Open Access Article
Open Access ArticleSynthesis of 1,4,5,6-tetrahydropyridazines and pyridazines via transition-metal-free (4 + 2) cycloaddition of alkoxyallenes with 1,2-diaza-1,3-dienes†
Qi Wu‡
a,
Pan-Lin Shao‡ *ab and
Yun He
*ab and
Yun He *a
*a
aChongqing Key Laboratory of Natural Product Synthesis and Drug Research, School of Pharmaceutical Sciences, Chongqing University, 55 Daxuecheng South Road, Shapingba, Chongqing 401331, People's Republic of China. E-mail: shaopl@cqu.edu.cn; yun.he@cqu.edu.cn
bCollege of Innovation and Entrepreneurship, Southern University of Science and Technology, Shenzhen 518000, People's Republic of China
First published on 10th July 2019
Abstract
We developed an economical and practical protocol for the synthesis of 1,4,5,6-tetrahydropyridazines. A diverse range of alkoxyallenes and 1,2-diaza-1,3-dienes undergo (4 + 2) cycloaddition to generate the desired products in excellent yields. The high efficiency, wide substrate scope and good functional group tolerance of this process, coupled with operational simplicity, render the method synthetically attractive. The utility of the cycloaddition is also demonstrated by the preparation of various pyridazines from 1,4,5,6-tetrahydropyridazines.
For several years, we have been developing the methodologies of cumulative dienes1 for the synthesis of heterocyclic compounds.2 In the past few decades, allenes have attracted significant attention in organic synthesis.3 By virtue of their reactive and cumulative double bonds, allenes are widely used as valuable C3-feedstocks.4 Functional groups at the double bonds of allene moieties strongly influence the reactivities, and thus allow site- and regioselective transformations. For example allenoates, bearing electron-withdrawing substituents (carboxylic ester groups) at the allene moieties, lead to preferred reactions with nucleophiles attacked on the central carbon, and have been thoroughly studied.5 Nevertheless, investigations of alkoxyallenes are still limited.6 As special enol ethers, alkoxyallenes were frequently employed as strong nucleophiles via deprotonations and metalations.7 Moreover, the electronic bias imposed by the alkoxyl groups makes them unique dienophiles; the electron-deficient or electron-rich double bonds could engage in cycloadditions.
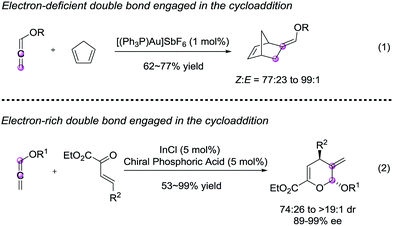
Recently, Goeke8 and Luo9 et al. developed (4 + 2) annulation of alkoxyallenes with cyclopentadienes and β,γ-unsaturated α-keto esters, respectively (Scheme 1). These established methods employed expensive heavy metals (Au, In), which maybe resulting in the contamination of medicinal products. Accordingly, there is a clear demand for the development of transition-metal-free protocols with high efficiency, operational simplicity, atom economy and general applicability.
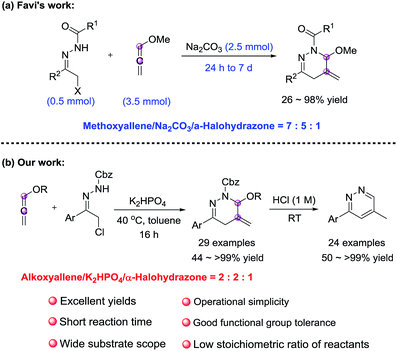
In 2015, Favi et al. developed (4 + 2) cycloaddition of alkoxyallene with α-halohydrazones (precursors of 1,2-diaza-1,3-dienes), but in which only methoxyallene could be employed as the dienophile (Scheme 2a).10 The approach allows access to 1,4,5,6-tetrahydropyridazines, which are versatile building blocks and prevalent in a large number of pharmacologically active molecules.11 However, the cyclization suffered from moderate conversion and narrow substrates scope, required long reaction time (up to 7 days) and high stoichiometric ratio of reactants (methoxyallene/Na2CO3/α-halohydrazone = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Currently, there is an increased drive to find new ways to maximize synthetic efficiency and minimize waste in chemical and pharmaceutical industries.12 As part of our group's continuous interest in cumulene chemistry and transition-metal-free synthesis,13 the (4 + 2) annulation of alkoxyallene with 1,2-diaza-1,3-dienes was thus systematically reinvestigated, and in this context, we demonstrate that the cyclization can proceed with a broad range of substrates, producing a wide variety of 1,4,5,6-tetrahydropyridazines in high efficiency. Besides, it was found that these adducts could further convert into pyridazines (Scheme 2b).
