Open Access Article
Open Access ArticleFacile stabilization of a cyclodextrin metal–organic framework under humid environment via hydrogen sulfide treatment†
Duo Keac,
Jun-Feng Fenga,
Di Wub,
Jun-Bo Houb,
Xiao-Qin Zhangb,
Bang-Jing Li *a and
Sheng Zhang*b
*a and
Sheng Zhang*b
aKey Laboratory of Mountain Ecological Restoration and Bioresource Utilization, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China. E-mail: libj@cib.ac.cn
bState Key Laboratory of Polymer Materials Engineering (Sichuan University), Polymer Research Institute of Sichuan University, Chengdu 610065, China. E-mail: zslbj@163.com
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 11th June 2019
Abstract
Increasing resistance to humid environments is a major challenge for the application of γ-CD-K-MOF (a green MOF) in real-world utilisation. γ-CD-K-MOF–H2S with enhanced moisture tolerance was obtained by simply treating MOF with H2S gas. XPS, Raman and TGA characterizations indicated that the H2S molecules coordinated with the metal centers in the framework. H2S acting as a newly available water adsorption potential well near the potassium centers protects the metal–ligand coordination bond from attack by water molecules and thus improves the moisture stability of MOF. After 7 days exposure in 60% relative humidity, γ-CD-K-MOF–H2S retained its crystal structure and morphology, while γ-CD-K-MOF had nearly collapsed. In addition, the formaldehyde uptake tests indicated that γ-CD-K-MOF retain their permanent porosity after interaction with H2S. This simple and facile one-step strategy would open a new avenue for preparation of moisture stable MOFs for practical applications.
Introduction
Metal–Organic Frameworks (MOFs), a new class of porous and crystalline materials synthesized by the assembly of organic ligands with metal ions have gained remarkable interest in recent years due to their ultrahigh surface areas, adjustable chemical functionality, and structural diversity.1–4 MOFs can be reasonably designed and prepared to satisfy desired versatile applications including catalysis,5 gas storage,6,7 separation,8 drug delivery,9 sensing,10,11 imaging12 and proton conductors.13,14 Their actual implementation depends on the stability of these materials against the environment. Unfortunately, most previously reported MOFs with weak metal–ligand coordination bonds are generally vulnerable to water molecules and collapse in humid and aqueous environments, which severely limit their practical application.15–17 However, the strategies employed to enhance the moisture resistance of MOFs have been addressed in a few studies. Currently, two main approaches have been developed to improve the water stability of MOFs. One is to directly synthesize stable MOFs by changing the chemical composition of the MOFs to increase the metal–linker bond strengths.18,19 However, this method is limited to the preparation of new materials, and does not permit enhancing the stability of those already available. The other effective method involves post-synthetic modifications, such as encapsulation of hydrophobic guest molecules,20,21 modification of the linkers with hydrophobic groups22–24 and functionalization of their external surfaces via hydrophobic coatings25–27 to protect the MOFs from water intrusion. Despite successful improving the water stability of MOFs, these approaches suffered from their respective limitations, including tedious procedure, complex instrumentation and reduced the functionality of materials.Recently, γ-cyclodextrin based MOF (γ-CD-K-MOF), which linked by coordination of the hydroxyl groups on γ-cyclodextrin with potassium ions to form body-centered cubic structure,28 have attracted broad attention. The six γ-CD units are combined by the coordination of K+ ions with C6 OH groups and glycosidic ring oxygen atoms of D-glucopyranosyl residues on the primary face of the γ-CD to formed (γ-CD)6 cube. The (γ-CD)6 cubes are attached to one another by the coordination of K+ ions to the C2 and C3 OH groups of the other set of alternating residues on the secondary face of the γ-CD to formed 3D extended frameworks. The γ-CD-K-MOF shows two main kinds of pores: spherical voids with diameters 1.7 nm and apertures with diameter 0.78 nm.29 Due to the porous structure, high local concentration of the hydroxyl groups and biocompatibility, this γ-CD-K-MOF holds prospective applications in gas adsorption,30 small molecules separation,31 sensing32,33 and biomedicine.34,35 In particular, the adsorption of toxic gases from breathable air for personal protection is an excellent example of MOFs in practical applications. Previously, our group have demonstrated that γ-CD-K-MOF showed high selective adsorption capability and speed for formaldehyde owing to the host–guest interactions between γ-CD and HCHO and hydrogen bonding with hydroxyl groups.36 However, these applications face severe challenges associated with the poor stability of γ-CD-K-MOF, which rapidly decomposed when in contact with moisture. Up to now, only few studies have been reported for the improvement of moisture stability of γ-CD-K-MOF. One is to obtain water insoluble γ-CD-K-MOF by cross-linking γ-CD with crosslinkers such as diphenyl carbonate or ethylene glycol diglycidyl.37,38 However, the synthesis and post-processing were complicated and time-consuming. Li et al. incorporated fullerene (C60) in the γ-CD-K-MOF matrices, the exposed surface of the trapped C60 imparted the γ-CD-K-MOF with enhanced hydrophobicity to improve their water stability.39 However, the occupancy of γ-CD cavities by C60 might reduce the capacity of γ-CD-K-MOF to load drugs. Recently, Singh et al. grafted a layer of hydrophobic cholesterol on the external surface of γ-CD-K-MOF.40 The water resistance of the functionalized MOF is higher than the bare MOF, while such chemistry modification was also time-consuming (taking 24 h at 45 °C) and used organic solvent N,N-dimethyl formamide. Thus, it is still an urgent need to develop more facile and efficient strategies to improve the moisture resistance of γ-CD-K-MOF.
