Open Access Article
Open Access ArticleRational design of functionalized covalent organic frameworks and their performance towards CO2 capture†
Shuhao An,
Ting Xu,
Changjun Peng *,
Jun Hu
*,
Jun Hu and
Honglai Liu*
and
Honglai Liu*
State Key Laboratory of Chemical Engineering and School of Chemistry & Molecular Engineering, East China University of Science and Technology, Shanghai, 200237, China. E-mail: cjpeng@ecust.edu.cn; hlliu@ecust.edu.cn
First published on 10th July 2019
Abstract
We describe the design and synthesis of two new functionalized covalent organic frameworks, named Cz-COF and Tz-COF, by using monomers containing carbazole and benzobisthiazole as building blocks. The resultant materials possess high crystallinity, permanent porosities as well as abundant heteroatom activated sites in the framework. As solid adsorbents, both COFs exhibit excellent CO2 uptake (11.0 wt% for Cz-COF and 15.4 wt% for Tz-COF), high adsorption selectivity for CO2 over N2 and good recyclability.
Introduction
Covalent organic frameworks (COFs)1 with periodically ordered structures are a new emerging type of porous crystalline material, and have gained great attention in recent years.2–4 Owing to their features of robust framework, inherent porosity and tailor-made functionalities, COFs have recently been seen as a new porous platform for extensive applications such as gas storage and separation,5–7 optoelectronics,8–10 catalysis,11–13 and chemical sensor.14–16 Thus far, in order to achieve excellent performance, a pore channel post-modification strategy has been seen as an effective means of introducing various functional moieties to modify the surface properties of the COF skeleton.17–20 However, this method tends to reduce the intrinsic porosity and crystallinity of the materials aiming to afford a high density of functional groups, and also suffers from the cleavage of grafted parts during recycling. Correspondingly, the direct construction of functionalized COFs by pre-designed monomers will reduce multistep synthetic procedure and allow the in situ introduced active sites to be more evenly dispersed in the framework.21,22 Due to the solubility and geometric symmetry of building blocks have to be taken into account, the development of functionalized COFs still remains difficult and it is therefore strongly desired based on this method.On the other hands, with rapid industrialization development and the progress of human society, the continuous emission of carbon dioxide (CO2) in the atmosphere has become a massive global problem. For this reason, developing effective technologies and strategies to effectively capture CO2 and alleviate this predicament is both challenging and urgent.23 Carbon capture and sequestration (CCS) technology is considered an economical and effective way to mitigate the trend of global warming.24–27 The development of novel nanoporous materials like metal–organic frameworks (MOFs)28 and porous organic polymers (POPs),29,30 has shown particular promise for CCS because of their cost-effective physical adsorption process, high efficiency and facile recyclability. Remarkably, various CO2-philic moieties including triazine,31 carbazole32 or benzothiazole33 have been incorporated into the polymeric networks to enhance the interaction between the material surfaces and CO2 molecules, consequently exhibiting excellent performance towards CO2 capture. Thus, rationally design and synthesis of functionalized COFs consist of these moieties not only extend the library of COFs but also investigate COF-based adsorbents for efficient CO2 capture.
Herein, we contribute to describe the synthesis and CO2 gas adsorption properties of two new functionalized COFs, Cz-COF and Tz-COF, prepared by using monomers containing carbazole (Cz) and benzobisthiazole (Tz) as building blocks. The crystalline structures and porous properties of obtained COFs are systematically examined through powder X-ray diffraction and nitrogen adsorption–desorption experiments. Most importantly, because of their permanent porosities and abundant heteroatom (N or S) polar sites, both COFs show excellent CO2 uptake, high adsorption selectivity for CO2 over N2 and good recyclability at ambient conditions.