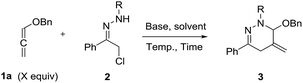
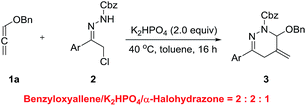
The investigations began with assaying the (4 + 2) cycloaddition between benzyloxyallene 1a and α-halohydrazone 2a, as shown in Table 1. A survey of solvents identified toluene as the most suitable media (entries 1–4). Protic solvent (MeOH) did not promote the reaction. Several bases were screened to evaluate their ability to promote the cyclization at ambient temperature (entries 4–9). Trace cycloadduct was generated by using organic base [TEA, DIPEA] (entries 5 and 6), whereas inorganic base [K2CO3, KOAc] afforded the desired product in good yields (entries 7–8). K2HPO4 was found to be most efficient and afforded predominantly the desired 1,4,5,6-tetrahydropyridazine 3aa in 94% yield at room temperature for 72 h (entry 9). Then, in order to speed up the cycloaddition, changes of temperature was made (entries 10 and 11). To our delight, a breakthrough was achieved. Raising the reaction temperature to 40 °C benefited the reaction rate dramatically and the full conversion was reached in only 16 h (entry 10). To our surprise, when the temperature was increased to 50 °C, the yield of 3aa decreased to 45% (entry 11). The negative impact on the yield may blame the low stability of 1a at higher temperature. Besides, α-halohydrazones 2 bearing different protecting groups [Cbz, Ac, Boc] were all suitable substrates, producing the corresponding products in uniformly high yields, and the yield is slightly higher when 2b was employed (entries 12–14). Meanwhile, we also investigated the effect of the stoichiometric ratio of 1a/2b (entries 15 and 16).
| Entry | R | 2 | X | Base | Solvent | Temp. (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (X equiv.), 2 (0.2 mmol), base (2.0 equiv., 0.4 mmol), solvent (2 mL).b Yield was that of the isolated product. NR: no reaction. | ||||||||
| 1 | Bz | 2a | 4.0 | Na2CO3 | MeOH | RT | 72 | NR |
| 2 | Bz | 2a | 4.0 | Na2CO3 | DCM | RT | 72 | 87 |
| 3 | Bz | 2a | 4.0 | Na2CO3 | CHCl3 | RT | 72 | 89 |
| 4 | Bz | 2a | 4.0 | Na2CO3 | Toluene | RT | 72 | 90 |
| 5 | Bz | 2a | 4.0 | TEA | Toluene | RT | 72 | <5 |
| 6 | Bz | 2a | 4.0 | DIPEA | Toluene | RT | 72 | <5 |
| 7 | Bz | 2a | 4.0 | K2CO3 | Toluene | RT | 72 | 72 |
| 8 | Bz | 2a | 4.0 | KOAc | Toluene | RT | 72 | 80 |
| 9 | Bz | 2a | 4.0 | K2HPO4 | Toluene | RT | 72 | 94 |
| 10 | Bz | 2a | 4.0 | K2HPO4 | Toluene | 40 | 16 | 92 |
| 11 | Bz | 2a | 4.0 | K2HPO4 | Toluene | 50 | 16 | 45 |
| 12 | Cbz | 2b | 4.0 | K2HPO4 | Toluene | 40 | 16 | 94 |
| 13 | Ac | 2c | 4.0 | K2HPO4 | Toluene | 40 | 16 | 68 |
| 14 | Boc | 2d | 4.0 | K2HPO4 | Toluene | 40 | 16 | 90 |
| 15 | Cbz | 2b | 2.0 | K2HPO4 | Toluene | 40 | 16 | 94 |
| 16 | Cbz | 2b | 1.0 | K2HPO4 | Toluene | 40 | 16 | 89 |
After extensive experimentation to reduce the amount of α-halohydrazone 2b, we identified the following optimal protocol: reaction of 1a and 2b with a stoichiometric ratio of 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 in the presence of K2HPO4 (2.0 equiv.) in toluene at 40 °C for 16 h (benzyloxyallene/K2HPO4/α-halohydrazone = 2
1.0 in the presence of K2HPO4 (2.0 equiv.) in toluene at 40 °C for 16 h (benzyloxyallene/K2HPO4/α-halohydrazone = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). It is also worth mentioning that all the reactions were conducted open to air with no need for exclusion of air or moisture.
