Open Access Article
Open Access ArticleFly ash based robust biocatalyst generation: a sustainable strategy towards enhanced green biosurfactant production and waste utilization
Zhiwen Zhua,
Baiyu Zhang *a,
Bing Chena,
Jingjing Linga,
Qinghong Caibc and
Tahir Husaina
*a,
Bing Chena,
Jingjing Linga,
Qinghong Caibc and
Tahir Husaina
aNRPOP Laboratory, Faculty of Engineering and Applied Science, Memorial University, St. John's, NL A1B 3X5, Canada. E-mail: bzhang@mun.cab
bBiotechnology Research Institute of the National Research Council of Canada, 6100 Royalmount Ave, Montreal, QC H4P 2R2, Canada
cDepartment of Natural Resource Sciences, Faculty of Agricultural and Environmental Sciences, McGill University, Macdonald Campus, 21111 Lakeshore Rd, Ste Anne-de-Bellevue, QC H9X 3V9, Canada
First published on 28th June 2019
Abstract
Biosurfactants have been well recognized as an environmentally friendly alternative to chemical surfactants. However, their production remains challenging due to low productivity, short-term microbe stability and the potentially toxic by-products generated in the growth media. To overcome these challenges, the emerging biofilm-based biosynthesis was investigated in this study. A fresh insight into the biosynthesis process was provided through using waste fly ash as a carrier material. The biofilm produced by biosurfactant producer B. subtilis N3-1P attached onto the surface of fly ash acted as a robust and effective biocatalyst. Zeta potential analysis and scanning electron microscope (SEM) characterization were conducted to help unravel the biocatalyst formation. High-value biosurfactant products were then produced in an efficient and sustainable manner. Stimulation by a fly ash assisted biocatalyst on biosurfactant production was confirmed. The biosurfactant yield was boosted over ten times after 24 hours, at a fly ash dosage of 0.5%. The highest biosurfactant yield was achieved after five days, with a final productivity of 305 critical micelle dilution. The underlying mechanism of fly ash assisted biosurfactant production was tracked through it exerting an effect on the quorum sensing system. Fourier-transform infrared (FTIR) spectroscopy and matrix assisted laser desorption/ionization-time of flight (MALDI-TOF) analysis demonstrated that the final biosurfactant product belonged to the lipopeptides. This research output is expected to accelerate the development of more effective bioreactors, and make a continuous contribution to high-value product generation and waste reduction.
1. Introduction
Surface active agents, such as lipopeptides, glycolipids, phospholipids, fatty acids, and neutral lipids, if produced by microorganisms during their growth, are named biosurfactants.1 Compared to chemically synthetic surfactants, biosurfactants offer the advantages of retaining a stable and effective performance even under extreme environmental conditions, and they cause little or no environmental impact due to their low toxicity and high biodegradability.2,3 They exhibit high surface activities and low critical micelle concentrations (CMCs) and have, therefore, attracted a lot of attention in recent years.4 As an environmentally friendly alternative to chemical surfactants, biosurfactants could serve as detergents, emulsifiers, and foaming, wetting, and dispersing agents in environmental, oil and pharmaceutical industries.5 Despite their environmentally favorable characteristics, the economic feasibility of biosurfactants remains problematic owing to their poor production rate, arising primarily from the complex regulation system during fermentation and limited number of effective production cells.6Zhi et al.7 indicated that biosurfactant producers such as Bacillus subtilis can generate surfactin, a lipopeptide, through biosynthetic regulation of a quorum sensing system. In this system, surfactin synthesis, competence development and sporulation are cross-linked within a complex network of pheromones and pleiotropic regulators. As a consequence of quorum sensing, surfactin synthesis is dependent on cell density, preventing constant production and limiting overall yields. Methodologies for increasing cell density and thus enhancing biosurfactant productivity are important.
Biofilm is an assemblage of microorganisms embedded in a self-produced matrix of extracellular polymeric substances (EPSs).8 They can self-immobilize and self-regenerate on all kinds of interfaces with well-organized metabolism and, in the meantime, offer protection for the microbes inside.9 Given that whole-cell mobilization has been suggested as an effective approach to reduce fermentation time,10 the robust and long-lasting features of biofilms make them attractive as biocatalysts for organic synthesis with high productivity, particularly when cell viability is affected by substrates and/or metabolites.11,12 The proper selection of a solid carrier can greatly improve the growth rate of biofilm, and thereby effectively increase the density of cells and stimulate the production of target metabolites.13 Recent findings have indicated that harnessing porous solid carriers with larger surface areas, such as activated carbon and expanded clay, could promote gas exchange, and provide larger cell attachment and more immobilization sites for microbes.6,14 Solid carriers could also provide a larger buffer capacity under extreme culturing conditions and hence protect the microbes from biotic and abiotic stresses.6,13
Fly ash (FA) is a municipal solid waste produced worldwide due to the combustion of coal at high temperature. Owing to the concentrated toxic heavy metals in the ash, FA is regarded as a hazardous waste. Research has centered on the treatment of FA through detoxification and potential resource recovery. For example, treated FA can be beneficially used as a natural absorbent after proper treatment, given its porous structure. However, no attempt has ever been made to use FA as a solid carrier for microbial growth.