Results and discussion
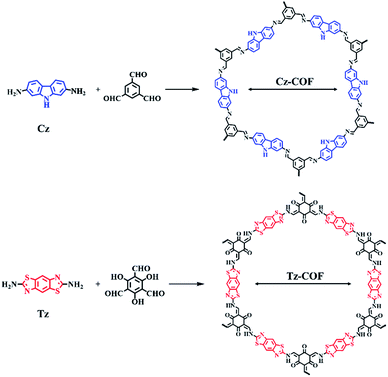
Although monomers containing Cz and Tz were employed as the scaffolds for the construction of amorphous POPs towards applications such as CO2 adsorption33,34 and semiconductor materials,35 to the best of our knowledge, no previous example of crystalline COFs based on Cz and Tz has been reported. As shown in Scheme 1, our strategy for preparing functionalized COFs involves imine-bond formation reactions together with typical [C3 + C2] schemes. After screening reaction conditions, Cz-COF was synthesized in dioxane/mesitylene/3 M AcOH (15/15/2 v/v) at 120 °C for 3 days and obtained as yellow solid in 85% isolated yield. Tz-COF was synthesized in dioxane/3 M AcOH (5/1 v/v) at 120 °C for 3 days and obtained as red solid in 84% isolated yield. These solid products were insoluble in water and common organic solvents such as tetrahydrofuran (THF), N,N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO). Thermogravimetric analysis (TGA) further revealed that both COFs exhibited high thermal stability up to 400 °C (Fig. S3†).The molecular structures of Cz-COF and Tz-COF were confirmed by Fourier transform infrared (FT-IR) spectroscopy and cross-polarization magic angle spinning (CP-MAS) 13C NMR spectroscopy. In the IR spectrum of Cz-COF (Fig. S4†), the strong absorption peaks for N–H (3405 cm−1) and C–N (1234 cm−1) indicated the presence of the carbazole moiety within the network, while a new characteristic stretching band for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (1629 cm−1) was observed.36 The IR spectrum of Tz-COF (Fig. S5†) indicated total consumption of the monomer based on the disappearance of the N–H absorption peaks of Tz. Moreover, a strong peak at 1596 cm−1 arising from the C
N (1629 cm−1) was observed.36 The IR spectrum of Tz-COF (Fig. S5†) indicated total consumption of the monomer based on the disappearance of the N–H absorption peaks of Tz. Moreover, a strong peak at 1596 cm−1 arising from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching band present in the keto form. It should be noted that the C
C stretching band present in the keto form. It should be noted that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak of Tz-COF were merged with the C
O peak of Tz-COF were merged with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching band at 1596 cm−1 as reported TpPa-1 and TpPa-2 COF.37 13C NMR spectrum of functionalized COFs also revealed the structures of their polymeric architectures very well (Fig. S6 and S7†). For Cz-COF, the peak at ca. 157 ppm corresponds to the carbon of the imine bonds (C
C stretching band at 1596 cm−1 as reported TpPa-1 and TpPa-2 COF.37 13C NMR spectrum of functionalized COFs also revealed the structures of their polymeric architectures very well (Fig. S6 and S7†). For Cz-COF, the peak at ca. 157 ppm corresponds to the carbon of the imine bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). The two peaks at ca. 110 ppm and 99 ppm in Cz-COF arise from the phenyl carbons ortho to the carbazole nitrogen substitution site.34 The Tz-COF in keto form was also confirmed by the peak at ca. 180 ppm ascribed to typical ketone carbon (C
N). The two peaks at ca. 110 ppm and 99 ppm in Cz-COF arise from the phenyl carbons ortho to the carbazole nitrogen substitution site.34 The Tz-COF in keto form was also confirmed by the peak at ca. 180 ppm ascribed to typical ketone carbon (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). The phenyl carbons from the benzobisthiazole were located at ca. 144 ppm and 128 ppm.33 The morphology of functionalized COFs was examined by scanning electron microscopy (SEM), in which Cz-COF and Tz-COF exhibited different morphology: Cz-COF possessed spherical particle aggregation, whereas Tz-COF showed a large quantity of uniform nanofibers (Fig. S8†).
O). The phenyl carbons from the benzobisthiazole were located at ca. 144 ppm and 128 ppm.33 The morphology of functionalized COFs was examined by scanning electron microscopy (SEM), in which Cz-COF and Tz-COF exhibited different morphology: Cz-COF possessed spherical particle aggregation, whereas Tz-COF showed a large quantity of uniform nanofibers (Fig. S8†).