1). It is also worth mentioning that all the reactions were conducted open to air with no need for exclusion of air or moisture.
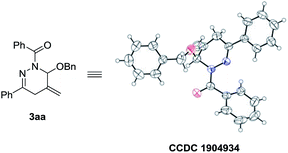
The structure of 3aa (CCDC 1904934) was unambiguously assigned by single crystal X-ray diffraction analysis (Fig. 1). The structure of 3 were assigned by analog.14
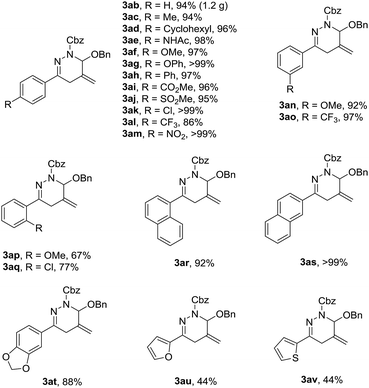
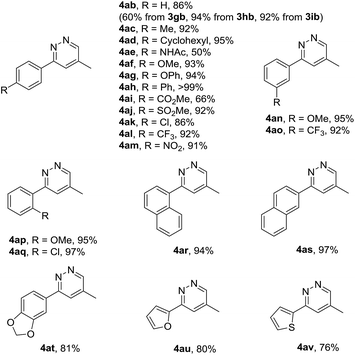
Having identified the optimized reaction conditions, the substrate scope of the cyclization was studied, and a variety of 1,4,5,6-tetrahydropyridazines 3 were synthesized in decent yields (Table 2). From a practical perspective, gram scale reaction was performed, 3ab was obtained without erosion of the yield, as shown in the parenthesis. We investigated the electronic effects of different aryl groups at the para positions of 1,2-diaza-1,3-dienes. All the α-halohydrazone 2 could well tolerate the existence of electron-neutral, electron-rich or electron-poor phenyl rings, and furnished the products 3ac–3am in uniformly excellent yields. In addition, we also demonstrated that the position of substituents at the meta positions of phenyl groups did not affect the reaction. However, when the substituents (MeO, Cl) were introduced at the ortho positions of the aromatic rings, the yields were decreased dramatically, maybe due to the steric hindrance on the aromatic rings (3ap–3aq). Besides, on replacement of the phenyl groups with α-naphthyl, β-naphthyl and 5-benzo[d][1,3]dioxolyl group, respectively, the reactions could still undergo smoothly, affording the desired 1,4,5,6-tetrahydropyridazines (3ar–3at) in high yields. In contrast, α-halohydrazone bearing 2-furanyl or 2-thiophenyl group showed lower reactivity for the cycloaddition, tended to give the cyclization product in diminished yields (3au–3av).
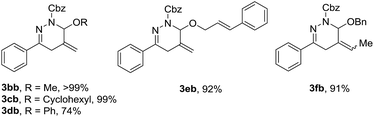
We next explored the possibility that the alkoxyallenes can bear other substituents, such as methyl (1b), cyclohexyl (1c), phenyl (1d) and cinnamyl (1e) groups. In fact, these alkoxyallenes proved to be compatible with the reaction conditions and led to smooth cyclization with α-halohydrazone 2b in good to excellent yields (Table 3). More interestingly, we also noticed that a similarly excellent yield was obtained when the reaction was conducted with racemic 3-methyl-substituted benzyloxyallene (1f).15 Unfortunately, despite vigorous efforts, a qualified single crystal of 3fb for X-ray crystallographic analysis could not be obtained to determine the relative configuration. Systematic experimentation of the annulation of 3-substituted alkoxyallenes is ongoing.