Therefore, in this work, FA was tried as a solid carrier for facilitating the cost-effective and highly efficient biosynthesis of biosurfactants for the first time. Two hypotheses regarding the role of FA on the biosynthesis process were examined: (1) the porous structure of FA could provide a larger surface area for the attachment of biocatalyst, thereby greatly stimulating biosurfactant production, and (2) the immobilized bacterial biofilm may have a positive effect on the detoxification of FA by means of a bioleaching process. The biosurfactant-producing microorganism applied was B. subtilis N3-1P, which was isolated from the Atlantic Ocean. The performance of FA on cell growth and biosurfactant production was investigated. The effect of FA dosage on biosurfactant production was examined using parameters including surface tension (ST), emulsification activity, and solution dilution as responses. The generated biosurfactant product was characterized by determining ST and CMC. Its structure was further characterized using Fourier-transform infrared (FTIR) spectroscopy and matrix assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS).
2. Materials and methods
2.1. FA as solid carrier
The FA to be used as a platform for biosurfactant production was obtained from the Corner Brook Pulp and Paper (CBPP) plants in Newfoundland and Labrador, Canada. Bunker C oil had been mixed with wasted pulpwood as a fuel during the thermal mechanical pulp process. Generated fly ash was then collected from the power boiler and subjected to air drying. The properties of the fly ash are listed in Table 1. FA was characterized before and after incubation with FTIR by the KBr pellet method and scanning electron microscopy (SEM).| pH | 12 |
| Density (g cm−3) | 0.45 |
| Moisture content (%) | 0.89 |
| Surface area (m2 g−1) | 249.4 |
| C/N ratio | 572.95 |
| Elemental content in solid (unit: mg kg−1) | |
|---|---|
| Mg | 511.65 |
| Al | 947.03 |
| Fe | 784.20 |
| P | 114.33 |
| Cl | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634 634 |
| Zn | 11.72 |
| Cu | 7.28 |
| Pb | 2.25 |
| V | 15.46 |
| Cr | 4.73 |
| Ni | 15.96 |
| Ca | 2656.36 |
2.2. Biosurfactant-producing microorganisms
B. subtilis is a motile, Gram-positive, rod-shaped endospore-forming bacterium widely studied in biofilm formation. It is famous for producing biosurfactants, especially effective lipopeptide biosurfactants. The bacterium used in this study, B. subtilis N3-1P, was screened from oily contaminated seawater samples.15 This strain was identified as a promising and economic biosurfactant producer among the screened bacteria, whose product possessed strong surface activity and high emulsification capacity.The composition of the culture medium for B. subtilis N3-1P was as follows: BD Difco™ Marine Broth (Fisher Scientific, Canada) 37.4 g in 1 L of distilled water. A loopful of a bacteria colony was transferred into a 125 mL Erlenmeyer flask containing 50 mL of inoculum broth. This seeded culture medium was initially grown on a rotary incubator shaker (VWR, Canada) at 200 rpm for 24 h at room temperature to reach its exponential growth phase. The biosurfactant production medium comprised (g L−1): sucrose (30), NH4NO3 (10), NaCl (15), KH2PO4 (3.4), K2HPO4·3H2O (4.4), MgSO4·7H2O (1.02), and yeast extract (0.5).
2.3. Biosurfactant production with FA
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min to remove the remaining cells. Filtrated FA particles were gently washed three times with PBS buffer solution and then subjected to zeta potential measurement. The growth behavior of the biofilm-based biocatalyst was quantified by the variation in zeta potential. The immobilized biocatalyst on FA particles was further characterized with FTIR and SEM. All the samples were collected and analyzed in triplicate. The cell-free filtrate was analyzed for biosurfactant productivity using critical micelle dilution (CMD) as an indicator.
000 rpm for 10 min to remove the remaining cells. Filtrated FA particles were gently washed three times with PBS buffer solution and then subjected to zeta potential measurement. The growth behavior of the biofilm-based biocatalyst was quantified by the variation in zeta potential. The immobilized biocatalyst on FA particles was further characterized with FTIR and SEM. All the samples were collected and analyzed in triplicate. The cell-free filtrate was analyzed for biosurfactant productivity using critical micelle dilution (CMD) as an indicator.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min to remove the remaining cells. The fermentation process was monitored by measuring parameters such as ST, pH, and CMD. The FA particles were collected and further examined to determine the effect of biosurfactant adsorption on final productivity. The residual FA particles were treated to separate the adsorbed biosurfactant following the method described by Dubey et al.16 The optimum FA level and incubation time obtained from the above tests were selected for batch-scale biosurfactant production. All the analyses in this study were performed in triplicate.
