the ileum, thus suggesting that up-regulated expression of the GPX1 mRNA of weaning piglets following putrescine intake may confirm increased GPX activity in this organ. Doses of 0.05% or 0.15% putrescine markedly increased mRNA levels of CAT and GST in the liver, and correlation analysis showed that CAT and GST activities were positively correlated with their respective mRNA levels (Table 10). This finding suggests that the benefits of putrescine intake on the activity of antioxidant enzymes (CAT and GST) may partially be ascribed to the enhancement of their respective mRNA levels in the livers of weaning piglets. A dose of 0.15% putrescine markedly increased the mRNA levels of SOD1, CAT and GPX1 in the thymus, and correlation analysis indicated that SOD, CAT and GPX activities were significantly positively associated with their respective mRNA expression (Table 10). This result indicates that putrescine increases the activities of antioxidant enzymes (T-SOD, CAT and GPX) by enhancing their respective mRNA levels in the thymus of weaning piglets. A dose of 0.15% putrescine also markedly increased the mRNA levels of GR and CAT in the spleen, and correlation analysis demonstrated that CAT activities were positively associated with CAT gene expression (Table 10). Correlation analysis further showed that GSH content was positively correlated with GR mRNA level in the spleen (Table 10), which supports the idea that GR can catalyse the reduction of oxidised glutathione to regenerate GSH.
Increases in antioxidant enzyme gene expression are related to the up-regulated transcription of the Nrf2 gene.17 Our data showed that 0.15% putrescine administration increased the transcription levels of the Nrf2 gene in the liver, and correlation analysis indicated that mRNA levels of CAT and GST are positively associated with Nrf2 gene expression in this organ (Table 10). Administration of 0.15% putrescine up-regulated the transcription levels of the Nrf2 gene in the thymus, and mRNA levels of CAT, GPX1 and SOD1 were positively correlated with Nrf2 gene expression (Table 10). This finding is consistent with previous results demonstrating that spermine supplementation remarkably increases SOD1, GPx1 and CAT mRNA levels by up-regulating Nrf2 gene transcription levels in the thymus of piglets.17 The results thus far reveal that modulation of the gene expression of antioxidant enzymes in the liver and thymus of weaning piglets by putrescine intake may be partially ascribed to the enhancement of Nrf2 mRNA expression.
Besides cellular membrane integrity, the structural integrity of immune organs is also associated with tight junction proteins.21,24 Tight junctions are the principal determinants of paracellular barrier functions.45 Tight junction proteins consist of ZOs, occludin and claudins. Up-regulation of ZO-1 and occludin attenuates the loss of intestinal barrier function in mice.46 In the present study, 0.10% putrescine supplementation up-regulated claudin 1 gene expression only in the ileum and thymus, while 0.15% putrescine supplementation up-regulated occludin, claudin 1, claudin 2, claudin 3, claudin 15 and claudin 16 mRNA expression in these organs. Occludin mRNA levels were positively correlated with claudin 3 gene expression in the ileum and thymus (Table 10), thus suggesting that enhanced occludin and claudin 3 mRNA levels may improve intestinal barrier functions in these organs.
TGF-β induces increases in T84 transepithelial resistance, which has been associated with an increase in claudin-1 expression.47 In the present study, 0.10% and 0.15% putrescine supplementation improved TGF-β1 gene expression (data not shown) in the ileum and thymus, and the mRNA levels of TGF-β1 were positively correlated with claudin 1 gene expression in these organs. These findings indicate that putrescine may improve claudin-1 levels by up-regulating anti-inflammatory cytokine (TGF-β1) mRNA levels in the ileum and thymus. Supplementation with 0.10% or 0.15% putrescine can improve tight junction protein mRNA levels in the ileum, thereby enhancing intestinal barrier functions.