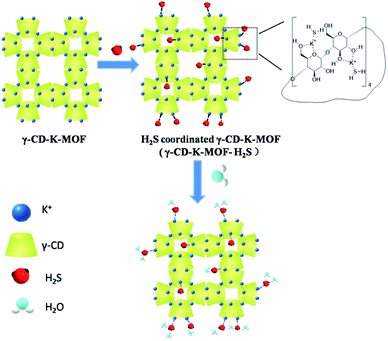
In this work, we report a very simple and efficient method to enhance the humidity tolerance of γ-CD-K-MOF by treating the γ-CD-K-MOF with hydrogen sulfide (H2S) atmosphere. Unlike other strategies employed to enhance the moisture resistance of MOFs, H2S stabilized γ-CD-K-MOF through coordinating with potassium metal sites and as new basins of attraction for the incoming water molecules, thus depleting the water content at the metal centers and improving its moisture resistance. It was found that H2S coordinated γ-CD-K-MOF remained the ability to rapidly adsorb formaldehyde and formaldehyde uptake capacity. To the best of our knowledge, this is the first work that improves moisture resistance of MOF via a facile gas treatment.
Experimental section
Materials and methods
γ-Cyclodextrin (99%), ferrous sulphide and barium hydroxide, cetyltrimethylammonium bromide (CTAB) were purchased from Sigma-Aldrich (Shanghai China). Potassium hydroxide (KOH), formaldehyde (HCHO), methyl alcohol and ethyl alcohol were produced from Chengdu Kelong Chemical Engineering Company (Chengdu China). All other reagents were of analytical grade and were used directly without further purification.Characterization
Scanning electron microscope (SEM) measurements were performed with Phenom pro, Phenom-world BV. X-Ray powder diffraction (XRD) data was collected on Panalytical B.V at room temperature from 3° to 40° (2θ). Thermal studies were conducted with Thermal Analyzer EXSTAR 6000 (Seiko Instruments Inc, Japan) in the temperature ranging 30–600 °C with heating rates of 10 °C min−1 under a N2 atmosphere. The FT-IR results were determined in Thermo Fisher Scientific Nicolet 6700 in the wavenumber range of 4000–400 cm−1. The Raman spectra were recorded using a Renishaw Raman Microscope spectrometer with laser emitting at 532 nm. The X-ray photoelectron spectroscopy (XPS) was carried on Thermo VG Multilab 2000 with Al Kα radiation in an ultra-high vacuum. The contact angle (CA) of water was measured at RT by using a CA meter (Data Physics, OCA20). Sessile water drops of 3 μL were used, and the value reported here was the average of three different positions.Results and discussion
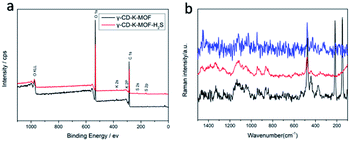
The H2S stabilized γ-CD-K-MOF was simply conducted via absorption of H2S. After absorption, the γ-CD-K-MOF loaded with H2S was heated at 50 °C under vacuum for 2 days to remove physically adsorbed H2S gas. For the convenience of our discussion, we use the notation γ-CD-K-MOF–H2S and γ-CD-K-MOF/H2S to refer to the H2S-loaded γ-CD-K-MOF with and without degassing respectively.The powder X-ray diffraction (XRD) patterns of γ-CD-K-MOF–H2S were identical to that of the parent γ-CD-K-MOF (Fig. S1†),28 indicating that γ-CD-K-MOF–H2S and γ-CD-K-MOF have the same crystalline structure. The X-ray photoelectron spectroscopy (XPS) proved the presence of S in γ-CD-K-MOF–H2S after γ-CD-K-MOF loaded with H2S degassed under vacuum at 323 K, indicating that H2S is bound to the framework of γ-CD-K-MOF through some interactions (Fig. 1a). The binding energy of K 2p in γ-CD-K-MOF–H2S exhibited an obvious shift to low binding energy compared to γ-CD-K-MOF, revealing the possible coordination bond formation between the K centers and S atoms from the H2S. In terms of S 2p, the peak (161.3 eV) assigned to S–K bond (Fig. S2†).41 It is reasonable to consider that H2S was coordinated to K metal ions in frameworks rather than agglomerating inside γ-CD-K-MOF pores or replacing γ-CD ligands and compromising its porous structure.