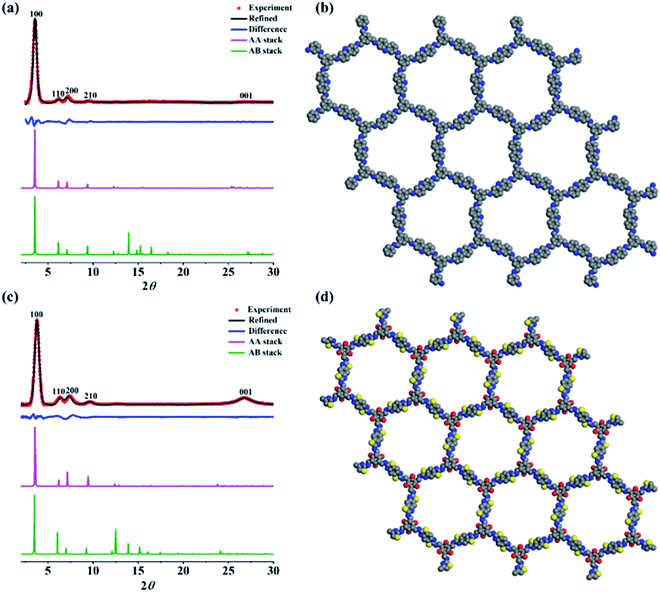
The powder X-ray diffraction (PXRD) analysis was subsequently used to investigate the crystallinity of the as-synthesized two COFs (Fig. 1). Cz-COF exhibited an intense peak at 3.6° and other three minor peaks at 6.4°, 7.4° and 9.7°, which correspond to the reflection from the (100), (110), (200) and (210) planes, respectively (Fig. 1a, red dot). The periodicity of the 2D sheets as a result of π–π interaction can also be inferred from the broad peak at ca. 26.9°, which is ascribed to the reflection from (001) plane.38 The diffraction pattern of Tz-COF exhibited signals at 3.8°, 6.2°, 7.2°, 9.6° and 26.7° that were assigned to the (100), (110), (200), (210) and (001) planes, respectively (Fig. 1c, red dot). Two types of extended structures based on eclipsed (AA) and staggered (AB) stacking models in the space group P6 were generated for both COFs by the Materials Studio software package and then simulated their powder PXRD patterns (Fig. 1a and c, pink and green curve, respectively). The calculated PXRD patterns based on AA stacking model shows excellent agreement with experimental results in terms of peak positions and intensities for both COFs (Fig. 1a and c, blue curve). The Pawley refinements were further conducted and yielded the unit cell parameters with good agreement factors (a = b = 28.79 Å, c = 3.51 Å, RWP = 5.63%, RP = 3.30% for Cz-COF and a = b = 28.55 Å, c = 3.78 Å, RWP = 4.79%, RP = 9.23% for Tz-COF). On the basis of these results, Fig. 1b and d illustrate the eclipsed AA stacking structures of Cz-COF and Tz-COF, respectively.
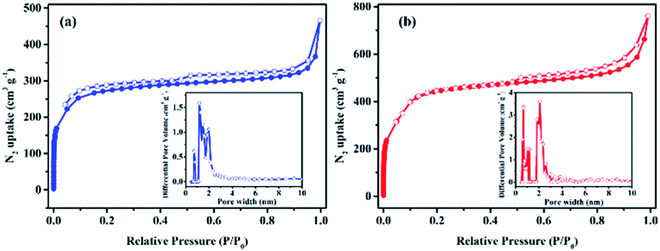
To investigate the permanent porosities of the functionalized COFs, nitrogen gas adsorption–desorption isotherms were measured at 77 K. The fresh sample was degassed at 120 °C and 1 × 10−5 Torr for 12 h prior to porosity measurement. As shown in Fig. 2, activated Cz-COF and Tz-COF show a sharp uptake under low relative pressure, which reflects the presence of micropores. The step observed at P/P0 = 0.10–0.20 indicates pore condensation in mesopores with a narrow distribution.39 The Brunauer–Emmett–Teller (BET) surface areas of the activated COFs were calculated to be 871 m2 g−1 for Cz-COF and 1439 m2 g−1 for Tz-COF. The total pore volumes were evaluated at P/P0 = 0.99 to be Vp = 0.72 cm3 g−1 for Cz-COF and 1.18 cm3 g−1 for Tz-COF. The pore size distributions (PSD) based on the nonlocal density functional theory (NLDFT) exhibit two dominant pore diameters of 1.2 nm and 2.0 nm for Cz-COF; 0.7 nm and 2.1 nm for Tz-COF (Fig. 2, insets). The difference between the PSD results with pore width predicted from the eclipsed AA stacking model (2.3 nm for Cz-COF; 2.6 nm for Tz-COF) mainly due to imperfect solid-state stacking of the two-dimension (2D) sheets in the framework that cannot be identified by PXRD studies as reported for many 2D COFs.40,41
 | ||
| Fig. 2 Nitrogen gas adsorption and desorption isotherms for (a) Cz-COF and (b) Tz-COF. Insets: pore size distributions. | ||
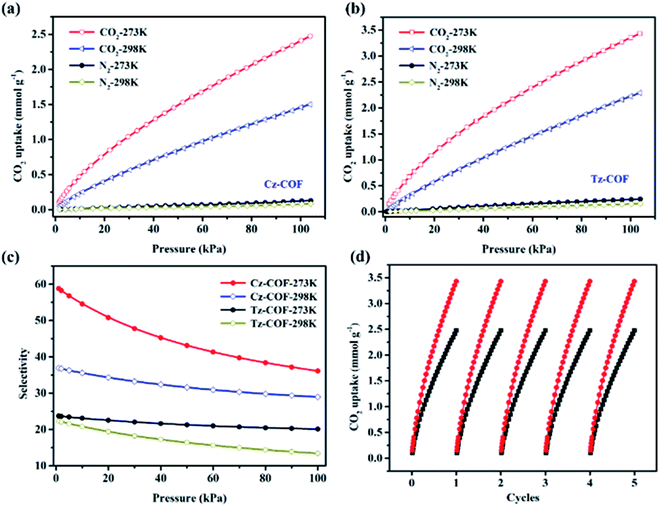