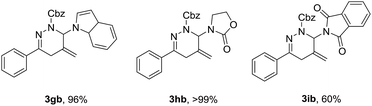
To further underscore the synthetic utility of the current method, we next sought to examine its application to nitrogen-substituted allenes,16 with the goal to provide a versatile approach for drug leads. As expected, the same reaction conditions could be utilized for the cycloaddition of allenamine (1g) and allenamide (1h) with α-halohydrazone 2b, respectively, providing the corresponding cycloadducts in excellent yields, albeit allenamide (1i) afforded a lower yield, maybe due to the strong electron-withdrawing effect of the phthalimido substituent (Table 4).
A possible mechanism for this cyclization was presented in Scheme 3. The 1,2-diaza-1,3-dienes is supposed to be produced in situ from 1,4-elimination of α-halohydrazones 2 under basic condition, and then intercepted by benzyloxyallene 1 to undergo inverse electron-demand aza-Diels–Alder reaction via transition state TS which finally results in the formation of 3.
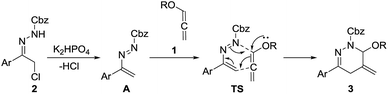 | ||
| Scheme 3 Proposed mechanism for the transition-metal-free (4 + 2) cycloaddition of alkoxyallenes with 1,2-diaza-1,3-dienes. | ||
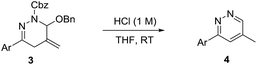
The high efficiency, wide substrate scope, good functional group tolerance of the above-mentioned processes, coupled with operational simplicity, make the cycloaddition an attractive method for the synthesis of 1,4,5,6-tetrahydropyridazines. Moreover, we found that the cycloadduct 3ab could be readily transformed into pyridazine 4ab by reduction in 60% yield or by hydrolysis in 86% yield. Furthermore, the hydrolysis in acidic condition (1 M HCl) proved to be an outstanding synthetic approach to pyridazines, since it was easily performed and provided the desired product in a transition-metal-free, efficient and economical manner, as shown in Scheme 4.
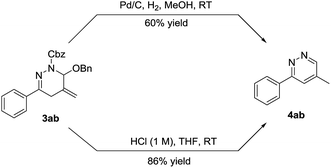 | ||
| Scheme 4 Synthesis of pyridazine. See the ESI† for details. | ||
It should be stressed in particular that the pyridazine skeletons are privileged structure moieties in many biologically active natural products, organocatalysts, ligands and synthetic materials.17 In order to explore the small heterocycles of pharmaceutical interest, we became interested in developing the hydrolysis method. When the cycloadducts 1,4,5,6-tetrahydropyridazines 3 were treated with hydrochloric acid at room temperature, a series of pyridazines were successfully delivered in good to excellent yield, regardless of the nature and the positions of the aryl substituent. In contrast, the desired compounds 4ae and 4ai were afforded in moderate yields, due to the susceptibility of amide (–NHAc) and ester (–CO2Me) groups to hydrolysis in the acidic condition (Table 5). What's more, pyridazine 4ab could be prepared likewise using 3gb, 3hb and 3ib as starting materials despite that 3gb underwent slower hydrolysis even employing higher concentration of HCl (3 M).
Conclusions
In summary, we have introduced a straightforward transition-metal-free method to synthesize 1,4,5,6-tetrahydropyridazines from alkoxyallenes and α-halohydrazones. This transformation proceeds with lower stoichiometric ratio of reactants in shorter reaction time, and produces a dramatic increase in yield and substrate scope, compared with the existing methods. The utility of the cycloaddition is demonstrated by the preparation of various pyridazines from the cycloadducts. As such, it is more applicable to the pharmaceutically promising 1,4,5,6-tetrahydropyridazines and pyridazines, specifically with respect to substitution pattern and functional group compatibility. Further studies to expand the application in drug synthesis are in progress.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (No. 21402150 and 21572027). We sincerely thank Dr Yong-Liang Shao (Lanzhou University) and Xiangnan Gong (Chongqing University) for the X-ray crystallographic analysis.Notes and references
- A. D. Allen and T. T. Tidwell, Chem. Rev., 2013, 113, 7287 CrossRef CAS.