000 rpm for 10 min to remove the remaining cells. The fermentation process was monitored by measuring parameters such as ST, pH, and CMD. The FA particles were collected and further examined to determine the effect of biosurfactant adsorption on final productivity. The residual FA particles were treated to separate the adsorbed biosurfactant following the method described by Dubey et al.16 The optimum FA level and incubation time obtained from the above tests were selected for batch-scale biosurfactant production. All the analyses in this study were performed in triplicate.2.4. Characterization of the generated biosurfactant product
The optimum FA addition level and incubation time derived from Section 2.2 were applied during batch-scale biosurfactant production. Biosurfactants in the FA particles were collected using the method described in Section 2.2. The FA-free culture broth was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min to remove the remaining cells. Cell-free culture broth was extracted using an equal volume of chloroform–methanol (1
000 rpm for 10 min to remove the remaining cells. Cell-free culture broth was extracted using an equal volume of chloroform–methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) solvent. The solvent was removed by rotary evaporation. Combined biosurfactant products were extracted from FA particles and culture medium, and then subjected to ST and CMC measurements. Their structures were further characterized using FTIR spectroscopy and MALDI-TOF-MS. All the characterization results were compared with those generated by the control.
2 v/v) solvent. The solvent was removed by rotary evaporation. Combined biosurfactant products were extracted from FA particles and culture medium, and then subjected to ST and CMC measurements. Their structures were further characterized using FTIR spectroscopy and MALDI-TOF-MS. All the characterization results were compared with those generated by the control.
2.5. Bioleaching of heavy metals from FA
Metal leachability from the FA particles during the biosurfactant production process was estimated. Cell-free culture mediums at a FA concentration of 1% and 2% collected from the process in Section 2.3 were further examined for their bioleaching behavior. Samples were collected and analyzed in duplicate, and a triplicate analysis was performed when the deviations were greater than 5%. The concentrations of leached heavy metals were examined using ICP-MS. The differences between FA-based medium and control samples were recorded.2.6. Sample analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) vHNO3 = 3
vHNO3 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added to the sample and heated at 70 °C for one day. The sample was eventually dried, cooled, and dissolved in 2% HNO3. The solution was then diluted and analyzed by ICP-MS.
1) was added to the sample and heated at 70 °C for one day. The sample was eventually dried, cooled, and dissolved in 2% HNO3. The solution was then diluted and analyzed by ICP-MS.3. Results and discussion
3.1. Effect of biocatalyst immobilization on microbe growth
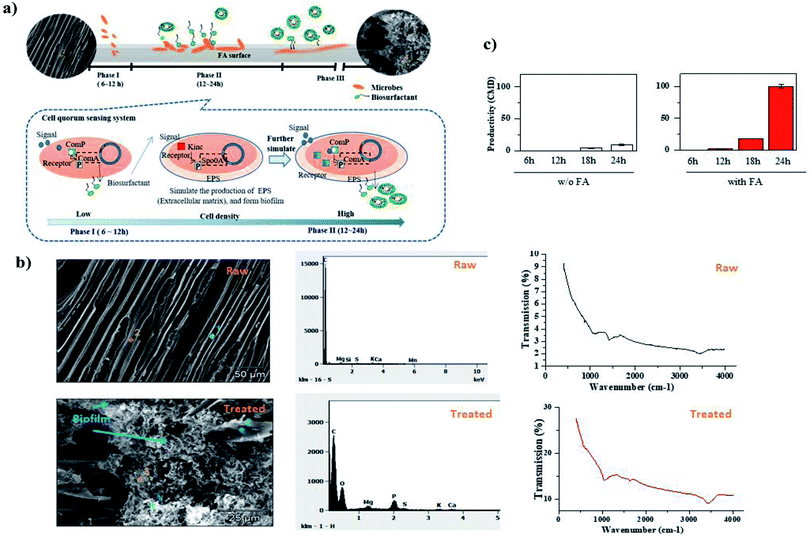
Previous studies have proved cell growth stimulation and product promotion through using certain types of porous carrier, such as α-cyclodextrin, filter paper, and silica gel.22 A mechanism for FA-enhanced biosurfactant production through the self-produced biocatalyst is proposed in Fig. 1(a). Biofilms, acting as a biocatalyst in this study, are microbial communities encased in a layer of self-produced matrix EPS and adhered to FA surfaces. As proposed in Fig. 1(a), those free-floating biosurfactant producers started to flow into the channels of the FA particles and initially attached to their surface within the first few hours. Those pioneers then quickly anchored themselves to the matrix via the production of pili, fimbriae, and exopolysaccharides.22 Following initial attachment, the proliferation and building of microcolonies on FA surface spontaneously occurred through the production of an extracellular matrix. The pore structure and surface charge of FA were major contributors to the bacterial adsorption process.23 Visual, elemental and spectroscopic analyses were carried out to provide multi-disciplinary evidence with SEM and FTIR about the microstructure and surface chemistry of FA before and after the incubation process. The results are presented in Fig. 1(b). The SEM image of raw CBPP FA demonstrated that it consisted of a highly porous, platelet and fiber-shaped structure. Its energy dispersion X-ray spectroscopy (EDX) analysis revealed a dominant amount of carbon (C) on the surface. After incubation, a layer of biofilm was identified on the FA surface from the SEM image. The EDX analysis proved the growth of biofilm on the surface of FA. The dominant components on the surface of FA were found to be P, O, C, and Mg, the major components of biofilm. Surface properties play an important role in initial cell attachment.24 The rough surface of FA, which Fig. 1(b) presents, has been considered to be an excellent solid carrier to promote cell settlement and biofilm growth on its surface, owing to the enhanced cell–surface interactions and strengthened protection from shear force.25 The absorbent properties of FA offered the biosurfactant producer better access to localized nutrients, which thus have higher metabolic activity than free-living ones. Recent research has confirmed that microbes tend to reside in biofilms, rather than as free-floating forms.26 Biofilm was able to provide biosurfactant producers with a stable environment under external stress (e.g. disinfectants and antibiotics) by reducing the diffusion of those compounds,27 thereby promoting their growth rate, and follow-up biosurfactant production.The FTIR spectra (Fig. 1(b)) provided qualitative characterization of the surface of FA, primarily by providing information about functional groups. The major peaks acquired from raw FA material were in agreement with those reported by Martins et al.28 The carbonate (870–1400 cm−1) group was recognized in this study. Its presence in wood-based FA has been widely acknowledged, as the combustion process mineralizes the organic compounds, and transforms the basic cations to their oxide forms. They are later hydrated and subsequently converted into the forms of carbonates and phosphates.29 The alkane C–H bond stretch (2700–3000 cm−1) and carboxylic and/or hydroxyl groups (3200–3600 cm−1) were identified from the FTIR analysis.30 The presence of Al and Fe as oxygen functional groups within Al–OH and Fe–OH (800–900 cm−1) can be confirmed from Putra et al.31 The FTIR spectrum of the FA samples after the incubation process is also presented in Fig. 1(b). This result showed an increased intensity of carboxylic and/or hydroxyl groups in 3200–3600 cm−1 and 800–1000 cm−1, verifying the existence of biofilm on its surface. It is recognized that biofilm is composed of up to 90% of water,32 while the rest consists of polysaccharide, protein, DNA, etc. The abundant carboxylic in FA and the biofilm surface may form a chemical bond structure with carbonate groups on the FA surface, thus leading to the disappearance of the stretching band at 1450 cm−1.
Fig. 1(c) provides biosurfactant productivity with the addition of FA. A rocketing biosurfactant production rate was observed after incubation for 18 hours. The assembled biocatalyst on FA particles accelerated the reaction by well over ten-fold. Biosurfactant concentration increased from 9 CMD in the control sample to 100 CMD in the FA sample after incubation for 24 hours. It is believed that biosurfactant production was stimulated through the quorum sensing system at this stage (Fig. 1(a)), not only to enhance the swarming motility of the biosurfactant producer, but also to alter the wettability and potential of the platform surface to facilitate their residence.22,33,34 The role of the lipopeptide biosurfactant as signaling molecules triggering robust biofilm formation for Bacillus strains under laboratory conditions has been identified.35 The intercellular communications within a biofilm further stimulated the up-and-down regulation of gene expression, enabling temporal adaptations, such as phenotypic variation and the ability to survive in nutrient-deficient conditions,36 thereby promoting the biosurfactant production. This study demonstrated the successful application of FA as a platform to stimulate the biosynthesis of lipopeptide through biofilm-encased cells and the finding in this study was comparable to that generated by Wigneswaran et al.37 This result further proved the enhanced production mechanism described in Fig. 1(a). Acting as molecular signal, biosurfactant was initially secreted to stimulate EPS production and biofilm formation at a relatively slow rate.35
It is acknowledged that cell attachment and biofilm formation will alter the physiochemical properties of a porous medium. The surface electrostatic charge of a porous medium will accordingly be affected by the attached biosurfactant production cells and the EPS matrix. Zeta potential measurement has been widely used to characterize the solid–liquid interface, obtaining the nature and charge information of a solid surface, and exhibiting the electrokinetic behavior of the solid–liquid interface. Therefore, the variation in zeta potential was investigated to shed light on the attachment of microbes and the growth of biofilm on the FA surface. The results are listed in Table 2. Zeta potentials of all FA particles were less than zero in the provided neutral buffer solution. Those with 2% FA dosage had the lowest starting zeta potential value, followed by 1% and then 0.5%. This negative zeta potential might due to the initial conditioning process, resulting from the attraction of mineral groups in the growth medium, such as PO43− or SO42−.38 A sharp decrease in zeta potential was identified during the first six hours, confirming the strong attachment of negatively charged biosurfactant producer to the FA surface. The hydrophobic FA surface tended to enhance bacterial attachment onto its surface by removing adsorbed surface water, and attracting bacteria with hydrophobic properties.39 Microbes then embedded into a self-produced extracellular matrix. When biofilm was gradually produced, its major component, namely neutral polysaccharide, shielded and/or neutralized the negatively charged surface functional groups, such as DNA and protein, thereby slowing the decrease in zeta potential.12 This explained the relatively stable zeta potential during the next few hours. The higher the concentration of FA particles, the longer the biosurfactant producer took to finish biofilm assembly. After that, a continuous decrease in zeta potential was observed due to the production and adsorption of produced biosurfactants on FA particles. At pH 7.4, most functional groups at the hydrophilic moiety of the produced anionic lipopeptide biosurfactant molecule were protonated or compensated by a counter ion, leaving limited acidic residues (e.g., Glu and Asp) working as effective negatively-charged carriers.40 The continuous accumulation of those produced anionic lipopeptide biosurfactants thus decreased the zeta potential of the FA surface.