In view of the fact that the functionalized COFs possess ordered structures, excellent porous characteristics, and in situ abundant CO2-philic heteroatom (N or S) sites among the pore wall, which promote us to investigate their CO2 uptake capacities. The CO2 adsorption isotherms were measured up to 1 bar at both 273 K and 298 K for both COFs (Fig. 3a and b). Remarkably, Cz-COF and Tz-COF show uptakes of 2.5 mmol g−1 (11.0 wt%) and 3.5 mmol g−1 (15.4 wt%), respectively, at 273 K (Table 1). The CO2 adsorption capacity of Tz-COF is not only higher than that of many previously reported COFs such as COF-103 (7.6 wt%, SBET = 3530 m2 g−1),5 ILCOF-1 (6.0 wt%, SBET = 2723 m2 g−1)42 and TDCOF-5 (9.2 wt%, SBET = 2497 m2 g−1)43 even though they have a much higher surface area, but also comparable to the reported excellent amorphous porous organic polymers like benzimidazole-linked polymers BILP-1 (18.8 wt%),44 microporous polycarbazole CPOP-1 (21.2 wt%)32 and microporous polyaminal networks PAN-2 (17.7 wt%)45 under the same conditions (Table S1†). Previous work has shown that heteroatom sites may have an important influence on CO2 adsorption besides the contribution of porous properties.46 To obtain a better understanding, we then calculated the isosteric heats of adsorption (Qst) for both functionalized COFs by fitting the CO2 adsorption isotherms obtained from different temperature and applying the Clausius–Clapeyron equation (Fig. S9†). At low adsorption values, Qst was calculated to be 20 kJ mol−1 for Cz-COF and 22 kJ mol−1 for Tz-COF, suggesting a strong dipole–quadrupole interaction between the COFs framework and CO2 molecules, which is similar to reported functionalized POPs32 and COFs.41
| COFs | SBET (m2 g−1) | Vtotala (cm3 g−1) | CO2 uptakeb (mmol g−1) 273 K/298 K | Qst (kJ mol−1) | Selectivityc 273 K/298 K |
|---|---|---|---|---|---|
a Total volumes calculated at P/P0 = 0.99.b Measured at the pressure of 1 bar.c IAST selectivity (1 bar) for CO2/N2 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 v 85 v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v). v). |
|||||
| Cz-COF | 871 | 0.72 | 2.5/1.5 | 20 | 36/28 |
| Tz-COF | 1439 | 1.18 | 3.5/2.3 | 22 | 20/12 |
The selectivity is another key parameter for COF-based adsorbents in gas separation field. We subsequently performed the N2 adsorption at both 273 and 298 K (1 bar) to examine the selective adsorption for CO2 over N2 (Fig. 3a and b). On the basis of Ideal Adsorption Solution Theory (IAST) method,47 the CO2/N2 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 v
85 v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) selectivity for the functionalized COFs were calculated to be 36 for Cz-COF and 20 for Tz-COF at 273 K and 1 bar (Fig. 3c). This value of Cz-COF surpasses reported TpPa-COF (32)48 or [EtNH2]50-H2P-COF (17)18 and is lower than the high values of micorporous organic polymers including azo-linked polymer azo-POP-2 (130),49 aromatic heterocyclic polymer Py-1 (117)50 and PPN-6-SO3H (150, 295 K)51 (Table S1†). At 298 K, the CO2/N2 selectivity for Cz-COF and Tz-COF decrease to 29 and 13, respectively. Moreover, the selectivity values for the functionalized COFs were further calculated to be 44 for Cz-COF and 24 for Tz-COF at 273 K using the ratio of