- (a) X.-L. Huang, L. He, P.-L. Shao and S. Ye, Angew. Chem., Int. Ed., 2009, 48, 192 CrossRef CAS; (b) P.-L. Shao, X.-Y. Chen and S. Ye, Angew. Chem., Int. Ed., 2010, 49, 8412 CrossRef CAS; (c) P.-L. Shao, X.-Y. Chen, L.-H. Sun and S. Ye, Tetrahedron Lett., 2010, 51, 2316 CrossRef CAS; (d) P.-L. Shao, L.-T. Shen and S. Ye, Chin. J. Chem., 2012, 30, 2688 CAS; (e) J.-Y. Liao, P.-L. Shao and Y. Zhao, J. Am. Chem. Soc., 2015, 137, 628 CrossRef CAS; (f) Z. Wang, H. Xu, Q. Su, P. Hu, P.-L. Shao, Y. He and Y. Lu, Org. Lett., 2017, 19, 3111 CrossRef CAS; (g) G. P. Y. Kok, P.-L. Shao, J.-Y. Liao, S. N. F. B. S. Ismail, W. Yao, Y. Lu and Y. Zhao, Chem.–Eur. J., 2018, 24, 10513 CrossRef CAS.
- (a) Modern Allene Chemistry, ed. N. Krause and A. S. K. Hashmi, Wiley-VCH, Weinheim, 2004 Search PubMed; for selected reviews on allene, see: (b) S. Ma, Acc. Chem. Res., 2003, 36, 701 CrossRef CAS; (c) S. Ma, Chem. Rev., 2005, 105, 2829 CrossRef; (d) Z. Wang, X. Xu and O. Kwon, Chem. Soc. Rev., 2014, 43, 2927 RSC; (e) F. López and J. L. Mascareñas, Chem. Soc. Rev., 2014, 43, 2904 RSC; (f) H. Ni, W.-L. Chan and Y. Lu, Chem. Rev., 2018, 118, 9344 CrossRef CAS; (g) T. Cañeque, F. M. Truscott, R. Rodriguez, G. Maestri and M. Malacria, Chem. Soc. Rev., 2014, 43, 2916 RSC.
- For recent studies on allene, see: (a) H. Zhou, Y. Wang, L. Zhang, M. Cai and S. Luo, J. Am. Chem. Soc., 2017, 139, 3631 CrossRef CAS; (b) T. M. Beck and B. Breit, Angew. Chem., Int. Ed., 2017, 56, 1903 CrossRef CAS; (c) N. Casanova, K. P. D. Rio, R. García-Fandiño, J. L. Mascareñas and M. Gulías, ACS Catal., 2016, 6, 3349 CrossRef CAS PubMed; (d) L. A. Schwartz, M. Holmes, G. A. Brito, T. P. Gonçalves, J. Richardson, J. C. Ruble, K.-W. Huang and M. J. Krische, J. Am. Chem. Soc., 2019, 141, 2087 CrossRef CAS; (e) M. Holmes, K. D. Nguyen, L. A. Schwartz, T. Luong and M. J. Krische, J. Am. Chem. Soc., 2017, 139, 8114 CrossRef CAS; (f) J. Liu, Q. Liu, R. Franke, R. Jackstell and M. Beller, J. Am. Chem. Soc., 2015, 137, 8556 CrossRef CAS PubMed; (g) L.-Y. Mei, Y. Wei, X.-Y. Tang and M. Shi, J. Am. Chem. Soc., 2015, 137, 8131 CrossRef CAS; (h) Y. Jiang, A. B. Diagne, R. J. Thomson and S. E. Schaus, J. Am. Chem. Soc., 2017, 139, 1998 CrossRef CAS; (i) W. Zhao and J. Montgomery, J. Am. Chem. Soc., 2016, 138, 9763 CrossRef CAS; (j) W. Yao, X. Dou and Y. Lu, J. Am. Chem. Soc., 2015, 137, 54 CrossRef CAS; (k) H. Wu, F. Haeffner and A. H. Hoveyda, J. Am. Chem. Soc., 2014, 136, 3780 CrossRef CAS.