| Time (h) | 0.5% FA | 1% FA | 2% FA | |||
|---|---|---|---|---|---|---|
| Zeta potential (ξ) | SD (%) | Zeta potential (ξ) | SD (%) | Zeta potential (ξ) | SD (%) | |
| 0 | −3.45 | 6 | −9.54 | 6 | −10.89 | 2 |
| 6 | −13.78 | 5 | −15.05 | 5 | −13.43 | 5 |
| 12 | −14.48 | 4 | −15.05 | 3 | −14.08 | 3 |
| 18 | −17.17 | 3 | −15.08 | 6 | −14.48 | 6 |
| 24 | −19.13 | 1 | −16.52 | 1 | −14.77 | 5 |
3.2. Effect of FA dosage on biosurfactant production
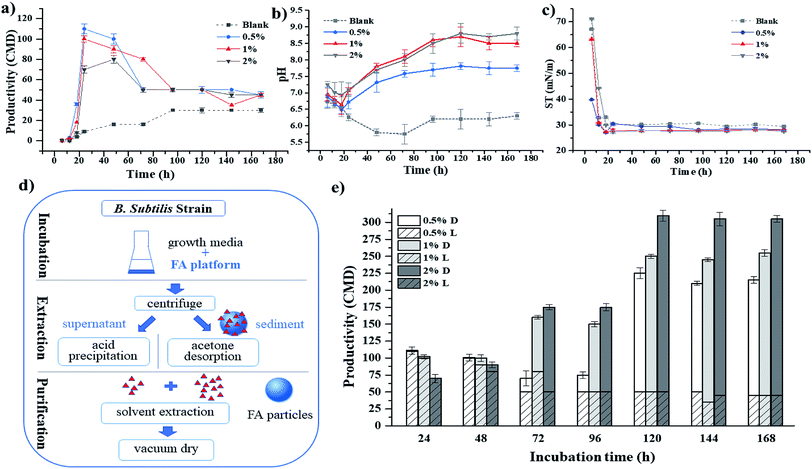
The effect of supplemental FA as a platform for stimulating the growth of biocatalyst upon the enhancement of biosurfactant production was assessed. The medium was supplemented by a fixed amount (i.e., 0.5%, 1% or 2%) of FA carrier. The biosurfactant production rate was obtained using the selected Bacillus strain, and the correlation between FA dosage and biosurfactant production rate was evaluated (Fig. 2). The results clearly demonstrated the remarkable advantage of using FA to promote biosurfactant production. As Fig. 2(a) shows, the lag phase of biosurfactant production was clearly affected by FA dosage. Acceleration of production firstly took place in incubation samples with 0.5% FA dosage and was last found in the samples with 2% dosage. After 24 hours, biosurfactant production was increased from 9 CMD to 110, 100 and 70 CMD, respectively. Afterwards, a reduction in biosurfactant production was recognized, accompanied by a reduction in pH (Fig. 2(b)), which was due to the generation of secondary acid metabolites, such as uronic acid, when using sugar as the carbon source.4 Biosurfactant was produced and led to a reduction in surface tension (Fig. 2(c)).The reduction in biosurfactant concentration in culture media may be attributed to the depletion of the carbon source, as Yeh et al.22 suggested. Produced surfactin might be assimilated as a carbon source for additional cell growth when another carbon source was not available. Dubey et al.,16 however, claimed that activated carbon could also be used as an absorbent to recover biosurfactants, thus contributing to the reduction in biosurfactant in the culture broth. In this study, biosurfactant product in the sediment was further separated from the fly ash through acetone desorption (Fig. 2(d)). The biosurfactant concentration in culture broth (0.5% L, 1% L, 2% L) and in FA particles (0.5% D, 1% D, 2% D) are shown in Fig. 2(e). The adsorption of biosurfactant on FA particles gradually increased until the maximum adsorption capacity of FA was reached on the fifth day. The addition of 2% FA gave the highest final biosurfactant yield, namely 305 CMD. The yields with the addition of 1% FA and 0.5% FA were 255 CMD and 170 CMD, respectively. A positive relationship between FA dosage and biosurfactant production yield was observed. A higher dosage of FA provided a larger surface area of biocatalyst. Through serving as a cell growth booster for the growth of B. subtilis in the media, a higher biosurfactant production yield was achieved. In addition, a higher FA dosage led to a higher content of iron inside (Table 1), which also contributed to the increase in biosurfactant production yield.41