the initial slopes of the CO2 and N2 adsorption isotherms (Fig. S10†), which is consistent with those obtained from IAST method. Interestingly, at 298 K, the CO2/N2 selectivity of Cz-COF and Tz-COF remain at a good level and reach 28 and 21, respectively. Furthermore, five times adsorption–desorption cycle was performed to test the recyclability of the functionalized COFs. As shown in Fig. 3d, there was no apparent loss of uptake amount in each cycling test for both COFs, revealing the good sustainability for functionalized COFs in CO2 capture process.
v) selectivity for the functionalized COFs were calculated to be 36 for Cz-COF and 20 for Tz-COF at 273 K and 1 bar (Fig. 3c). This value of Cz-COF surpasses reported TpPa-COF (32)48 or [EtNH2]50-H2P-COF (17)18 and is lower than the high values of micorporous organic polymers including azo-linked polymer azo-POP-2 (130),49 aromatic heterocyclic polymer Py-1 (117)50 and PPN-6-SO3H (150, 295 K)51 (Table S1†). At 298 K, the CO2/N2 selectivity for Cz-COF and Tz-COF decrease to 29 and 13, respectively. Moreover, the selectivity values for the functionalized COFs were further calculated to be 44 for Cz-COF and 24 for Tz-COF at 273 K using the ratio of the initial slopes of the CO2 and N2 adsorption isotherms (Fig. S10†), which is consistent with those obtained from IAST method. Interestingly, at 298 K, the CO2/N2 selectivity of Cz-COF and Tz-COF remain at a good level and reach 28 and 21, respectively. Furthermore, five times adsorption–desorption cycle was performed to test the recyclability of the functionalized COFs. As shown in Fig. 3d, there was no apparent loss of uptake amount in each cycling test for both COFs, revealing the good sustainability for functionalized COFs in CO2 capture process.
Conclusions
In conclusion, we have synthesized two new functionalized COFs, Cz-COF and Tz-COF, by using monomers containing carbazole and benzobisthiazole as building blocks. Due to their characterization of high crystallinity, permanent porosities as well as abundant heteroatom activated sites in the framework, both COFs exhibit excellent CO2 uptake (11.0 wt% for Cz-COF and 15.4 wt% for Tz-COF), high adsorption selectivity for CO2 over N2 and good recyclability. We expect that these two functionalized COFs successfully prepared in this study not only extend the library of COFs but also inspire us to investigate their potential application in semiconductor material and photocatalyst.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was supported by the National Natural Science Foundation of China (no. 91334203, 21476070) and the 111 Project of Ministry of Education of China (no. B08021).Notes and references
- A. P. Cote, A. I. Benin, N. W. Ockwig, M. O'keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef CAS PubMed.
- X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010–6022 RSC.
- S. Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548–568 RSC.
- M. S. Lohse and T. Bein, Adv. Funct. Mater., 2018, 28, 1705553 CrossRef.
- H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875–8883 CrossRef CAS.
- Y. Zeng, R. Zou and Y. Zhao, Adv. Mater., 2016, 28, 2855–2873 CrossRef CAS.