- (a) B. J. Cowen and S. J. Miller, Chem. Soc. Rev., 2009, 38, 3102 RSC; (b) N. Krause and C. Winter, Chem. Rev., 2011, 111, 1994 CrossRef CAS.
- For selected reviews on alkoxyallene, see: (a) R. Zimmer and H.-U. Reissig, Chem. Soc. Rev., 2014, 43, 2888 RSC; (b) M. Brasholz, H.-U. Reissig and R. Zimmer, Acc. Chem. Res., 2009, 42, 45 CrossRef CAS PubMed; (c) M. A. Tius, Chem. Soc. Rev., 2014, 43, 2979 RSC; (d) H.-U. Reissig and R. Zimmer, Synthesis, 2017, 49, 3291 CrossRef CAS.
- For recent studies on alkoxyallene, see: (a) H. Zhou, Z. Wei, J. Zhang, H. Yang, C. Xia and G. Jiang, Angew. Chem., Int. Ed., 2017, 56, 1077 CrossRef CAS; (b) B. M. Trost, J. Xie and J. D. Sieber, J. Am. Chem. Soc., 2011, 133, 20611 CrossRef CAS; (c) H. Kim, W. Lim, D. Im, D. G. Kim and Y. H. Rhee, Angew. Chem., Int. Ed., 2012, 51, 12055 CrossRef CAS; (d) W. Lim, J. Kim and Y. H. Rhee, J. Am. Chem. Soc., 2014, 136, 13618 CrossRef CAS PubMed; (e) Z. Wang, C. Nicolini, C. Hervieu, Y.-F. Wong, G. Zanoni and L. Zhang, J. Am. Chem. Soc., 2017, 139, 16064 CrossRef CAS; (f) J. Zhang, L. Zhu, K. Shen, H. Yang, X.-C. Hang and G. Jiang, Chem. Sci., 2019, 10, 1070 RSC; (g) N. W. Mszar, F. Haeffner and A. H. Hoveyda, J. Am. Chem. Soc., 2014, 136, 3362 CrossRef CAS; (h) Y. Tani, T. Fujihara, J. Terao and Y. Tsuji, J. Am. Chem. Soc., 2014, 136, 17706 CrossRef CAS PubMed; (i) T. H. Meyer, J. C. A. Oliveira, S. C. Sau, N. W. J. Angand and L. Ackermann, ACS Catal., 2018, 8, 9140 CrossRef CAS; (j) R. Kuppusamy, R. Santhoshkumar, R. Boobalan, H.-R. Wu and C.-H. Cheng, ACS Catal., 2018, 8, 1880 CrossRef CAS.
- G. Wang, Y. Zou, Z. Li, Q. Wang and A. Goeke, Adv. Synth. Catal., 2011, 353, 550 CrossRef CAS.
- S. Li, J. Lv and S. Luo, Org. Chem. Front., 2018, 5, 1787 RSC.
- O. A. Attanasi, G. Favi, F. Mantellini, S. Mantenuto, G. Moscatelli and S. Nicolini, Synlett, 2015, 26, 193 CAS.
- (a) S. M. M. Lopes, A. L. Cardoso, A. Lemos and T. M. V. D. Pinho e Melo, Chem. Rev., 2018, 118, 11324 CrossRef CAS PubMed; (b) A. M. Shelke and G. Suryavanshi, Org. Lett., 2016, 18, 3968 CrossRef CAS; (c) P. J. Rybczynski, D. W. Combs, K. Jacobs, R. P. Shank and B. Dubinsky, J. Med. Chem., 1999, 42, 2403 CrossRef CAS; (d) H. Huo, R. A and Y. Gong, J. Org. Chem., 2019, 84, 2093 CrossRef CAS; (e) X. Zhong, J. Lv and S. Luo, Org. Lett., 2015, 17, 1561 CrossRef CAS; (f) S. M. M. Lopes, A. F. Brigas, F. Palacios, A. Lemos and T. M. V. D. P. e Melo, Eur. J. Org. Chem., 2012, 2152 CrossRef CAS.
- Y. Hayashi, Chem. Sci., 2016, 7, 866 RSC.
- (a) P.-L. Shao, Z.-R. Li, Z.-P. Wang, M.-H. Zhou, Q. Wu, P. Hu and Y. He, J. Org. Chem., 2017, 82, 10680 CrossRef CAS; (b) Z.-P. Wang, Y. He and P.-L. Shao, Org. Biomol. Chem., 2018, 16, 5422 RSC.
- CCDC 1904934 (3aa) contain the crystallographic data for this paper. Selected data are also included in the ESI.†.
- (a) T. Pecchioli, F. Cardona, H.-U. Reissig, R. Zimmer and A. Goti, J. Org. Chem., 2017, 82, 5835 CrossRef CAS; (b) A. Hausherr and H.-U. Reissig, Synthesis, 2018, 50, 2546 CrossRef CAS.
- (a) T. Lu, Z. Lu, Z.-X. Ma, Y. Zhang and R. P. Hsung, Chem. Rev., 2013, 113, 4862 CrossRef CAS; (b) R. Blieck, R. A. A. Abdine, M. Taillefer and F. Monnier, Org. Lett., 2018, 20, 2232 CrossRef CAS; (c) L. Perego, R. Blieck, A. Groué, F. Monnier, M. Taillefer, I. Ciofini and L. Grimaud, ACS Catal., 2017, 7, 4253 CrossRef CAS; (d) R. Blieck, J. Bahri, M. Taillefer and F. Monnier, Org. Lett., 2016, 18, 1482 CrossRef CAS PubMed; (e) X.-X. Li, L.-L. Zhu, W. Zhou and Z. Chen, Org. Lett., 2012, 14, 436 CrossRef CAS; (f) S. Pang, X. Yang, Z.-H. Cao, Y.-L. Zhang, Y. Zhao and Y.-Y. Huang, ACS Catal., 2018, 8, 5193 CrossRef CAS; (g) Y. Liu, A. De Nisi, A. Cerveri, M. Monari and M. Bandini, Org. Lett., 2017, 19, 5034 CrossRef CAS.
- (a) M. Borger and J. H. Frederich, Org. Lett., 2019, 21, 2397 CrossRef CAS; (b) M. Saldías, N. Guzmán, F. Palominos, C. Sandoval-Altamirano, G. Günther, N. Pizarro and A. Vega, ACS Omega, 2019, 4, 4679 CrossRef; (c) C. Liu, J. Lin, R. Moslin, J. S. Tokarski, J. Muckelbauer, C. Chang, J. Tredup, D. Xie, H. Park, P. Li, D.-R. Wu, J. Strnad, A. Zupa-Fernandez, L. Cheng, C. Chaudhry, J. Chen, C. Chen, H. Sun, P. Elzinga, C. D'arienzo, K. Gillooly, T. L. Taylor, K. W. McIntyre, L. Salter-Cid, L. J. Lombardo, P. H. Carter, N. Aranibar, J. R. Burke and D. S. Weinstein, ACS Med. Chem. Lett., 2019, 10, 383 CrossRef CAS; (d) H. R. Kalhor and A. N. Khodadadi, Chem. Res. Toxicol., 2018, 31, 1092 Search PubMed; (e) M. Li, Y. Yuan and Y. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 1237 CrossRef CAS; (f) A. T. Londregan, D. W. Piotrowski and L. Wei, ACS Comb. Sci., 2016, 18, 651 CrossRef CAS; (g) A. Tamaki, S. Kojima and Y. Yamamoto, J. Org. Chem., 2016, 81, 8710 CrossRef CAS; (h) H. B. Abed, O. Mammoliti, O. Bande, G. Van Lommen and P. Herdewijn, J. Org. Chem., 2013, 78, 7845 CrossRef PubMed; (i) T. J. Ritchie, S. J. F. Macdonald, S. Peace, S. D. Pickett and C. N. Luscombe, Med. Chem. Commun., 2012, 3, 1062 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data, copies of 1H and 13C NMR spectra. CCDC 1904934 (3aa). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra02712b |
| ‡ These authors contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2019 |