3.3. Characterization of the biosurfactant product
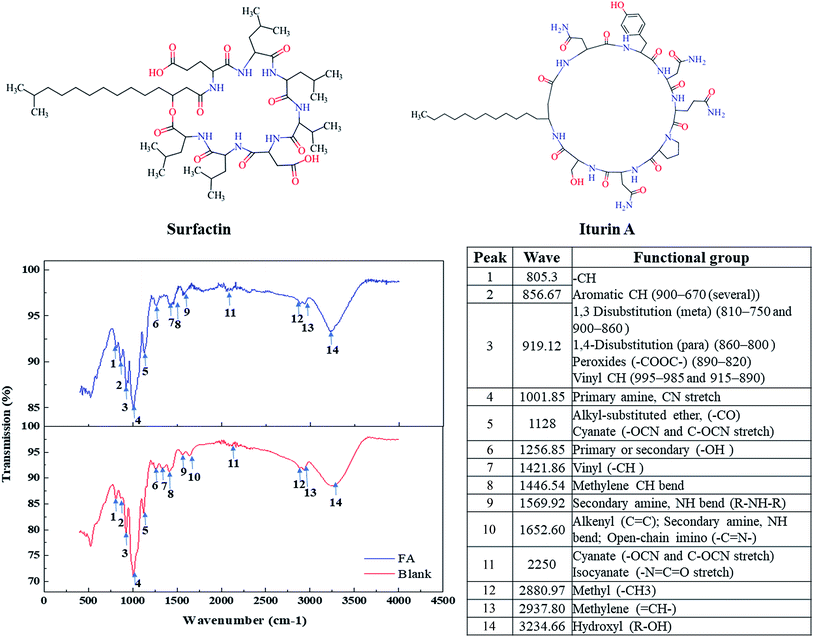
The surface activity properties of the generated biosurfactant product were characterized in this study by measuring the ST and CMC values. The results indicated that biosurfactant products generated by both FA-based medium and control sample could reduce the ST of water from 75 to 27.8 mN m−1. The CMC value of the biosurfactant generated by FA-based medium was 0.407 g L−1, lower than that generated by the control (0.524 g L−1). It was assumed that the attachment of biosurfactant production cells on the FA surface eased the purification process. Biosurfactant was more easily desorbed from FA particles than other impurities. Its purity was therefore enhanced.16FTIR was further examined in this study to acquire information about the chemical bonds (functional groups) of generated biosurfactant product. Fig. 3 presents the FTIR spectra of the biosurfactant products generated by FA-based medium and control samples in the region of 400–4500 cm−1. Both products had similar spectra, indicating that they share the same functional groups. The characteristic absorbance peaks at 700–950 cm−1 (peaks 1, 2, and 3) revealed the presence of an aliphatic long fatty acid chain. The stretching mode identified from the band range 1000–1250 cm−1 was evidence for the presence of carbonyl (peak 6) and amide groups (amide I band) (peaks 4 and 5). The peaks corresponded to the linkage group between the amine and carboxylic groups of the amino acids and to the carboxylic group of the fatty acid.42 FTIR results displayed absorbance in the range of 1600 to 1700 cm−1 (peaks 9 and 10), due to the deformation mode of the N–H bond combined with the C–N stretching mode, indicating the existence of an amide II band. A typical C–H stretching vibration in the alkyl chain was identified from the band range 2700–2900 cm−1 (peaks 12 and 13). The FTIR results confirmed that the biosurfactant was lipopeptide in nature.
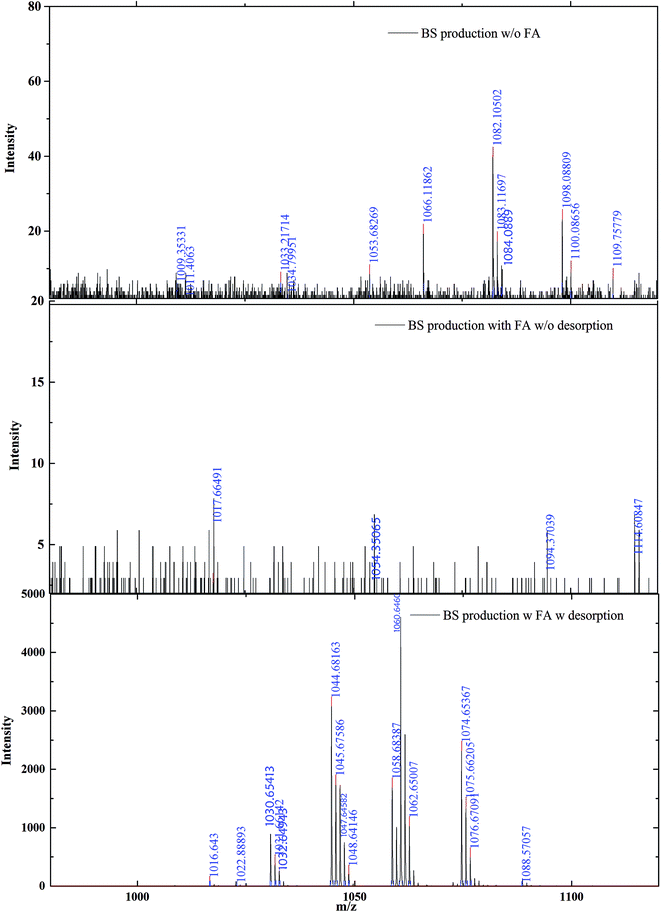
The structure of the lipopeptide biosurfactant was elucidated based on MALDI-TOF spectral analysis and the results (Fig. 4) are in accordance with those generated by Yang et al.43 This study compared the biosurfactant products generated in an FA-based culture medium with and without a desorption process, and those generated in the control sample using FA-free growth medium. The results were in accordance with the CMD result generated in Section 3.2. Comparing the low intensity and limited identified biosurfactant product in FA-free culture broth, the FA-based medium showed higher productivity. The productivity of the biosurfactant product was increased almost 100 times after the desorption process. This result clearly indicated the existence of three groups of lipopeptide biosurfactants: namely surfactin (m/z 1008, 1016, 1030, 1044, 1058, and 1060), itruin (1026, 1043, 1065, 1079, 1093), and fengycin (1463, 1485, 1499, 1587).