- Y. Pramudya and J. L. Mendoza-Cortes, J. Am. Chem. Soc., 2016, 138, 15204–15213 CrossRef CAS PubMed.
- S. Wan, J. Guo, J. Kim, H. Ihee and D. Jiang, Angew. Chem., Int. Ed., 2008, 120, 8958–8962 CrossRef.
- X. Feng, L. Liu, Y. Honsho, A. Saeki, S. Seki, S. Irle, Y. Dong, A. Nagai and D. Jiang, Angew. Chem., Int. Ed., 2012, 124, 2672–2676 CrossRef.
- M. Dogru and T. Bein, Chem. Commun., 2014, 50, 5531–5546 RSC.
- S. Y. Ding, J. Gao, Q. Wang, Y. Zhang, W. G. Song, C. Y. Su and W. Wang, J. Am. Chem. Soc., 2011, 133, 19816–19822 CrossRef CAS.
- Q. Fang, S. Gu, J. Zheng, Z. Zhuang, S. Qiu and Y. Yan, Angew. Chem., Int. Ed., 2014, 126, 2922–2926 CrossRef.
- S. Lu, Y. Hu, S. Wan, R. McCaffrey, Y. Jin, H. Gu and W. Zhang, J. Am. Chem. Soc., 2017, 139, 17082–17088 CrossRef CAS.
- W. Li, C. X. Yang and X. P. Yan, Chem. Commun., 2017, 53, 11469–11471 RSC.
- S. Y. Ding, M. Dong, Y. W. Wang, Y. T. Chen, H. Z. Wang, C. Y. Su and W. Wang, J. Am. Chem. Soc., 2016, 138, 3031–3037 CrossRef CAS.
- Z. Li, N. Huang, K. H. Lee, Y. Feng, S. Tao, Q. Jiang, Y. Nagao, S. Irle and D. Jiang, J. Am. Chem. Soc., 2018, 140, 12374–12377 CrossRef CAS PubMed.
- A. Nagai, Z. Guo, X. Feng, S. Jin, X. Chen, X. Ding and D. Jiang, Nat. Commun., 2011, 2, 536 CrossRef.
- N. Huang, R. Krishna and D. Jiang, J. Am. Chem. Soc., 2015, 137, 7079–7082 CrossRef CAS.
- Q. Lu, Y. Ma, H. Li, X. Guan, Y. Yusran, M. Xue, Q. Fang, Y. Yan, S. Qiu and V. Valtchev, Angew. Chem., Int. Ed., 2018, 57, 6042–6048 CrossRef CAS PubMed.
- H. Guo, J. Wang, Q. Fang, Y. Zhao, S. Gu, J. Zheng and Y. Yan, CrystEngComm, 2017, 19, 4905–4910 RSC.
- H. S. Xu, S. Y. Ding, W. K. An, H. Wu and W. Wang, J. Am. Chem. Soc., 2016, 138, 11489–11492 CrossRef CAS.
- G. Lin, H. Ding, R. Chen, Z. Peng, B. Wang and C. Wang, J. Am. Chem. Soc., 2017, 139, 8705–8709 CrossRef CAS.
- R. S. Haszeldine, Science, 2009, 325, 1647–1652 CrossRef CAS PubMed.
- M. E. Boot-Handford, J. C. Abanades, E. J. Anthony, M. J. Blunt, S. Brandani, N. Mac Dowell, J. R. Fernández, M. C. Ferrari, R. Gross and J. P. Hallett, Energy Environ. Sci., 2014, 7, 130–189 RSC.
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, P. S. Fennell, S. Fuss, A. Galindo and L. A. Hackett, Energy Environ. Sci., 2018, 11, 1062–1176 RSC.
- Z. Li, X. Feng, Y. Zou, Y. Zhang, H. Xia, X. Liu and Y. Mu, Chem. Commun., 2014, 50, 13825–13828 RSC.
- Y. F. Zhi, P. P. Shao, X. Feng, H. Xia, Y. M. Zhang, Z. Shi, Y. Mu and X. M. Liu, J. Mater. Chem. A, 2018, 6, 374–382 RSC.
- Y. Lin, C. Kong, Q. Zhang and L. Chen, Adv. Energy Mater., 2017, 7, 1601296 CrossRef.
- R. Dawson, E. Stöckel, J. R. Holst, D. J. Adams and A. I. Cooper, Energy Environ. Sci., 2011, 4, 4239–4245 RSC.
- R. He, S. Cong, J. Wang, J. Liu and Y. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 4338–4344 CrossRef CAS.
- A. K. Sekizkardes, S. Altarawneh, Z. Kahveci, T. İslamoğlu and H. M. El-Kaderi, Macromolecules, 2014, 47, 8328–8334 CrossRef CAS.
- Q. Chen, M. Luo, P. Hammershøj, D. Zhou, Y. Han, B. W. Laursen, C. G. Yan and B. H. Han, J. Am. Chem. Soc., 2012, 134, 6084–6087 CrossRef CAS.
- V. S. P. K. Neti, X. Wu, P. Peng, S. Deng and L. Echegoyen, RSC Adv., 2014, 4, 9669–9672 RSC.
- X. Zhu, S. M. Mahurin, S. H. An, C. L. Do-Thanh, C. Tian, Y. Li, L. W. Gill, E. W. Hagaman, Z. Bian and J. H. Zhou, Chem. Commun., 2014, 50, 7933–7936 RSC.
- A. P. Katsoulidis, S. M. Dyar, R. Carmieli, C. D. Malliakas, M. R. Wasielewski and M. G. Kanatzidis, J. Mater. Chem. A, 2013, 1, 10465–10473 RSC.
- M. Grigoras and N. C. Antonoaia, Eur. Polym. J., 2005, 41, 1079–1089 CrossRef CAS.
- S. Kandambeth, A. Mallick, B. Lukose, M. V. Mane, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2012, 134, 19524–19527 CrossRef CAS PubMed.
- S. Dalapati, M. Addicoat, S. Jin, T. Sakurai, J. Gao, H. Xu, S. Irle, S. Seki and D. Jiang, Nat. Commun., 2015, 6, 7786 CrossRef CAS.
- Q. Fang, Z. Zhuang, S. Gu, R. B. Kaspar, J. Zheng, J. Wang, S. Qiu and Y. Yan, Nat. Commun., 2014, 5, 4503 CrossRef.
- B. Lukose, A. Kuc and T. Heine, Chem.–Eur. J., 2011, 17, 2388–2392 CrossRef CAS.
- Z. Li, Y. Zhi, X. Feng, X. Ding, Y. Zou, X. Liu and Y. Mu, Chem.–Eur. J., 2015, 21, 12079–12084 CrossRef CAS.
- M. G. Rabbani, A. K. Sekizkardes, Z. Kahveci, T. E. Reich, R. Ding and H. M. El-Kaderi, Chem.–Eur. J., 2013, 19, 3324–3328 CrossRef CAS.
- Z. Kahveci, T. Islamoglu, G. A. Shar, R. Ding and H. M. El-Kaderi, CrystEngComm, 2013, 15, 1524–1527 RSC.
- M. G. Rabbani and H. M. El-Kaderi, Chem. Mater., 2012, 24, 1511–1517 CrossRef CAS.
- G. Li, B. Zhang, J. Yan and Z. Wang, Macromolecules, 2014, 47, 6664–6670 CrossRef CAS.
- R. Dawson, D. J. Adams and A. I. Cooper, Chem. Sci., 2011, 2, 1173–1177 RSC.
- A. L. Myers and J. M. Prausnitz, AIChE J., 1965, 11, 121–127 CrossRef CAS.
- H. Wei, S. Chai, N. Hu, Z. Yang, L. Wei and L. Wang, Chem. Commun., 2015, 51, 12178–12181 RSC.
- H. A. Patel, S. H. Je, J. Park, D. P. Chen, Y. Jung, C. T. Yavuz and A. Coskun, Nat. Commun., 2013, 4, 1357 CrossRef.
- Y. Luo, B. Li, W. Wang, K. Wu and B. Tan, Adv. Mater., 2012, 24, 5703–5707 CrossRef CAS.
- W. Lu, D. Yuan, J. Sculley, D. Zhao, R. Krishna and H. C. Zhou, J. Am. Chem. Soc., 2011, 133, 18126–18129 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra03487k |
| This journal is © The Royal Society of Chemistry 2019 |