 | ||
| Fig. 1 (a) XPS spectra of γ-CD-K-MOF and γ-CD-K-MOF–H2S (b) Raman spectra of the γ-CD-K-MOF (red), γ-CD-K-MOF–H2S (black) and γ-CD-K-MOF–H2S heated under vacuum at 130 °C for 7 h (blue). | ||
Thermogravimetric (TG) analysis was performed on γ-CD-K-MOF and γ-CD-K-MOF–H2S and the results were shown in Fig. S3.† It is observed that pristine γ-CD-K-MOF has a mass loss of 8.8% at low temperature and then is stable to temperatures as high as 200 °C. The γ-CD-K-MOF–H2S showed two mass losses, a 4.5% mass loss at low temperatures similarly to pristine γ-CD-K-MOF followed by a gradual mass loss at higher temperature (120–200 °C), which might be assigned to H2S coordinated to the γ-CD-K-MOF. From TG analysis it was estimated an 8% H2S coordinated to the metal ions of γ-CD-K-MOF.
Fig. 1b compared the Raman spectrum of the γ-CD-K-MOF and γ-CD-K-MOF–H2S. The bare γ-CD-K-MOF exhibited two bands centred at 438 and 478 cm−1, which shifted to 442 and 475 cm−1 respectively upon H2S interaction. At lower frequency, the additional component, observed at 374, 217 and 149 cm−1 and absent in both pristine γ-CD-K-MOF and γ-CD-K-MOF–H2S heated under vacuum at 130 °C for 7 h (coordinated H2S would be released at 130 °C according to TGA profiles), could be associated to the vibration of the K–S bond of the coordinated H2S. The above results demonstrated that H2S was coordinated to the potassium ion in the γ-CD-K-MOF.
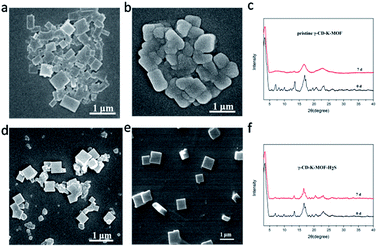
Interestingly, the moisture stability of γ-CD-K-MOF was significantly enhanced after interaction with H2S. The moisture resistance of pristine γ-CD-K-MOF and γ-CD-K-MOF–H2S were investigated with exposure to air at 60% relative humidity for 7 days. As shown in Fig. 2, the morphology and structure of all the samples were monitored by scanning electron microscopy (SEM) and XRD respectively. SEM image showed that γ-CD-K-MOF lost its cubic shapes and the edges become rounder after staying in humid environment for 7 days (Fig. 2a and b). In sharp contrast, the γ-CD-K-MOF–H2S showed the identical morphology before and after the 7 days treatment in humid environment (Fig. 2d and e). Powder XRD results showed that the characteristic peaks at 4.1°, 5.7° and 7.0° for the γ-CD-K-MOF disappeared and the peak broaden at 16.4° after treated in humidity (RH 60%) for 7 days, revealing the destruction of the initial structure. In contrast, the framework and crystallinity of γ-CD-K-MOF–H2S retained after the same treatment. In addition, treatment of γ-CD-K-MOF and γ-CD-K-MOF–H2S at higher humidity of 92% RH for 5 days, SEM images showed that the cubic crystals of γ-CD-K-MOF collapsed completely, while γ-CD-K-MOF–H2S remained regular cubic shape (Fig. S4†). The above results unambiguously demonstrated that the coordination of H2S on γ-CD-K-MOF effectively improved its moisture resistance.