3.4. Bioleaching of heavy metals from FA
The leachability of heavy metals from FA by the biosurfactant producer is illustrated in Fig. 5(a)–(c), and the principal component analyses of the behaviors of the elements contained in FA are illustrated in Fig. 5(d). The behavior in the culture medium can be attributed to two groups. One group was bacterial growth-related elements, such as Cl, P, Mg, Ca, and Al, while the other was heavy metals such as Cr, Pb and Zn. Increased leachability was identified for all three heavy metals (Cr, Pb and Zn). Previous research revealed that the Zn extraction process was faster than the others.44 This trend was also proved in this study. The slow extraction process for Pb and Cr might be due to the relatively high pH value in the growth medium, as bio-acidic dissolution was preferred in the bioleaching process. The bioleaching attempt in this study indicated that FA could be detoxicated after several runs of the incubation process and ease its disposal and treatment process.45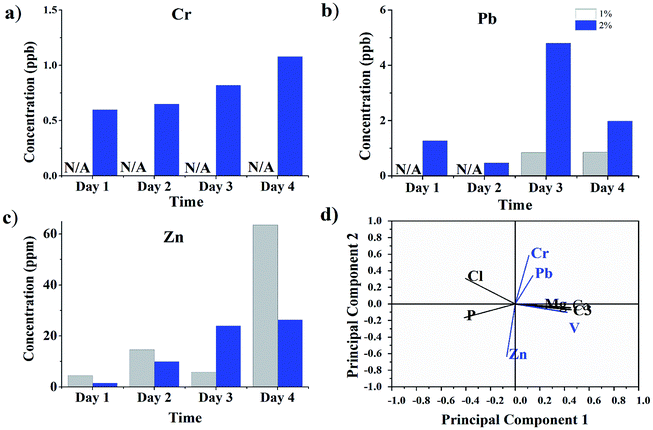 | ||
| Fig. 5 (a–c) Bioleaching of heavy metal from FA surface; (d) principal component analysis of leachate metals. | ||
4. Conclusion
This study examined an environmentally-friendly and cost-effective way to produce biosurfactant through applying a self-produced biocatalyst immobilized on FA surface. The results indicated that the addition of FA particles at 2% w/v ratio triggered the growth of biofilm, thus remarkably increasing the biosurfactant production rate. The application of FA further enhanced biosurfactant purity, resulting in a lower CMC value. FTIR and MALDI-TOF characterized the product as a lipopeptide. The findings improved the understanding of the cultivation setups and shed light on the application of a fixed-bed biofilm reactor for catalyzing bioproduct generation.Conflicts of interest
The authors have no conflicts of interest to declare.Acknowledgements
The authors would like to express their gratitude to the Natural Science and Engineering Research Council of Canada (NSERC), the Canada Foundation for Innovation (CFI), Canada Research Chairs (CRC) Program and Fisheries and Oceans Canada (DFO) for their support.References
- S. Shekhar, A. Sundaramanickam and T. Balasubramanian, Crit. Rev. Environ. Sci. Technol., 2015, 45, 1522–1554 CrossRef CAS.
- P. Jimenez-Penalver, M. Castillejos, A. Koh, R. Gross, A. Sanchez, X. Font and T. Gea, J. Cleaner Prod., 2018, 172, 2735–2747 CrossRef CAS.
- F. Fan, B. Zhang, P. L. Morrill and T. Husain, RSC Adv., 2018, 8(47), 26596–26609 RSC.
- Z. Zhu, B. Zhang, B. Chen, Q. Cai and W. Lin, Water, Air, Soil Pollut., 2016, 227, 328 CrossRef.
- X. Li, F. Fan, B. Zhang, K. Zhang and B. Chen, Int. Biodeterior. Biodegrad., 2018, 132, 216–225 CrossRef CAS.
- C.-Y. Chen and J.-S. Chang, Process Biochem., 2006, 41, 2041–2049 CrossRef CAS.
- Y. Zhi, Q. Wu and Y. Xu, Sci. Rep., 2017, 7, 40976 CrossRef CAS PubMed.
- C. Kokare, S. Chakraborty, A. Khopade and K. R. Mahadik, Indian J. Biotechnol., 2009, 8(2), 159–168 CAS.
- R. Karande, L. Debor, D. Salamanca, F. Bogdahn, K. H. Engesser, K. Buehler and A. Schmid, Biotechnol. Bioeng., 2016, 113, 52–61 CrossRef CAS PubMed.
- B. Zhou, G. J. Martin and N. B. Pamment, Biotechnol. Bioeng., 2008, 100, 627–633 CrossRef CAS PubMed.
- B. Halan, K. Buehler and A. Schmid, Trends Biotechnol., 2012, 30, 453–465 CrossRef CAS PubMed.
- Y. Liu and J. Li, Environ. Sci. Technol., 2007, 42, 443–449 CrossRef.
- D. A. A. El-Fattah, W. E. Eweda, M. S. Zayed and M. K. Hassanein, Annals of Agricultural Sciences, 2013, 58, 111–118 CrossRef.
- F. B. Rebah, R. Tyagi and D. Prevost, Bioresour. Technol., 2002, 83, 145–151 CrossRef PubMed.