A previous study demonstrated that up-regulation of MLCK could down-regulate the mRNA levels of ZO-1 and occludin in human Caco-2 cells.48 In the present study, putrescine down-regulated MLCK gene expression in the ileum and thymus of weaning piglets. The mRNA level of MLCK was negatively correlated with claudin 16 gene expression in the ileum (Table 10), thus suggesting that putrescine up-regulates claudin 16 mRNA levels by down-regulating MLCK mRNA levels in this organ. The mRNA level of MLCK was also negatively correlated with ZO-1, ZO-2, occludin, claudin 2 and claudin 3 mRNA levels in the thymus (Table 10), thus suggesting that up-regulation of ZO-1, ZO-2, occludin, claudin 2 and claudin 3 mRNA levels by the putrescine may be due to the down-regulation of MLCK mRNA levels in this organ. Putrescine supplementation reduced MLCK gene expression in the spleen, and MLCK gene expression was negatively correlated with claudin 1 and claudin 2 mRNA levels in this organ (Table 10). MLCK gene expression was positively correlated with claudin 15 mRNA levels in the spleen (Table 10). These results suggest that up-regulation of claudin 1 and claudin 2 mRNA levels and down-regulation of claudin 15 mRNA levels by the diamine may be due to the down-regulation of MLCK mRNA levels in the spleen.
In the current study, compared with findings in the control group, 0.05% putrescine supplementation improved mRNA levels of claudin 1 and claudin 14 in the liver, while 0.10% putrescine supplementation increased ZO-1 and claudin 1 levels in the liver and ZO-1 and claudin 3 levels in the spleen. Moreover, 0.15% putrescine supplementation increased ZO-1, ZO-2, occludin, claudin 1, claudin 2 and claudin 3 levels in the liver and spleen. Exogenous IL-10 administration has been observed to up-regulate the gene expression levels of ZO-1 and occludin in rat intestinal epithelial cells.46 In the current study, supplementation with 0.10% or 0.15% putrescine up-regulated the gene expression levels of IL-10 in the liver (data not shown), and the mRNA level of IL-10 was positively correlated with occludin and ZO-1 gene expression in this organ (data not shown). Taken together, these results suggest that putrescine enhances immune organs' physical barrier in a dose- and tissue-dependent and independent manner.
Conclusion
This study provides novel evidence demonstrating that putrescine supplementation can protect the health of weaning piglets' organs by enhancing organ barrier function as evidenced by increasing ZOs, occludin, claudins, which was associated with the MLCK signalling pathway, and the antioxidant defense as evidenced by increasing enzymatic antioxidant capacity, GSH content and clearance of oxygen radicals in a dose- and tissue-dependent and independent manner.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to all teammates of this research for their continuing assistance. This project was supported by the Program for Discipline Construction in Sichuan Agricultural University (to G. Liu, No. 03570126).References
- V. D. Leibbrandt, R. C. Ewan, V. C. Speer and D. R. Zimmerman, J. Anim. Sci., 1975, 40, 1077–1080 CrossRef.
- C. H. Hu, K. Xiao, Z. S. Luan and J. Song, J. Anim. Sci., 2013, 91, 1094–1101 CrossRef CAS PubMed.
- L. H. Zhu, K. L. Zhao, X. L. Chen and J. X. Xu, J. Anim. Sci., 2012, 90, 2581–2589 CrossRef CAS PubMed.
- A. Hernandez, C. F. Hansen, B. P. Mullan and J. R. Pluske, Anim. Feed Sci. Technol., 2009, 154, 102–111 CrossRef CAS.
- J. Wang, L. Chen, P. Li, X. Li, H. Zhou, F. Wang, D. Li, Y. Yin and G. Wu, J. Nutr., 2008, 138, 1025–1032 CrossRef CAS PubMed.
- W. Cao, G. Liu, T. Fang, X. Wu, G. Jia, H. Zhao, X. Chen, C. Wu, J. Wang and J. Cai, RSC Adv., 2015, 5, 76607–76614 RSC.
- P. R. O'Quinn, D. A. Knabe and G. Wu, J. Anim. Sci., 2002, 80, 467–474 CrossRef PubMed.
- G. Wu, N. E. Flynn and D. A. Knabe, Am. J. Physiol.: Endocrinol. Metab., 2000, 279, E395–E402 CrossRef CAS PubMed.
- F. Madeo, T. Eisenberg, S. Büttner, C. Ruckenstuhl and G. Kroemer, Autophagy, 2010, 6, 160–162 CrossRef PubMed.
- M. Farriol, T. Segovia-Silvestre, J. M. Castellanos, Y. Venereo and X. Orta, Nutrition, 2001, 17, 934–938 CrossRef CAS.
- S. Nagoshi and K. Fujiwara, Hepatology, 1994, 20, 725–730 CrossRef CAS PubMed.
- S. R. Girdhar, J. R. Barta, F. A. Santoyo and T. K. Smith, J. Nutr., 2006, 136, 2319–2324 CrossRef CAS PubMed.
- K. D. Priya, P. Selvaraj, K. Nanjappan, S. Jayachandran and P. Visha, Turk. J. Vet. Anim. Sci., 2010, 6, 250–254 Search PubMed.
- S. M. Hashemi, T. C. Loh, H. L. Foo, I. Zulkifli and M. H. Bejo, Anim. Feed Sci. Technol., 2014, 194, 151–156 CrossRef CAS.
- S. R. Chowdhury and T. K. Smith, Poult. Sci., 2001, 80, 1208–1214 CrossRef CAS PubMed.
- J. Bird, S. Mohd-Hidir and D. A. Lewis, Agents Actions, 1983, 13, 342–347 CrossRef CAS PubMed.
- W. Cao, X. Wu, G. Jia, H. Zhao, X. Chen, C. Wu, J. Tang, J. Wang, J. Cai and G. Liu, Arch. Anim. Nutr., 2017, 71, 175–191 CrossRef CAS PubMed.
- M. F. Cesta, Toxicol. Pathol., 2006, 34, 455–465 CrossRef PubMed.
- G. Pearse, Toxicol. Pathol., 2006, 34, 504–514 CrossRef PubMed.
- J. Y. Jeremy, R. A. Jones, A. J. Koupparis, M. Hotston, G. D. Angelini, R. Persad and N. Shukla, J. Urol., 2006, 175, 1175–1176 CrossRef.
- H. J. Xu, W. D. Jiang, L. Feng, Y. Liu, P. Wu, J. Jiang, S. Y. Kuang, L. Tang, W. N. Tang, Y. A. Zhang and X. Q. Zhou, Fish Shellfish Immunol., 2016, 52, 111–138 CrossRef CAS PubMed.
- T. Fang, G. Jia, H. Zhao, X. Chen, J. Tang, J. Wang and G. Liu, Anim. Nutr., 2016, 2, 370–375 CrossRef PubMed.
- M. Zhang, C. An, Y. Gao, R. K. Leak, J. Chen and F. Zhang, Prog. Neurobiol., 2013, 100, 30–47 CrossRef CAS PubMed.
- X. Mao, X. Zeng, S. Qiao, G. Wu and D. Li, Front. Biosci., 2011, 3, 1192–1200 Search PubMed.
- J. Wang, G. R. Li, B. E. Tan, X. Xiong, X. F. Kong, D. F. Xiao, L. W. Xu, M. M. Wu, B. Huang, S. W. Kim and Y. L. Yin, J. Anim. Sci., 2015, 93, 1679–1688 CrossRef CAS PubMed.
- S. Basuroy, P. Sheth, C. M. Mansbach and R. K. Rao, Am. J. Physiol., 2005, 289, G367–G375 CAS.
- X. Guo, J. N. Rao, L. Liu, T. Zou, K. M. Keledjian, D. Boneva, B. S. Marasa and J. Y. Wang, Am. J. Physiol., 2005, 288, G1159–G1169 CAS.
- T. K. Smith, Proc. Soc. Exp. Biol. Med., 1990, 194, 332–336 CrossRef CAS PubMed.
- T. T. Fang, G. M. Liu, W. Cao, X. J. Wu, G. Jia, H. Zhao, X. L. Chen, C. M. Wu and J. Wang, RSC Adv., 2016, 6, 31323–31335 RSC.
- B. Özmen, D. Özmen, E. Erkin, I. Güner, S. Habif and O. Bayindir, Clin. Biochem., 2002, 35, 69–72 CrossRef.
- M. Y. Hung, T. Y. C. Fu, P. H. Shih, C. P. Lee and G. C. Yen, Food Chem. Toxicol., 2006, 44, 1424–1431 CrossRef CAS PubMed.
- W. Cao, G. M. Liu, T. T. Fang, X. J. Wu, G. Jia, H. Zhao, X. L. Chen, C. M. Wu, J. Y. Cai and J. Wang, Food Funct., 2016, 7, 2303–2311 RSC.
- G. Boudry, V. Péron, I. Le Huërou-Luron, J. P. Lallès and B. Sève, J. Nutr., 2004, 134, 2256–2262 CrossRef CAS PubMed.
- R. M. Peace, J. Campbell, J. Polo, J. Crenshaw, L. Russell and A. Moeser, J. Nutr., 2011, 141, 1312–1317 CrossRef CAS PubMed.
- A. J. Moeser, K. A. Ryan, P. K. Nighot and A. T. Blikslager, Am. J. Physiol., 2007, 293, G413–G421 CAS.
- N. Gahalain, J. Chaudhary, A. Kumar, S. Sharma and A. Jain, Int. J. Appl. Pharm. Sci. Res., 2011, 2, 2757–2766 CAS.
- G. Liu, W. Cao, G. Jia, H. Zhao, X. Chen, J. Wang and C. Wu, J. Anim. Feed Sci., 2016, 25, 335–342 CrossRef.
- W. Ding, L. G. Hudson and K. J. Liu, Mol. Cell. Biochem., 2005, 279, 105–112 CrossRef CAS PubMed.
- T. Fang, J. Zheng, W. Cao, G. Jia, H. Zhao, X. Chen, J. Cai, J. Wang and G. Liu, Animal, 2017, 25, 1–9 Search PubMed.
- A. M. Kafy, C. G. Haigh and D. A. Lewis, Agents Actions, 1986, 18, 555–559 CrossRef CAS.
- B. Ozmen, D. Ozmen, E. Erkin, I. Güner, S. Habif and O. Bayindir, Clin. Biochem., 2002, 35, 69–72 CrossRef CAS.
- C. C. Yang, J. Y. Fang, T. L. Hong, T. C. Wang, Y. E. Zhou and T. C. Lin, Int. Immunopharmacol., 2013, 15, 106–113 CrossRef CAS PubMed.
- X. Wu, W. Cao, G. Jia, H. Zhao, X. Chen, C. Wu, J. Tang, J. Wang and G. Liu, Anim. Nutr., 2017, 3, 85–90 CrossRef PubMed.
- W. Ren, Y. Yin, G. Liu, X. Yu, Y. Li, G. Yang, T. Li and G. Wu, Amino Acids, 2012, 42, 2089–2094 CrossRef CAS PubMed.
- J. R. Turner, Nat. Rev. Immunol., 2009, 9, 799–809 CrossRef CAS PubMed.
- X. Sun, H. Yang and K. Nose, Am. J. Physiol., 2008, 294, G139–G147 CrossRef CAS PubMed.
- K. L. Howe, C. Reardon, A. Wang, A. Nazli and D. M. McKay, Am. J. Pathol., 2005, 167, 1587–1597 CrossRef CAS PubMed.
- L. Nahidi, S. T. Leach, D. A. Lemberg and A. S. Day, Dig. Dis. Sci., 2013, 58, 3144–3155 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra02674f |
| This journal is © The Royal Society of Chemistry 2019 |