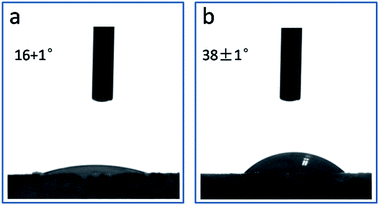
Contact angle provided quantitative information on the wettability of the solid surface. For powder materials, the measured contact angle is usually smaller due to the roughness and porosity of the pellets produced.42 Therefore, to provide approximate estimation of the hydrophobic/hydrophilic characteristics of γ-CD-K-MOF and γ-CD-K-MOF–H2S, the measured sample was obtained by applying pressure (under 1 Mpa for 30 s). As shown in Fig. 3, the water contact angle for γ-CD-K-MOF is 16 ± 1°. After the H2S treatment, the corresponding γ-CD-K-MOF–H2S exhibited a water contact angles as 38 ± 1°, which are more than twice with respect to γ-CD-K-MOF. These results confirmed that γ-CD-K-MOF become more hydrophobic after coordinated to H2S.
The water adsorption capacity of γ-CD-K-MOF and γ-CD-K-MOF–H2S was performed on exposure to a moist environment at 298 K. γ-CD-K-MOF showed a rapid increase in mass as the material adsorbs water and no further change over the remainder of the experiment (up to 4 days) (Fig. S5†). The γ-CD-K-MOF–H2S showed a similar immediate mass gain as the water adsorption, but water uptake significant decrease with respect to γ-CD-K-MOF. These results are in complete agreement with the observed lower solvent loss on γ-CD-K-MOF–H2S from TGA profiles, which could be attributed to the more hydrophobic features of the γ-CD-K-MOF–H2S that decrease the incorporation of water molecules inside the framework cavities. In order to get further insight into the improvement of moisture resistance after H2S coordinated to the γ-CD-K-MOF, we also studied the solubility of γ-CD-K-MOF and γ-CD-K-MOF–H2S in aqueous solution. Due to the weak metal–ligand coordination, γ-CD-K-MOF decomposes in water and release γ-CD. Thus, the maximum degradation of materials could be quantified by the amount of γ-CD in the solution. The measured concentration of samples that not increased with the addition of samples was taken as the solubility of the tested material. It was found that γ-CD-K-MOF rapidly dissolved in water and showed solubility as large as 76 mg mL−1 (Fig. S6†), while γ-CD-K-MOF–H2S exhibited slow dissolution rate and the solubility is 0.4 mg mL−1. The γ-CD-K-MOF/H2S also showed lower solubility of 0.3 mg mL−1. The solubility of the γ-CD-K-MOF–H2S is significantly decreased after interaction with H2S.
The γ-CD-K-MOF showed extreme sensitivity to moisture because it is believed that the weak potassium–oxygen coordination allows for an attack by water molecules. Water aggregation has been identified as an important prerequisite for some hydrolysis reactions and Fuchs et al. demonstrated that metal–ligand coordination bond could be broken after water formed clusters around the metal centers.43,44 In addition, López et al. showed that the introduction of polar groups into the IRMOFs structure could enhance their hydrothermal stability, because the polar functional group as a new adsorption site competed with the metal centers for the adsorption of water molecules so as to effectively prevent the attack from water molecules to the vulnerable coordination bonds.45 In this study, H2S molecules bind to the γ-CD-K-MOF by the coordination of H2S with metal ions. The presence of H2S endowed γ-CD-K-MOF–H2S with significantly enhanced moisture stability primarily attributed to the fact that the polar H2S as a new available water adsorption potential well near the potassium centers draws water away from the metal centers to prevent the formation of water clusters at the potassium site and protect the a vulnerable metal–ligand coordination bonds from the attack of water molecules (Fig. 4). The γ-CD-K-MOF/H2S showed higher hydrophobicity than γ-CD-K-MOF–H2S as shown in Fig. S8,† while γ-CD-K-MOF/H2S exhibited similar solubility to γ-CD-K-MOF–H2S, which excludes the hydrophobicity of γ-CD-K-MOF–H2S is mainly responsible for its water stability. This result further demonstrated that the water stability enhancement of γ-CD-K-MOF–H2S mainly attributed to the coordinated H2S that preferentially adsorb incoming water molecules to diminish the aggregation of water molecules in metal centers. In addition, the hydrophobicity enhancement of γ-CD-K-MOF–H2S also contributed to improvement of the moisture resistance.
 | ||
| Fig. 4 Schematic illustration of mechanism of improving the moisture resistance of γ-CD-MOF by H2S treatment. | ||
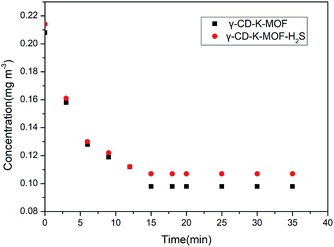
The data of γ-CD-K-MOF–H2S BET surface area might be inaccurate since coordinated H2S might be released during the degassed process of the measurement. Therefore, we investigated the porosities of γ-CD-K-MOF–H2S by measuring its formaldehyde adsorption capability. It has been demonstrated that γ-CD-K-MOF showed effective formaldehyde adsorption capability.36 Fig. 5 showed the formaldehyde adsorption behaviour of γ-CD-K-MOF and γ-CD-K-MOF–H2S. In this study, formaldehyde gas was obtained by heating formaldehyde solution. Taking into account the high-speed formaldehyde adsorption of γ-CD-K-MOF, it is not surprising that the maximum concentration of formaldehyde measured by the formaldehyde detector was lower than theoretical maximum concentration of formaldehyde (2.643 mg m−3), because partial formaldehyde gas has been adsorbed during the heating process. It can be seen that γ-CD-K-MOF–H2S exhibited similar adsorbing behaviour with γ-CD-K-MOF at this situation, demonstrating that γ-CD-K-MOF remained their permanent porosity after interaction with H2S.
We also studied their saturated formaldehyde loading capacity. It was found that H2S treatment makes γ-CD-K-MOF lost around 60% formaldehyde adsorption capacity (Table S1†), which may be since two reasons: (1) it has been reported that hydroxyl groups in γ-CD-K-MOF contribute to formaldehyde adsorption via forming hydrogen binds with formaldehyde molecules, but coordinated H2S form hydrogen bonds with hydroxyl groups firstly and then intervene the formaldehyde adsorption; (2) the coordinated H2S occupied the some of the pores in the MOF. But it should be noticed that γ-CD-K-MOF–H2S still showed a formaldehyde adsorption capacity three times higher than that of activated carbon.36
Conclusions
In summary, we have reported a facile and efficient strategy to improve moisture stability of γ-CD-K-MOF via simple introduction of H2S. The H2S coordinated with the potassium ions in the frameworks preferentially adsorb incoming water to diminish water cluster–γ-CD-K-MOF interactions, avoiding the linker displacement. The coordinated H2S not only greatly enhances MOFs' moisture resistance but also improve its hydrophobicity. When exposed to humid environment (RH = 60%) for 7 days, γ-CD-K-MOF–H2S retained its crystal structure and morphology, while the as-synthesized γ-CD-K-MOF quickly collapsed. The formaldehyde uptake tests indicated that γ-CD-K-MOF remained its permanent porosity after interaction with H2S. It is the first time to enhance MOF moisture stability via introducing polar gas into the MOFs' structure to form vulnerable coordination bonds. This simply and facile one-step strategy would open a new avenue for preparation of moisture stable MOFs for practical applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by National Natural Science Foundation of China (Grant No. 51573187), the Biological Resources Network of Chinese Academy of Sciences (ZSTH-030) and State Key Laboratory of Polymer Materials Engineering (Grant No. sklpme2017-2-05).Notes and references
- F. Hiroyasu, K. E. Cordova, O. K. Michael and O. M. Yaghi, Science, 2013, 341, 974–989 Search PubMed.
- E. Mohamed, K. Jaheon, R. Nathaniel, V. David, W. Joseph, O. K. Michael and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef PubMed.
- Z. G. Gu, G. Sylvain, B. S. Stefan, W. L. Christof and H. Lars, Chem. Commun., 2015, 51, 8998–9001 RSC.
- W. G. Lu, Z. W. Wei, Z. Y. Gu, T. F. Liu, P. Jinhee, P. Jihye, T. Jian, M. W. Zhang, Q. Zhang and G. Thomas, Chem. Soc. Rev., 2014, 43, 5561–5593 RSC.
- L. Jeongyong, O. K. Farha, R. John, K. A. Scheidt, S. B. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- S. Krause, V. Bon, I. Senkovska, U. Stoeck, D. Wallacher, D. M. Többens, S. Zander, R. S. Pillai, G. Maurin and F. X. Coudert, Nature, 2016, 532, 348–352 CrossRef CAS PubMed.
- S. Q. Ma and H. C. Zhou, Chem. Commun., 2010, 46, 44–53 RSC.
- J. R. Li, K. J. Ryan and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- T. Simon-Yarza, A. Mielcarek, P. Couvreur and C. Serre, Adv. Mater., 2018, 30, 1707365–1707380 CrossRef PubMed.
- Z. S. Dou, J. C. Yu, Y. J. Cui, Y. Yang, Z. Y. Wang, D. Yang and G. D. Qian, J. Am. Chem. Soc., 2014, 136, 5527–5530 CrossRef CAS PubMed.
- X. Zhu, H. Y. Zheng, X. F. Wei, Z. Y. Lin, L. H. Guo, B. Qin and G. N. Chen, Chem. Commun., 2013, 49, 1276–1278 RSC.
- H. Patricia, C. Tamim, S. Christian, G. Brigitte, S. Catherine, B. Tarek, J. F. Eubank, H. Daniela, C. Pascal and K. Christine, Nat. Mater., 2010, 9, 172–178 CrossRef PubMed.
- G. K. H. Shimizu, J. M. Taylor and S. R. Kim, Science, 2013, 341, 354–355 CrossRef CAS PubMed.
- R. Padmini, N. E. Wong and G. K. H. Shimizu, Chem. Soc. Rev., 2014, 43, 5913–5932 RSC.
- H. Sang Soo, C. Seung-Hoon and A. van Duin, Chem. Commun., 2010, 46, 5713–5715 RSC.
- J. J. Low, A. I. Benin, J. Paulina, J. F. Abrahamian, S. A. Faheem and R. R. Willis, J. Am. Chem. Soc., 2009, 131, 15834–15842 CrossRef CAS PubMed.
- J. A. Greathouse and M. D. Allendorf, J. Am. Chem. Soc., 2006, 128, 10678–10679 CrossRef CAS PubMed.
- M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632–6640 CrossRef CAS.
- F. Hiroyasu, G. Felipe, Y. B. Zhang, J. C. Jiang, W. L. Queen, M. R. Hudson and O. M. Yaghi, J. Am. Chem. Soc., 2014, 136, 4369–4391 CrossRef PubMed.
- S. J. Yang, J. Y. Choi, H. K. Chae, J. H. Cho, K. S. Nahm and R. P. Chong, Chem. Mater., 2009, 21, 1893–1897 CrossRef CAS.
- J. B. Decoste, G. W. Peterson, M. W. Smith, C. A. Stone and C. R. Willis, J. Am. Chem. Soc., 2012, 134, 1486–1489 CrossRef CAS PubMed.
- H. Li, Z. Lin, X. Zhou, X. Wang, Y. Li, H. Wang and Z. Li, Chem. Eng. J., 2017, 307, 537–543 CrossRef CAS.
- J. G. Nguyen and S. M. Cohen, J. Am. Chem. Soc., 2010, 132, 4560–4561 CrossRef CAS PubMed.
- T. Wu, L. Shen, M. Luebbers, C. Hu, Q. Chen, Z. Ni and R. I. Masel, Chem. Commun., 2010, 46, 6120–6122 RSC.
- J. Castells-Gil, F. Novio, N. M. Padial, S. Tatay, D. Ruíz-Molina and C. Martí-Gastaldo, ACS Appl. Mater. Interfaces, 2017, 9, 44641–44648 CrossRef CAS PubMed.
- Z. Wang, Y. L. Hu, J. Ge, H. L. Jiang and S. H. Yu, J. Am. Chem. Soc., 2014, 136, 16978–16981 CrossRef PubMed.
- C. S. Arnau, K. C. Stylianou, C. Carlos, N. Majid, I. Inhar and M. Daniel, Adv. Mater., 2015, 27, 869–873 CrossRef PubMed.
- R. A. Smaldone, R. S. Forgan, H. Furukawa, J. J. Gassensmith, A. M. Z. Slawin, O. M. Yaghi and J. F. Stoddart, Angew. Chem., Int. Ed., 2010, 49, 8630–8634 CrossRef CAS PubMed.
- R. S. Forgan, R. A. Smaldone, J. J. Gassensmith, H. Furukawa, D. B. Cordes, Q. Li, C. E. Wilmer, Y. Y. Botros, R. Q. Snurr, A. M. Z. Slawin and J. F. Stoddart, J. Am. Chem. Soc., 2012, 134, 406–417 CrossRef CAS PubMed.
- J. J. Gassensmith, F. Hiroyasu, R. A. Smaldone, R. S. Forgan, Y. Y. Botros, O. M. Yaghi and S. J. Fraser, J. Am. Chem. Soc., 2011, 133, 15312–15315 CrossRef CAS PubMed.
- K. J. Hartlieb, J. M. Holcroft, P. Z. Moghadam, N. A. Vermeulen, M. M. Algaradah, M. S. Nassar, Y. Y. Botros, R. Q. Snurr and J. F. Stoddart, J. Am. Chem. Soc., 2016, 138, 2292–2301 CrossRef CAS PubMed.
- J. J. Gassensmith, K. Jeung Yoon, J. M. Holcroft, O. K. Farha, S. J. Fraser, J. T. Hupp and J. Nak Cheon, J. Am. Chem. Soc., 2014, 136, 8277–8282 CrossRef CAS PubMed.
- D. Shen, G. Wang, Z. Liu, P. Li, K. Cai, C. Cheng, Y. Shi, J. M. Han, C. W. Kung, X. Gong, Q. H. Quo, H. Chen, A. C. H. Sue, Y. Y. Botros, A. Facchetti, O. K. Farha, T. J. Marks and J. F. Stoddart, J. Am. Chem. Soc., 2018, 140, 11402–11407 CrossRef CAS PubMed.
- W. Michida, M. Ezaki, M. Sakuragi, G. Guan and K. Kusakabe, Cryst. Res. Technol., 2015, 50, 556–559 CrossRef CAS.
- K. J. Hartlieb, D. P. Ferris, J. M. Holcroft, I. Kandela, C. L. Stern, M. S. Nassar, Y. Y. Botros and J. F. Stoddart, Mol. Pharm., 2017, 14, 1831–1839 CrossRef CAS PubMed.
- L. Wang, X. Y. Liang, Z. Y. Chang, L. S. Ding, S. Zhang and B. J. Li, ACS Appl. Mater. Interfaces, 2017, 10, 42–46 CrossRef CAS PubMed.
- V. Singh, T. Guo, L. Wu, J. H. Xu, B. T. Liu, R. Gref and J. W. Zhang, RSC Adv., 2017, 7, 20789–20794 RSC.
- Y. Furukawa, T. Ishiwata, K. Sugikawa, K. Kokado and K. Sada, Angew. Chem., Int. Ed., 2012, 51, 10566–10569 CrossRef CAS PubMed.
- H. Li, M. R. Hill, R. Huang, C. Doblin, S. Lim, A. J. Hill, R. Babarao and P. Falcaro, Chem. Commun., 2016, 52, 5973–5976 RSC.
- V. Singh, T. Guo, H. T. Xu, L. Wu, J. K. Gu, C. B. Wu, R. Gref and J. W. Zhang, Chem. Commun., 2017, 53, 9246–9249 RSC.
- M. Fantauzzi, B. Elsener, D. Atzei, A. Rigoldi and A. Rossi, RSC Adv., 2015, 5, 75953–75963 RSC.
- E. Chibowski and R. Perea-Carpio, Adv. Colloid Interface Sci., 2002, 98, 245–264 CrossRef CAS PubMed.
- B. Luca, C. Juan Manuel, V. Thijs, C. Sofía and L. Núria, Chem.–Eur. J., 2012, 18, 12260–12266 CrossRef PubMed.
- T. M. De, R. Jonchiere, P. Pullumbi, F. X. Coudert and A. H. Fuchs, ChemPhysChem, 2012, 13, 3497–3503 CrossRef PubMed.
- L. Bellarosa, J. J. Gutiérrez-Sevillano, S. Calerob and N. López, Phys. Chem. Chem. Phys., 2013, 15, 17696–17704 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra03079d |
| This journal is © The Royal Society of Chemistry 2019 |