- Q. Cai, B. Zhang, B. Chen, Z. Zhu, W. Lin and T. Cao, Mar. Pollut. Bull., 2014, 86, 402–410 CrossRef CAS PubMed.
- K. V. Dubey, A. A. Juwarkar and S. Singh, Biotechnol. Prog., 2005, 21, 860–867 CrossRef CAS PubMed.
- J. D. Sheppard and C. N. Mulligan, Appl. Microbiol. Biotechnol., 1987, 27, 110–116 CrossRef CAS.
- M. Shavandi, G. Mohebali, A. Haddadi, H. Shakarami and A. Nuhi, Colloids Surf., B, 2011, 82, 477–482 CrossRef CAS PubMed.
- Q. Cai, Z. Zhu, B. Chen and B. Zhang, Water Res., 2019, 149, 292–301 CrossRef CAS PubMed.
- A. M. Akgün, Citeseer, 2005 Search PubMed.
- L. Li, S. Liu and J. Liu, J. Hazard. Mater., 2011, 192, 683–690 CrossRef CAS PubMed.
- M. S. Yeh, Y. H. Wei and J. S. Chang, Biotechnol. Prog., 2005, 21, 1329–1334 CrossRef CAS PubMed.
- O. Rendueles and J. M. Ghigo, FEMS Microbiol. Rev., 2012, 36, 972–989 CrossRef CAS PubMed.
- C. Goller and T. Romeo, in Bacterial Biofilms, Springer, 2008, pp. 37–66 Search PubMed.
- X. Z. Li, B. Hauer and B. Rosche, Appl. Microbiol. Biotechnol., 2007, 76, 1255–1262 CrossRef CAS PubMed.
- M. R. Frederick, C. Kuttler, B. A. Hense and H. J. Eberl, Theor. Biol. Med. Modell., 2011, 8, 8 CrossRef PubMed.
- M. Berlanga and R. Guerrero, Microb. Cell Fact., 2016, 15, 165 CrossRef PubMed.
- F. M. Martins, J. M. Martins, L. C. Ferracin and C. J. da Cunha, J. Hazard. Mater., 2007, 147, 610–617 CrossRef CAS PubMed.
- A. Demeyer, J. V. Nkana and M. Verloo, Bioresour. Technol., 2001, 77, 287–295 CrossRef CAS PubMed.
- W. J. Weber, J. Tang and Q. Huang, Environ. Sci. Technol., 2006, 40, 1650–1656 CrossRef CAS PubMed.
- E. K. Putra, R. Pranowo, J. Sunarso, N. Indraswati and S. Ismadji, Water Res., 2009, 43, 2419–2430 CrossRef CAS PubMed.
- J. Schmitt and H.-C. Flemming, Water Sci. Technol., 1999, 39, 77–82 CrossRef CAS.
- I. A. Ribeiro, M. R. Bronze, M. F. Castro and M. H. Ribeiro, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2012, 899, 72–80 CrossRef CAS PubMed.
- S. Gélis-Jeanvoine, A. Canette, M. Gohar, T. Caradec, C. Lemy, M. Gominet, P. Jacques, D. Lereclus and L. Slamti, Research in Microbiology, 2016 Search PubMed.
- H. Zeriouh, A. Vicente, A. Pérez-García and D. Romero, Environ. Microbiol., 2014, 16, 2196–2211 CrossRef CAS PubMed.
- T. R. Garrett, M. Bhakoo and Z. Zhang, Prog. Nat. Sci., 2008, 18, 1049–1056 CrossRef CAS.
- V. Wigneswaran, K. F. Nielsen, C. Sternberg, P. R. Jensen, A. Folkesson and L. Jelsbak, Microb. Cell Fact., 2016, 15, 181 CrossRef PubMed.
- F. Julien, M. Baudu and M. Mazet, Water Res., 1998, 32, 3414–3424 CrossRef CAS.
- M. Van Loosdrecht, J. Lyklema, W. Norde, G. Schraa and A. Zehnder, Appl. Environ. Microbiol., 1987, 53, 1893–1897 CAS.
- H. Y. Fan, M. Nazari, G. Raval, Z. Khan, H. Patel and H. Heerklotz, Biochim. Biophys. Acta, Biomembr., 2014, 1838, 2306–2312 CrossRef CAS PubMed.
- Y. H. Wei, L. F. Wang and J. S. Chang, Biotechnol. Prog., 2004, 20, 979–983 CrossRef CAS PubMed.
- A. Gordillo and M. C. Maldonado, Chromatogr. Its Appl., 2012, 11, 201–225 Search PubMed.
- H. Yang, X. Li, X. Li, H. Yu and Z. Shen, Anal. Bioanal. Chem., 2015, 407, 2529–2542 CrossRef CAS PubMed.
- B. Xin, W. Jiang, X. Li, K. Zhang, C. Liu, R. Wang and Y. Wang, Bioresour. Technol., 2012, 112, 186–192 CrossRef CAS PubMed.
- X. Zeng, S. Wei, L. Sun, D. A. Jacques, J. Tang, M. Lian, Z. Ji, J. Wang, J. Zhu and Z. Xu, J. Soils Sediments, 2015, 15, 1029–1038 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |