Open Access Article
Open Access ArticleFunctionalized gold nanostructures: promising gene delivery vehicles in cancer treatment
Sanjay Kumar
,
Anchita Diwan
,
Parinita Singh
,
Shikha Gulati
 *,
Devanshu Choudhary
,
Ayush Mongia
,
Shefali Shukla
and
Akanksha Gupta
*,
Devanshu Choudhary
,
Ayush Mongia
,
Shefali Shukla
and
Akanksha Gupta
Department of Chemistry, Sri Venkateswara College, University of Delhi, Delhi-110021, India. E-mail: shikha2gulati@gmail.com
First published on 1st August 2019
Abstract
Surface-modified gold nanoparticles are recognized as promising gene delivery vehicles in the treatment of cancer owing to their excellent biocompatibility with biomolecules (like DNA or RNA) and their unique optical and structural properties. In this context, this review article focuses on the diverse transfection abilities of the gene to the targeted cell on the basis of different shapes and sizes of gold nanoparticles in order to promote its effective expression for cancer treatment. In addition, recent trends in gold nanoparticle mediated gene silencing, gene delivery, detection and combinatory therapies are highlighted considering their cytotoxic effects on healthy human cells.
1. Introduction
Cancer, with high incidence and deregulated cell growth, has caused death all over the world. Conventionally, cancer treatment relied mainly upon tumour resection along with chemotherapy, where there is non-specific accumulation of drugs and/or radiotherapy resulting in various side effects. A large number of cancer cells have become drug resistant towards these therapies mostly due to the molecular mechanism. These drawbacks of the traditional approach towards treatment of cancer can be overcome by combining gene therapy with other related therapies such as chemotherapy, photothermal therapy, photodynamic therapy, etc.1–4 Gene delivery vectors reported in recent years, include polymer-based, lipid-based, and inorganic nanoparticle-based systems.5–9 Ideal non-viral gene vectors offer numerous advantages, such as chemical stability, biocompatibility, effective targeting, stimulus responsiveness and feasibility for combined treatments.2–4 Due to irregular signalling pathways and genetic alterations, the concept of novel molecularly targeted therapeutic strategies such as gene-silencing or knockdown therapies have been introduced.10–14 RNA interference (RNAi), one of the significant gene therapy approaches followed by tumour resection has fascinated researchers in cancer treatment,15 where microRNA (miRNA) and plasmid DNA (pDNAs) are frequently utilized because of their higher stability and specificity than those of small interfering RNA (siRNA).16,17 Surface-modified gold nanomaterials are acknowledged as favourable gene delivery vehicles,18–21 in consequence of their small size, general non-toxicity, ease of functionalization and high surface to volume ratio, localized surface plasmon resonance (LSPR) of gold nanoparticles resulting in improved consideration from non-viral siRNA gene delivery.11,22,23 Additionally, these nanoparticles can also be used for imaging of tumours by computed tomography, a leading radiologic technology in the field of biomedical imaging.24 In continuation of our research work in the field of nanomaterials and cancer treatment,25–30 the current review focuses on advanced accomplishments of gene therapy in conjugation with gold nanoparticles that stand out as potential candidates for anti-oncological approaches. In addition, detailed mechanisms like gene silencing, gene delivery along with detection and imaging are discussed both in vitro and in vivo.2. Evaluation of gold nanoparticles in gene silencing
The commendable contributions of AuNPs in gene silencing have enhanced the treatment of cancer drastically. Various recent reports have shown effectiveness utilizing this approach as shown in Table 1. Table 1 shows the cellular recognition and entry of the nanoparticles, of different size and shape at tumor sites for knockdown. RNA interference (RNAi), one of the gene therapy approaches offered has attracted interest in cancer treatment due to its high specificity and efficiency and the relatively minor upshots.31,32 Over the decade, a novel strategy for detection and gene knockdown has been introduced using RNA interference.33,34 Telomerase which is highly expressed in many types of cancer cells whose activity inhibition using human telomerase reverse transcriptase (hTERT) has been investigated in oncology.35 However, there are challenges that hinder the efficient delivery of hTERTsiRNA at the tissue level as well as the intracellular levels (Fig. 1).36–38| S. No. | Type of gold nanoparticles (shape & size) | Type of cancer treated/targeted cells | Delivery vehicle | Targeted Functions | Reference |
|---|---|---|---|---|---|
| 1 | Gold nanoparticles (∼50 nm) | Breast cancer | AuNP-PEI/eEF-2K siRNA | Gene knockdown in vitro and in vivo and evaluated antitumor efficacy in a TNBC tumor model | 33 |
| 2 | Gold nanorods (∼70 nm × ∼30 nm) | HepG2 cancer cells | PEI-Au/siRNA and PEI-Au/siRNA@ZGOC | Protection of SiRNA for gene silencing and LDDS gene vector system of PEI-Au/siRNA@ZGOC which binds siRNA for gene silencing | 39 |
| 3 | Gold nanoparticles (∼50 nm) | Gastric cancer | HAG5PAMAMAuMETase | Suppressed the growth of tumor via targeting CD44(+) cells | 56 |
| 4 | Gold nanoparticles (65–128 nm) | Breast (MCF-7), and cervical (KB) cells | Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G5D and Au G5D and Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G5D G5D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FA NPs FA NPs |
Affords good protection to the pDNA against nucleases | 60 |
| 5 | Gold nanostars (72.5 ± 3.2 nm) | Hepatocellular carcinoma cells (HepG2) and SGC7901 cells | siRNA/9R/DG-AuNS (hydrazone) | Gene silencing and inhibiting the cell growth rate | 61 |
| 6 | Gold nanoclusters (2.6 ± 0.5 nm) | Panc-1 in vitro, pancreatic tumours in vivo | AuNC-siRNA | Suppressed pancreatic tumour progression | 62 |
| 7 | Gold nanostars (AuNS TRITON: 80–90 nm; AuNS LSB: 50–70 nm; shrunk AuNS LSB: 20–30 nm) | MCF-7/Luc cells | AuNS-POLYMER-siRNA | Suppressed luciferase expression | 63 |
| 8 | Gold nanoshells (159 ± 11 nm) | Triple-negative breast cancer | LbL-NS | Suppressed cancer cell growth | 64 |
| 9 | Gold nanorods (48 × 16 nm) | Ovarian cancer cell line (SKOV-3) | DTC-anchored siRNA duplex | Efficient knockdown | 66 |
| 10 | Gold nanoassembly (20–25 nm) | HeLa cells | Rf, RfSH and Rf@AuNPs | DNA damage and apoptosis | 69 |
| 11 | Gold nanoparticles (30.42 ± 0.29 nm) | MCF-7 and MDA-MB-453 cells | AuNP@PEG@anti-RAB27A | Gene silencing and decrease of exosome release | 74 |
| 12 | Gold nanoparticles (∼6 nm) | A375 human melanoma cells | AuNP-SX/SS/SN-DNA and AuNP-TSX/TSS/TSN-plasmid complexes | To modify cellular protein expression | [81] |
| 13 | Gold nanocages (∼50 nm) | Hepatocellular carcinoma (HCC) | HA/anti-miR-21/PPAuNCs | Reversing MDR (multidrug resistance) | 85 |
| 14 | Gold nanoclusters (103.7 ± 3.8 nm) | A375 cells | TAT-AuNS/Cas9 protein/sgRNA | Inhibited the tumour growth | 93 |
| 15 | Gold nanoparticles (5.3 nm) | Lung cancer, breast cancer, pancreas cancer, skin cancer, ovary cancer | TDDS (Au-TR-DX-si) | Knockdown of erbB2 oncogene in vitro and tumor supression in vivo | 98 |
| 16 | Gold nanoparticles (65–128 nm) | HEK293, HepG2, Caco-2, MCF-7, KB | Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G5D, Au G5D, Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G5D G5D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FA, G5D, G5D FA, G5D, G5D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FA FA |
Apoptosis, enhanced transfection efficiency | 127 |
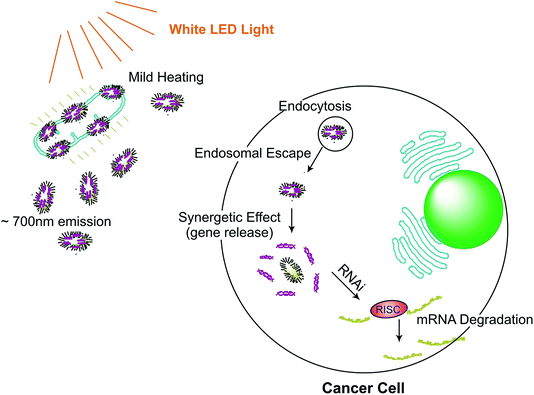 | ||
| Fig. 1 Gene silencing on targeted cancer cell site using white LED light (∼700 nm emission). Gold nanorods-assembled ZnGa2O4–Cr nanofibers for LED-amplified gene silencing.39 | ||
Under the influence of LED radiation, the Chromium-doped zinc gallate nanoparticles ZnGa2-xO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Crx (ZGOC) nanofiber emission could be absorbed selectively by gold nanorods (AuNRs), which induces a mild photothermal effect that in turn triggers the liberation of the particles from ZGOC nanofibers, localized drug delivery systems (LDDSs), and enhances the cellular uptake and the therapeutic effect. The new therapeutic platform, with LED-amplified gene silencing has therefore inspired another potential system to enhance gene therapy in cancer treatment. The successful RNAi lies in the effective release of siRNA from the endosomes, which can destroy the target mRNA in the cytoplasm and inhibit the expression of the target protein.39 Post conjugation of mRNA, siRNA instigates degradation of mRNA, hence significantly reducing protein expression by targeting the gene.34,40 In a study, to therapeutically target eukaryotic elongation factor 2 kinase (eEF-2K), which has been identified as an important oncogenic pathways for promoting breast cancer. Kezban et al. synthesized a highly monodisperse and stable nano-formulation using polyethylenimine-modified gold nanoparticles (AuNP-PEI) and conjugated them with eEF-2K siRNA. Hence, down regulation of triple negative breast cancer cells (TNBC) using AuNP-PEI/eEF-2K siRNA was observed in vitro.33
Crx (ZGOC) nanofiber emission could be absorbed selectively by gold nanorods (AuNRs), which induces a mild photothermal effect that in turn triggers the liberation of the particles from ZGOC nanofibers, localized drug delivery systems (LDDSs), and enhances the cellular uptake and the therapeutic effect. The new therapeutic platform, with LED-amplified gene silencing has therefore inspired another potential system to enhance gene therapy in cancer treatment. The successful RNAi lies in the effective release of siRNA from the endosomes, which can destroy the target mRNA in the cytoplasm and inhibit the expression of the target protein.39 Post conjugation of mRNA, siRNA instigates degradation of mRNA, hence significantly reducing protein expression by targeting the gene.34,40 In a study, to therapeutically target eukaryotic elongation factor 2 kinase (eEF-2K), which has been identified as an important oncogenic pathways for promoting breast cancer. Kezban et al. synthesized a highly monodisperse and stable nano-formulation using polyethylenimine-modified gold nanoparticles (AuNP-PEI) and conjugated them with eEF-2K siRNA. Hence, down regulation of triple negative breast cancer cells (TNBC) using AuNP-PEI/eEF-2K siRNA was observed in vitro.33
To make sure the better detection and therapeutic effect, antimiR-21 strands are fully modified by 2′-O-methyl to increase binding affinity with tumor gene and improve stability and resistance to exonucleases to prolonged inhibition of miRNAs.41 Recent studies have shown that Taxol, a chemotherapeutic drug initially induces apoptosis of cancer cells, but on prolonged treatment, the cancer cells become immune to this drug and to other drugs as well.42,43 This resistance of cancer cells towards these chemotherapeutic drugs is called multidrug resistance (MDR).44 Various studies have reported that P-glycoprotein (P-gp) is overexpressed in MDR cell lines.45 The reduction in the expression of P-gp could be done using hyperthermia.46 Also, microRNA-21 (miR-21) a type of miRNA could be downregulated resulting in inhibiting tumor progression and invasion.47,48 miRNA, is advantageous in promoting cell invasion by regulating various genes, including programmed cell death 4 (PDCD4) gene, tissue inhibitor of metallopeptidase inhibitor 3 (TIMP3), phosphatase and tensin homolog (PTEN) gene in a variety of tumors, such as lung cancer, glioblastoma, and breast cancer.49 AuNPs are functionalized with miRNA-21 inhibitors that are hybridized with fluorophore-labeled DNA molecules named “flares”, miRNA-21 inhibitors are 2′-O-methyl modified antisense oligonucleotide that are complementary to the mature miRNA-21 to suppress its function, resulting in cells growth inhibition and apoptotic cells death, as shown in Fig. 2.50 Cancer stem cell (CSC) with the mark of CD44 plays an important role in gastric cancer (GC). The transfection of rMETase carried by HA-G5 PAMAM-Au visibly inhibited the escalation and tumor sphere formation of GC cells through promoting METase activity. Elevated level of methionine (MET) promotes self-growth of cancer cells. Suppression of CD44(+) GC resulted because of downregulation of METase activity, which lead to increase of Cyc C and ROS levels. For instance, the p53 and HSV-TK suppressed the proliferation and promoted apoptosis of cancer cells.51,52 No effect on healthy cells was observed due to the low concentration of MET.53 Owing to the excellent structural properties (PAMAM) is found to be suitable in conjugation with metallic nanoparticles.54 Improvement in efficacy of gene transfecting COS-7 cells and shape maintenance of dendrimers was caused through G5-PAMAM encapsulated gold nanoparticles.55 Additionally, hyaluronic acid (HA) was conjugated onto PAMAM for targeting CD44 + gastric cancer stem cells specifically.56
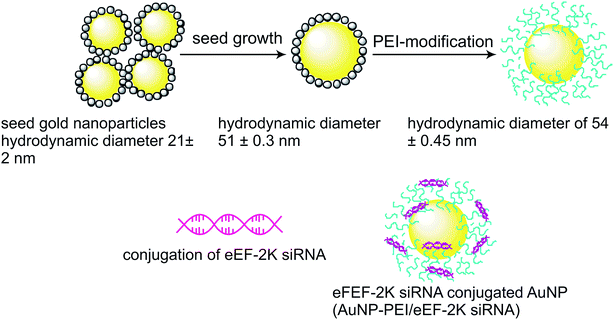 | ||
| Fig. 2 Schematic illustration for modification of AuNPs via polyethyleneimine.33 | ||
Another instance of this approach is demonstrated by the use of folic acid (FA) due to its replication control, cell proliferation, synthesis of proteins and biocompatibility.58 Moreover, it has high affinity towards FA receptors which aremainly overexpressed in breast (MCF-7), and cervical (KB) cells.59 Gold nanoparticle conjugated generation five dendrimers (Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G5D) and Au
G5D) and Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G5D
G5D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FA NPs proved to be highly efficient in combining with pCMV-Luc DNA and transporting it. The pDNA were safeguarded from nucleases corresponding to formation of stable nanocomplexes.60 siCOX-2/9R/DG-AuNS (hydrazone) showed best gene silencing and inhibition effect on tumor cell growth amongst siCOX-2/AuNS, siCOX-2/9R-AuNS, siCOX-2/9R/DG-AuNS, and siCOX-2/9R/DG-AuNS (hydrazone) groups in both HepG2 and SGC7901 cells.61 In comparison to free siRNA, gold nanocages conjugated siRNA (AuNC-siRNA) entered the Panc-1 cells in much larger quantity, showed lysosomal escape and effective gene silencing. In addition, it enhanced circulation lifetime, increased accumulation on tumour sites showed high safety.62 PPE-LA coating showed better gene silencing of MCF-7 cells than AuNS (gold nanostar) coated with SH-PEG3000-NH2. Although, siRNA complexes coated with AuNS showed better results than siRNA alone.63 Using Western blotting, it was analysed that miR-34a-LbL-NS significantly decreased Bcl-2 and SIRT1 expression in MDA-MB-231 cells in comparison to control groups and also reduced metabolic activities in MDA-MB-231 cells.64 Transglutaminase 2 (TG2) plays a vital role in metastasis of recurrent ovarian cancers, hence becomes a critical target.65 Successful gene knockdown by AuNR based DTC-siRNA, was observed using colorimetric assay.66 Flavoprotein enzyme assembling is done mainly by riboflavin (Rf) and is overexpressed in prostate and human breast cancers.67,68 Abhishek Sau and team prepared thiol-modified riboflavin-gold nanoparticles (RfS@AuNPs), targeting HeLa cells hence causing DNA damage and apoptosis in them.69 Exosomes which are found in body fluids, containing protein and nucleic acids, secreted by both normal and malignant cells.70–72 The presence of these in higher content is responsible for tumour growth and evasion.71,73 AuNP@PEG@anti-RAB27A silence higher gene expression than that of RAB27A, also inhibiting formation of Rab27, a protein from RAB27A mRNA.74 Xinyan Chen et al. used “ship in a bottle strategy” and encapsulated AuNRs inside the hollow cavity of HSN (hollow silica nanoparticles). SF (hydrophobic anti-proliferative and antiangiogenic drug) release was observed due to the detachment of CD-PGEA when NIR radiation was applied, leading to apoptosis in both in vitro as well as in vivo.75
FA NPs proved to be highly efficient in combining with pCMV-Luc DNA and transporting it. The pDNA were safeguarded from nucleases corresponding to formation of stable nanocomplexes.60 siCOX-2/9R/DG-AuNS (hydrazone) showed best gene silencing and inhibition effect on tumor cell growth amongst siCOX-2/AuNS, siCOX-2/9R-AuNS, siCOX-2/9R/DG-AuNS, and siCOX-2/9R/DG-AuNS (hydrazone) groups in both HepG2 and SGC7901 cells.61 In comparison to free siRNA, gold nanocages conjugated siRNA (AuNC-siRNA) entered the Panc-1 cells in much larger quantity, showed lysosomal escape and effective gene silencing. In addition, it enhanced circulation lifetime, increased accumulation on tumour sites showed high safety.62 PPE-LA coating showed better gene silencing of MCF-7 cells than AuNS (gold nanostar) coated with SH-PEG3000-NH2. Although, siRNA complexes coated with AuNS showed better results than siRNA alone.63 Using Western blotting, it was analysed that miR-34a-LbL-NS significantly decreased Bcl-2 and SIRT1 expression in MDA-MB-231 cells in comparison to control groups and also reduced metabolic activities in MDA-MB-231 cells.64 Transglutaminase 2 (TG2) plays a vital role in metastasis of recurrent ovarian cancers, hence becomes a critical target.65 Successful gene knockdown by AuNR based DTC-siRNA, was observed using colorimetric assay.66 Flavoprotein enzyme assembling is done mainly by riboflavin (Rf) and is overexpressed in prostate and human breast cancers.67,68 Abhishek Sau and team prepared thiol-modified riboflavin-gold nanoparticles (RfS@AuNPs), targeting HeLa cells hence causing DNA damage and apoptosis in them.69 Exosomes which are found in body fluids, containing protein and nucleic acids, secreted by both normal and malignant cells.70–72 The presence of these in higher content is responsible for tumour growth and evasion.71,73 AuNP@PEG@anti-RAB27A silence higher gene expression than that of RAB27A, also inhibiting formation of Rab27, a protein from RAB27A mRNA.74 Xinyan Chen et al. used “ship in a bottle strategy” and encapsulated AuNRs inside the hollow cavity of HSN (hollow silica nanoparticles). SF (hydrophobic anti-proliferative and antiangiogenic drug) release was observed due to the detachment of CD-PGEA when NIR radiation was applied, leading to apoptosis in both in vitro as well as in vivo.75
3. Analyzing targeted gene delivery using modified gold nanoparticles
The main goal of gene therapy is to select an acceptable carrier to specifically transport the target gene to the desired target cell and promote its effective expression for treatment. The carrier of gene therapy mainly includes two categories: viral vector and non-viral vector.56 Surface-modified Gold nanomaterials are considered as efficient gene delivery vehicles.76,77 However, for gene therapy in general, siRNA molecules are phagocytosed and hence need to overcome extracellular and intracellular obstacles and be released from the endosome after being internalized by tumor cells.78 Scientists have reported the efficiency of gold nanoparticles in analyzing targeted gene delivery and these are summarized in Table 2. The Covalently coupled nucleic acids (such as single monodentate thiol (SX), bidentate dual thiol (SS), or mixed bidentate thiol plus amine (SN) coordination of nucleic acids) on the surface of gold nanoparticles (AuNP) have been used as an effective gene therapy agent to modify cellular protein expression. It is anticipated that the therapeutic effect depends on length of time the nucleic acid sequence resides in the endo-lysosomal pathway once the cell is transfected. It is believed that it depends on the linkage chemistry of the DNA to the AuNP surface.79 LDDSs, which are designed to manipulate the tumor microenvironment are normally injected (or implanted), have intrinsic drawbacks in achieving effective gene therapy. Therefore, by combining LDDSs and particulate gene carriers (i.e. gold nanorods) unique opportunities might be offered allowing controlled delivery and release of a targeted therapeutic concentration and effective phagocytosis of siRNA molecules for enhanced gene therapy. Polyethyleneimine (PEI) is a well-known non-viral nucleic acid vector with high transfection efficiency both in vitro and in vivo due to the high density of positively charged amino groups distributed along the backbones of the molecular chains.80 The positively-charged polyethyleneimine conjugated gold nanorods (Au-PEI NRs) possess the ability to electrostatically bind to negatively-charged siRNA. The protection of siRNA by PEI-Au/siRNA and PEI-Au/siRNA@ZGOC vectors was further verified by the addition of RNaseA to mimic the human serum environment. The PEI-Au/siRNA and PEI-Au/siRNA@ZGOC vectors were found to protect the siRNA, effectively, from the degradation effects of RNaseA.39 The permeability barrier imposed by stratum corneum makes an extreme challenge for the topical delivery of plasmid DNA (pDNA), which is widely used in gene therapy.81 Dendritic macromolecules are highly branch monodisperse macromolecules, presenting a sphere with cavity structure, hence are easy to combine with DNA to form nanoscale complex, which can protect DNA to achieve the purpose of transfection, so as the viral carrier, which is widely used.82 A group of researchers performed surface modification on polyamidoamine (PAMAM) dendrimers to decrease cytotoxicity and increase transfection efficiency.83 Hyaluronic acid (HA), with good biocompatibility and aqueous solubility, was reported to be a receptor of CD44.84 HA can target the site with high-expression CD44 tumor for targeted drug delivery to CD44-overexpressing cancer cell.46 A novel gene delivery vector was developed, hyaluronic acid (HA)-modified, polyethylenimine (PEI)-conjugated PEGylated gold nanocages (AuNCs), which was designated as HA/PPAuNCs. This system possessed relative high photothermal conversion efficiency upon near infrared (NIR) laser irradiation, low cytotoxicity, and high targeted delivery ability. Moreover, anti-miR-21 could be condensed by PEI onto the surface of HA/PPAuNCs and then delivered into target cells. Therefore, HA/anti-miR-21/PPAuNCs might effectively overcome DOX resistance of HCC and provide new insights for cancer treatment.85| S. no. | Type of gold nanoparticles (shape & size) | Type of cancer treated | Delivery vehicle | Targeted functions | Reference |
|---|---|---|---|---|---|
| 1 | Gold nanorods (∼30 nm × 10 nm) | HEK293, HepG2 cells | AHP, sm-AHP, CD-PGEA | Apoptosis, enhanced transfection efficiency | 75 |
| 2 | Gold nanoparticles (2.9 nm and 3.1 nm) | EGFP, Luc cells | Au DENPs-mPEG and Au DENPs-PEG-FA | Low immunogenicity and enhanced gene transfection efficiency | 142 |
3.1 Employing gold nanoparticles for gene delivery in vitro
Various scientists have studied the role of AuNPs in vitro to improve gene delivery in a targeted manner. In this context, modification of AuNPs with cationic polymers bound effectively to negatively surface charged siRNA molecules via a layer-by-layer approach has been studied. Due to their unique combination of surface plasmon properties and nanometer-scale dimensions, effective intracellular delivery of Au nanoparticles can be achieved along with transfected siRNA in vitro.39 Presently, using multifunctional carrier AuNP, the perforation and transfection effect of pDNAs in the melanoma cells at the subcutaneous site were investigated.81 Polyethyleneimine (PEI) having abundant surface amine groups is found to be showing high efficacy due to its ability to compact DNA efficiently,86,87 however it has high levels of cytotoxicity. Hence, PEG has been used along with gold nanoparticles targeting HeLa.88 Cyclooxygenase-2 (COX-2) is a rate-limiting enzyme overexpression of which is a sign of tumorigenesis, promotion of malignant tumor cells and is expressed in vascular endothelial and adjacent normal tissues.89,90 Hongyan Zhu et al. have prepared siCOX-2/AuNS, siCOX-2/9R-AuNS, siCOX-2/9R/DG-AuNS, and siCOX-2/9R/DG-AuNS (hydrazone) groups in combination with siRNA to down-regulate COX-2 expression, targeting HepG2 (hepatocellular carcinoma cells) and SGC7901 cells.61 Plk1 gene is overexpressed in many tumor tissues for e.g., A375 cells, inhibition of which is a good strategy for tumor therapy.91,92 Peng Wang et al. prepared LGCP (polyethylene glycol-lipid/AuNS/Cas9 protein/sgPlk1 plasmid) to suppress Plk1 gene.87 The folate receptor (FR) is overexpressed in abundant of cancers such as: human ovarian, brain, cervical, epithelial, and breast cancer amongst others.94 Jude Akinyelu and Moganavelli Singh prepared folic acid (FA) and chitosan (CS) functionalization with Au-PLGA NPs for downregulation of FR.95 Heregulin-2 (HER2) is overexpressed in human ovarian cancer cell line and are known to be resistant to chemotherapy and aggressive.96,97 Rajesh Kotcherlakota and team prepared TDDS (Au-TR-DX-si) to target the same.98 Pancreatic cancer being one of the dangerous cancers, its prominent cause being perineural invasion has its adverse effects such as increased neurite densities.99,100 Targeting NGF gene is the most important to tackle pancreatic cancer.62 Hence, Yifeng Lei et al. synthesized AuNC-siRNA62 because siRNA has the ability to cause sequence-specific gene silencing.101,102 Carla Sardo and team used a different shape of gold nanoparticle, i.e., gold nanostar and conjugated it with SH-PEG3000-NH2 and PPE-LA targeting MCF-7/Luc cells.63 As depicted in Table 2, AHP has shown better transfection efficiency than sm-AHP in HEK293 and HepG2 cells, which was visualized using enhanced green fluorescent protein (EGFP). This could be due to the shape and size of gold nanorods, and also rough surface of AHP.75 miRNA regulates gene expression to control cell metabolic activity.103 In an experiment conducted by R. Goyal et al., LbL-NS was developed to deliver miR-34a into triple-negative breast cancer cells.64 Poly-L-lysine (PLL) shows good endosomal escape,104 since loss of expression of miR-34a takes place in TNBC.105,106 Therefore, naked miRNA cannot passively target due to its large size and negative charge.107 In a study carried out by Jianxin Wang et al., DTC-anchored siRNA duplex loaded onto AuNRs was used, which targeted ovarian cancer cell line (SKOV-3). DTC (dithiocarbamates) are resistant to nonspecific desorption reducing off target effects.66 Nucleic acid is a great tool for detection as well as treatment purposes.108,109 MicroRNA (miRNA) plays vital roles in cell proliferation, differentiation,110,111 and tumors,112,113 and one of it's example is Lethal-7 (Let-7).114 One of its members is Let-7a which can suppress the tumor cell growth and metastasis.115,116 Although DNA and RNA are similar compounds, a major difference is in their structure. DNA contains only one hydroxyl group, while RNA contains two hydroxyl groups. So, PBA shows low binding effect for compounds containing one hydroxyl group.117Isocitrate dehydrogenase 1 (IDH1) which is highly expressed in Glioblastoma multiforme (GBM) is disruptive form found in brain tumors and can be targeted using siRNA.118,119 Jun Yue et al., modified the siRNA with AuNPs (stars and spheres) to target the Isocitrate dehydrogenase 1 (IDH1) expression.120 The N/P ratio of 2.5 reveals the best transfection efficiency which was observed using flow cytometry and confocal microscopy. It could be due to the compaction ability and strong interaction of vectors with pDNA.142
Baoji Du et al., have used GDD (gold nanoparticles/dimethyldioctadecylammonium bromide (DODAB)/dioleoylphosphatidylethanolamin) and GF (gold nanoparticles/DODAB/DOPE/DOPE-folic acid) with different ratios of DOPE-FA targeting MCF-7 and A549 cells both in vitro and in vivo which is portrayed in the above figure121
3.2 In vivo delivery of genes via gold nanoparticles
The emerging need to explore recent nanoparticles capable of altering the collective state is worthy of attention;122 that is, how such vehicles can be modified for the development of in vivo obstacles that AuNPs face.123 Previously, the interaction of small AuNPs with DNA was studied at low [NaCl], concluding that the reversible groove binding interaction was governed by solvation and viscosity factors.124 Recently, in the study of the structural characterization of the gold colloid-DNA interaction, it was shown that AuNPs was able to interact with DNA via groove binding or intercalation, depending on the ethanol content and the R ratio.125 According to Elia et al. previous structural approach,125 their study has demonstrated that ethanol modifies AuNPs/DNA interaction from intercalation to groove binding by two simple mechanisms: gold nanoparticle aggregation and change in DNA conformation from B to C-form.126 Particulate systems can be associated with the circulation of excessively high concentrations of drugs in vivo. (LDDSs) offer the potential to target the drug more effectively.127 In their work, Xiang et al. reported a new type of localized gene delivery system with LED-responsive properties based on the combination of the LDDSs and nanoparticle gene vectors. In their system, hTERT siRNA was grafted on to AuNRs and these were attached to the surface of electrospun ZGOC nanofibers as depicted in Fig. 3. Au nanorods with an absorption peak of 700 nm matching the emission of ZGOC nanofibers were prepared using a seed growth method. Gold nanorods modified with a cationic polymer (PEI) were electrostatically bound with negatively charged siRNA.39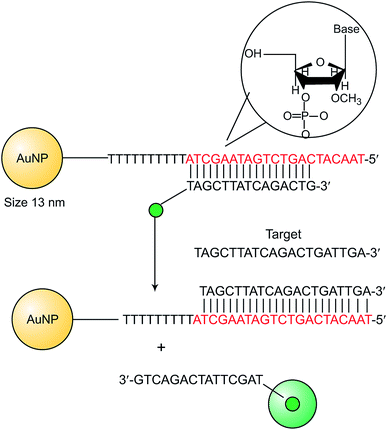 | ||
| Fig. 3 Schematic representation for miRNA detection using AuNP – 2′-OMe-DNA probes.57 | ||
To facilitate the skin penetration of pDNA deeply into the melanoma tissues, Jiang et al. presented a cell penetrating peptide and cationic polymer (PEI) conjugated gold nanoparticle (AuNP) that can compact the pDNAs into cationic nanocomplexes and penetrate through the intact stratum corneum without any additional enhancement used. Moreover, the AuNP is highly efficient in stimulating the intracellular uptake and nuclear targeting of the pDNAs in cells, which guarantees the effective transfection. It was shown that not only miR-222 but also miRNA-221 reduces viability and induce apoptosis mediated by the KIT, AKT and BCL2 signaling cascade.128 miRNA-221 has been proposed as a potential tumor suppressor for melanoma therapy. Moreover, topical delivery miRNA-221 inhibitor gene can avoid or decrease reticulo endothelial system (RES) uptake, reduce systemic toxicity, and provide targeted gene delivery to the tumor site located at the skin subcutaneous layer. However, stratum corneum always poses a formidable challenge to biomacromolecules penetration. To circumvent these problems that confront the current methods, herein, for the first time, they presented a novel strategy for the cutaneous melanoma therapy by topical delivery a pDNA encoded with miRNA-221 inhibitor gene through HIV-1 twinarginine translocation peptide (TAT) conjugated cationic gold nanoparticles.33
4. Combinatory therapies in cancer treatment using modified gold nanoparticles
In general, ideal non-viral gene vectors offer characteristics, such as chemical stability, biocompatibility, effective targeting, stimulus responsiveness and feasibility for combined treatments (i.e. chemotherapy, photothermal therapy, photodynamic therapy, etc.).38,78,144 Gene delivery vectors reported in the recent years, include polymer-based, lipid-based, and inorganic nanoparticle-based systems.129,130 Surface-modified Au nanomaterials are recognized as promising gene delivery vehicles.76,77 The anti-cancer effects of the PEI-Au/siRNA and PEI-Au/siRNA@ZGOC samples were assessed via in vitro cell culture for 48 h and 72 h, using exposure to LED light. LED radiation did not appear to induce any clear effect on the PEI-Au/siRNA group but, in contrast the PEI-Au/siRNA@ZGOC group showed a significant decrease in cell viability under LED irradiation.81 pAuNPs revealed to have high photothermal effect and loading capacity by Au-thiol linkage. Hence, FAM-Dz/TAT-pAuNPs was prepared to target hepatitis C virus (HCV) human hepatocarcinoma cells using hyperthermic treatment via photothermal therapy. The loading was successful which proved to be biocompatible and resulted in low cytotoxicity.131 Combinatorial approach using gene therapy and chemotherapeutic drugs has been successfully done in combination with AuNPs which successfully lead to tumor suppression and ErbB2 gene silencing.985. Role of modified gold nanoparticles in detection and imaging of tumors
One of the major aspects in treating cancer is accurate detection and imaging of oncogenic cells which enables various therapeutic techniques to eliminate them effectively, many of which are discussed in Table 3. Various gold nanoparticles of different shapes like, gold nanospheres and sizes have been depicted, which illustrate the cellular uptake by cell lines. Cellular uptake is usually observed using confocal microscopy, flow cytometry, Western blot analysis, TEM. Deep tissue imaging with a long period has been achieved in vivo using repeated excitation with low-energy LEDs.132–134 Several cellular uptake detections can be done using the confocal laser scanning microscopy (CLSM) as mentioned in few examples. A comparison between the cellular uptake of PEI and PEG-Au PENPs has been done using the confocal laser scanning microscopy (CLSM), proving that L25/pDNA polyplex has almost the same cellular uptake as that of PEI/pDNA polyplex.88 Also, siRNA/9R/DG-AuNS (hydrazone)-treated cells showed greater fluorescence intensity amongst AuNS, 9R-AuNS, 9R/DG-AuNS and 9R/DG-AuNS (hydrazone).61 LGCP was successfully detected using confocal microscopy indicating the successful endosomal escape of the LGCP as the separation of the green/red fluorescence from the blue fluorescence took place.93 Transfection analysis of FA-CS-PLGA NPs, CS-PLGA NPs, Au-CS-PLGA NPs and FA-Au-CSPLGA NPs only to be proven that Au functionalized has high cellular uptake than others because it has high atomic number hence easily identified using TEM.135 Also it has high zeta potential indicating its stability and increased adsorption of FA and chitosan onto the PLGA.60 Direct sequencing method which is used for traditional detection of gene mutations has various setbacks such as complexity, time-consuming, and expensive.136,137 So in an experiment done by Xihong Zhao and Chii-Wann Lin., they used colorimetric assays to detect various PNA/DNA complex in AuNPs solution. When PNA is added, colour of the AuNP solution changes from brick-red wine colour to dark purple, on addition of PNA–DNAcomp complex it retains its colour back to brick-red wine colour, and on adding PNA–DNAnc mixture it shows purple colour.138 Furthermore, Rajesh Kotcherlakota et al., used confocal microscopy to detect the effect of TDDS on SKOV-3 (HER2+) and MDA-MB-231 (HER2-) cells.98 Endosomal escape was also observed using TEM.98 Siyu Qian et al., reported an experiment in which they used PBA-AuNPs for detection of miRNA using SPR sensing system as illustrated in Fig. 4.139 RNA induces low response in SPR detection as it has lower mass, hence AuNPs are used to produce effective results for detection as it has high mass.140,141| S. no. | Type of gold nanoparticles (shape & size) | Type of cancer treated | Delivery vehicle | Inference | Reference |
|---|---|---|---|---|---|
| 1 | Gold nanoparticles (L25, L50, L100, L200) | HeLa cells | PEG-Au PENPs | L25 has high cellular uptake | 88 |
| 2 | Gold nanoparticles (199.4 ± 25.2 nm) | MCF-7, HepG2 and HEK293 cells | Au-CS-PLGA NPs and FA-Au-CSPLGA NPs | High cellular uptake | 95 |
| 3 | Gold nanosphere (∼13 and 50 nm), star (∼40 nm) | U87 cells | siRNA | High cellular uptake | 118 |
| 4 | Gold nanoparticles (∼10 nm) | MCF-7, A549 cells | GDD, GF | Enhanced cellular uptake | 119 |
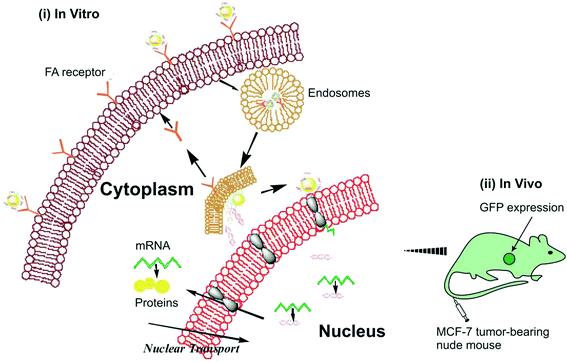 | ||
| Fig. 4 Depiction of targeted gene delivery of GF/DNA (i) in vitro and (ii) in vivo.121 | ||
Western Blot and Nano Tracking Analysis (NTA) were the techniques used for AuNP@PEG@anti-RAB27A to evaluate the exosomes release. Consequence of it was the reduction of exosomes secretion and also exported the nanoparticles to recipient cells, hence decrease of the exosome release.74 Confocal microscopy and flow cytometry (FCM) were used to evaluate the gene transfection abilities of GDD and GFs.119 Results showed that GF2.5 showed higher gene transfection efficiency for MCF-7 cells due to enhanced cellular uptake efficiency mediated by the FA targeting ability, but did not so in A549 (FA-receptor-negative cells) (Fig. 5 and 6).119
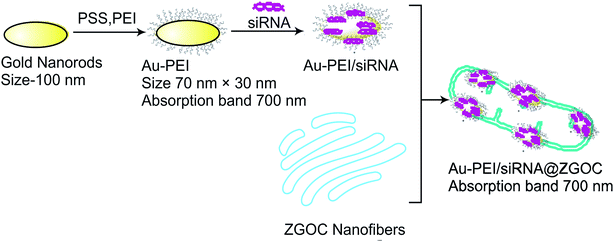 | ||
| Fig. 5 Schematic illustration of PEI-Au/siRNA onto ZGOC nanofibers.39 | ||
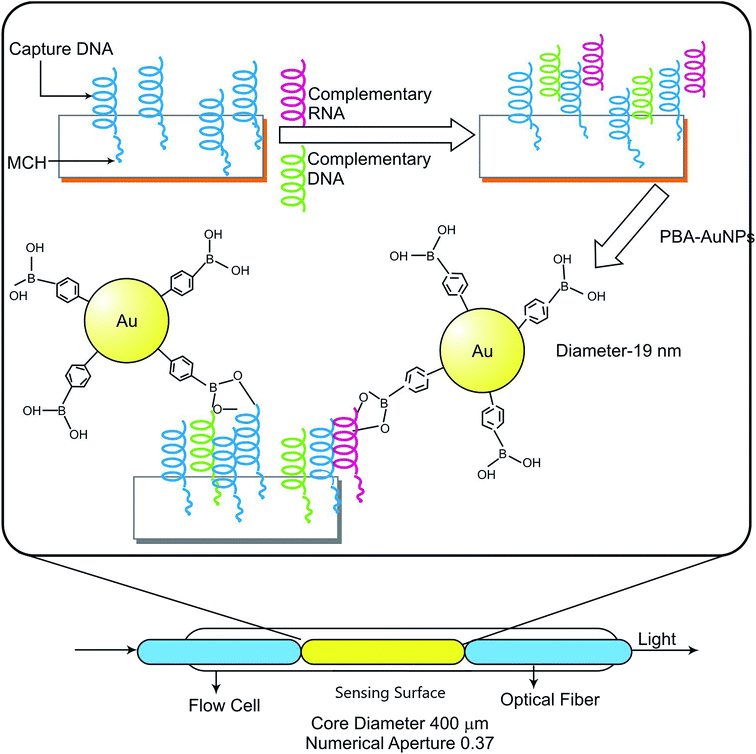 | ||
| Fig. 6 Combinatory therapy representing schematic illustration of SPR sensing system for detecting miRNA.139 | ||
Confocal microscopy and flow cytometry were used to assess the cellular uptake of both Au DENPs-mPEG and Au DENPs-PEG-FA, implying that FA modification and enhanced the cellular uptake.142 EGFP (Enhanced green fluorescent protein) was performed to visualize the gene transfection of AHP in HepG2 cells, hence demonstrating it to have highest transfection amongst CD-PGEA and PEI.75 Also, flow cytometry assay was used to further evaluate the cellular uptake and revealed AHP to have the highest trends.75
6. Cytotoxic assays of gene delivery vectors on healthy cells
The detection of cytotoxic effects of gene vectors and nanoparticles for both tumor and normal cells is critical for their translation to clinics. This indicates biocompatibility of gene delivery vehicles and nanoparticles for gene delivery. Haematological and biochemical parameters are main indicators to assess the cytoxic effects of nanoparticles. Various methods for detection of cytotoxicity levels are MTT assay, cell counting kit-8 (CCK-8), cell viability test, and many more. Moreover, the HA-G5 PAMAM-Au-METase could markedly inhibit the tumor sphere formation rather than HA-G5 PAMAM-METase.56 The cytotoxicity assay in an experiment conducted by Aijun Li and team was carried out using Cell Counting Kit-8 (CCK-8) assay, proving that polyethylenimine (PEI) alone is more cytotoxic than PEG-Au PENPs at different concentrations. Cytotoxic levels were found to be in the order PEI > L25 > L50 > L100 > L200.88 Another method used is detection using MTT reduction assay showing that all FA-CS-PLGA NPs, CS-PLGA NPs, Au-CS-PLGA NPs and FA-Au-CSPLGA NPs are non-toxic.95 MTT assay tested the cytotoxicity of Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G5D
G5D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA, Au
DNA, Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G5D
G5D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FA
FA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA, G5D
DNA, G5D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA and G5D
DNA and G5D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FA
FA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA and found Au
DNA and found Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G5D
G5D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA, Au
DNA, Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G5D
G5D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FA
FA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA to be less toxic than G5D
DNA to be less toxic than G5D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA and G5D
DNA and G5D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FA
FA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA.60 MTT assay was used to evaluate the cytotoxicity of GDD and GFn, GF2.5 demonstrated to be least cytotoxic.119 CCK-8 assay was used to evaluate the cell viability of non-viral vectors indicating the reduction of cytotoxicity using both Au DENPs-mPEG and Au DENPs-PEG-FA.142 Using MTT assay it was evaluated that cell viabilities of HEK293 and HepG2 cell lines was above ≈60%, suggesting the compromised cytotoxicity of AHP.75 Many combinatory aspects of employing AuNPs are depicted in Table 4.
DNA.60 MTT assay was used to evaluate the cytotoxicity of GDD and GFn, GF2.5 demonstrated to be least cytotoxic.119 CCK-8 assay was used to evaluate the cell viability of non-viral vectors indicating the reduction of cytotoxicity using both Au DENPs-mPEG and Au DENPs-PEG-FA.142 Using MTT assay it was evaluated that cell viabilities of HEK293 and HepG2 cell lines was above ≈60%, suggesting the compromised cytotoxicity of AHP.75 Many combinatory aspects of employing AuNPs are depicted in Table 4.
| S. No. | Type of gold nanoparticles (shape & size) | Type of cancer treated/targeted cells | Delivery vehicle | Targeted Functions | Reference |
|---|---|---|---|---|---|
| 1 | Gold nanoparticles (13 nm ± 2 nm) | Breast cancer | AuNP-2′-OMe-DNA probes | miRNA-21 detection and inhibition leading to apoptotic cells death | 57 |
| 2 | Gold nanoplates | HCV genomic human hepatocarcinoma | TAT/FDz-pAuNPs | To investigate the synergistic effect of NIR irradiation with pAuNPs-based chemotherapy | 123 |
| 3 | Gold nanobeacon (∼13 nm) | Colorectal carcinoma | Thiol-DNA-hairpin Cy3 sequences: antisense gold nanobeacon: nonsense gold nanobeacon | To detect and inhibit KRAS gene for a theranostic approach in colorectal carcinoma cells | 143 |
7. Conclusion
Gene therapy is a tool for treatment of cancer and genetic diseases. In this review, recent research work done in the field of cancer treatment using gene therapy, via surface modified gold nanoparticles has been discussed. There are numerous more therapies like radiotherapy, chemotherapy, immunotherapy, targeted therapy, and hormone therapy. For future prospects, these therapies in combination with gene therapy have better and safer treatments against cancer. Combinatory therapies along with gene therapy using AuNPs as delivery vehicles can be used effectively for the treatment of cancer since most cancers are recurring or have become immune to single therapies used. Also, therapies like chemotherapy and radiotherapy have various side effects which in synergy with gene therapy could reduce their cytotoxicity. Viral and non-viral vectors are commonly used for transfection of therapeutic genes to tumor. Since, viral vectors have less loading capacity, limited mass production, are cytotoxic and show strong unwanted immune activity. Hence, non-viral vectors have wider scope in the future. Their advantages include low cytotoxicity, good binding ability to nucleic acids, nucleic acids release controlling ability, strong binding efficiency to nucleic acids and high cellular uptake. Transfections of nucleic acids and various biomolecules in conjugation with gold nanoparticles to tumor sites have proven to inhibit tumor growth. This suggests the translational approaches toward cancer gene therapy via gold nanostructures. Higher cellular uptake, biocompatibility, low cytotoxicity and detection are some of the parameters required for biomolecules as well as gold nanoparticles for translation to clinics. Currently, some of the liposome based nanoparticles are in clinical trials for gene silencing.Conflicts of interest
There are no conflicts to declare.References
- S. Duarte, G. Carle, H. Faneca, M. C. P. de Lima and V. Pierrefite-Carle, Cancer Lett., 2012, 324, 160–170 CrossRef CAS PubMed.
- H. J. Kim, A. Kim, K. Miyata and K. Kataoka, Adv. Drug Deliv. Rev., 2016, 104, 61–77 CrossRef CAS PubMed.
- H. Kim and W. J. Kim, Small, 2014, 10, 117–126 CrossRef CAS.
- C. Ma, L. Shi, Y. Huang, L. Shen, H. Peng, X. Zhu and G. Zhou, Biomater. Sci., 2017, 5, 494–501 RSC.
- S. Rietwyk and D. Peer, ACS Nano, 2017, 11, 7572–7586 CrossRef CAS PubMed.
- M. W. Amjad, P. Kesharwani, M. C. I. Mohd Amin and A. K. Iyer, Prog. Polym. Sci., 2017, 64, 154–181 CrossRef CAS.
- J. Li, C. W. T. Leung, D. S. H. Wong, J. Xu, R. Li, Y. Zhao, C. Y. Y. Yung, E. Zhao, B. Z. Tang and L. Bian, ACS Appl. Mater. Interfaces, 2019, 11(25), 22074–22084 CrossRef CAS PubMed.
- D. Lee, K. Upadhye and P. N. Kumta, J. Mater. Sci. Eng. B, 2012, 177, 289–302 CrossRef CAS.
- C. Zhang, Y. Yong, L. Song, X. Dong, X. Zhang, X. Liu, Z. Gu, Y. Zhao and Z. Hu, Adv. Healthcare Mater., 2016, 5, 2776–2787 CrossRef CAS PubMed.
- D. Bumcrot, M. Manoharan, V. Koteliansky and D. W. Sah, Nat. Chem. Biol., 2006, 2(12), 711–719 CrossRef CAS PubMed.
- R. Shahbazi, B. Ozpolat and K. Ulubayram, Nanomedicine, 2016, 11(10), 1287–1308 CrossRef CAS PubMed.
- B. Ozpolat, A. K. Sood and G. Lopez-Berestein, J. Intern. Med., 2010, 267(1), 44–53 CrossRef CAS PubMed.
- M. E. Davis, J. E. Zuckerman, C. H. J. Choi, D. Seligson, A. Tolcher, C. A. Alabi, Y. Yen, J. D. Heidel and A. Ribas, Nature, 2010, 464(7291), 1067–1070 CrossRef CAS PubMed.
- A. Fire, S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver and C. C. Mello, Nature, 1998, 391(6669), 806–811 CrossRef CAS PubMed.
- K. V. Morris and D. J. Looney, Science, 2004, 305, 1289–1292 CrossRef CAS PubMed.
- V. Winstel, P. Kuhner, B. Krismer, A. Peschel and H. Rohde, Appl. Environ. Microbiol., 2015, 81, 2481–2488 CrossRef CAS PubMed.
- L. Smith, M. Wloch, M. Ye, L. Reyes, S. Boutsaboualoy, C. Dunne, J. Chaplin, D. Rusalov, A. Rolland, C. Fisher, M. Al-Ibrahim, M. Kabongo, R. Steigbigel, R. Belshe, E. Kitt, A. Chu and R. Moss, Vaccine, 2010, 28, 2565–2572 CrossRef CAS PubMed.
- J. Shen, H. C. Kim, C. Mu, E. Gentile, J. Mai, J. Wolfram, L. N. Ji, M. Ferrari, Z. W. Mao and H. Shen, Adv. Healthcare Mater., 2014, 3, 1629–1637 CrossRef CAS PubMed.
- Z. Yang, T. Liu, Y. Xie, Z. Sun, H. Liu, J. Lin, C. Liu, Z. W. Mao and S. Nie, Acta Biomater., 2015, 25, 194–204 CrossRef CAS PubMed.
- J. H. Choi, H. J. Hwang, S. W. Shin, J. W. Choi, S. H. Um and B. K. Oh, Nanoscale, 2015, 7, 9229–9237 RSC.
- B. K. Wang, X. F. Yu, J. H. Wang, Z. B. Li, P. H. Li, H. Wang, L. Song, P. K. Chu and C. Li, Biomaterials, 2016, 78, 27–39 CrossRef CAS PubMed.
- E. Boisselier and D. Astruc, Chem. Soc. Rev., 2009, 38(6), 1759–1782 RSC.
- M. S. Draz, B. A. Fang, P. Zhang, Z. Hu, S. Gu, K. C. Weng, J. W. Gray and F. F. Chen, Theranostics, 2014, 4(9), 872–892 CrossRef CAS PubMed.
- M. Shilo, T. Reuveni, M. Motiei and R. Popovtzer, Nanomedicine, 2012, 7(2), 257–269 CrossRef CAS PubMed.
- R. K. Sharma, S. Gulati and S. Mehta, J. Chem. Educ., 2012, 89, 1316–1318 CrossRef CAS.
- R. K. Sharma, S. Sharma, S. Gulati and A. Pandey, Anal. Methods, 2013, 5, 1414–1426 RSC.
- P. Pant, R. Bansal, S. Gulati, S. Kumar and R. Kodwani, Journal of Nanostructures in Chemistry, 2016, 6, 145–157 CrossRef CAS.
- S. Kumar, Anti-Cancer Agents Med. Chem., 2016, 16, 200–211 CrossRef.
- B. Madan, S. Batra and B. Ghosh, Mol. Pharmacol., 2000, 58, 526–534 CrossRef CAS PubMed.
- M. Sardana, V. Agarwal, A. Pant, V. Kapoor, K. C. Pandey and S. Kumar, Asian Pac. J. Trop. Biomed., 2018, 8(5), 268–272 CrossRef.
- K. V. Morris and D. J. Looney, Science, 2004, 305, 1289–1292 CrossRef CAS PubMed.
- L. Aagaard and J. J. Rossi, Adv. Drug Delivery Rev., 2007, 59, 75–86 CrossRef CAS PubMed.
- R. Shahbazi, E. Asik, N. Kahraman, M. Turk, B. Ozpolat and K. Ulubayram, Nanomedinice, 2017, 12, 16 Search PubMed.
- R. Shahbazi, B. Ozpolat and K. Ulubayram, Nanomedicine, 2016, 11(10), 1287–1308 CrossRef CAS PubMed.
- Y. Xie, H. Qiao, Z. Su, M. Chen, Q. Ping and M. Sun, Biomaterials, 2014, 35, 7978–7991 CrossRef CAS PubMed.
- Y. Deng, C. C. Wang, K. W. Choy, Q. Du, J. Chen, Q. Wang, L. Li, T. K. Chung and T. Tang, Gene, 2014, 538, 217–227 CrossRef CAS PubMed.
- S. J. Lee, M. J. Kim, I. C. Kwon and T. M. Roberts, Adv. Drug Deliv. Rev., 2016, 104, 2–15 CrossRef CAS PubMed.
- R. Acharya, S. Saha, S. Ray, S. Hazra, M. K. Mitra and J. Chakraborty, Mater. Sci. Eng., C, 2017, 76, 1378–1400 CrossRef CAS PubMed.
- L. Qin, P. Yan, C. Xie, J. Huang, Z. Ren, X. Li, S. Best, X. Cai and G. Hana, Nanoscale, 2018, 10, 13432–13442 RSC.
- P. Resnier, T. Montier, V. Mathieu, J. P. Benoit and C. Passirani, Biomaterials, 2013, 34(27), 6429–6443 CrossRef CAS PubMed.
- G. Meister, M. Landthaler, Y. Dorsett and T. Tuschl, RNA, 2004, 10, 544–550 CrossRef CAS PubMed.
- R. H. Wang, J. Bai, J. Deng, C. J. Fang and X. Chen, ACS Appl. Mater. Interfaces, 2017, 9(7), 5828–5837 CrossRef CAS PubMed.
- Q. Cheng, L. Du, L. Meng, S. Han, T. Wei, X. Wang, Y. Wu, X. Song, J. Zhou, S. Zheng, Y. Huang, X. J. Liang, H. Cao, A. Dong and Z. Liang, ACS Appl. Mater. Interfaces, 2016, 8(7), 4347–4356 CrossRef CAS PubMed.
- Y. Zhao, M. L. Huan, M. Liu, Y. Cheng, Y. Sun, H. Cui, D. Z. Liu, Q. B. Mei and S. Y. Zhou, Sci. Rep., 2016, 6, 35267 CrossRef CAS PubMed.
- Y. Zhao, X. Qi, J. Chen, W. Wei, C. Yu, H. Yan, M. Pu, Y. Li, L. Miao, C. Li and J. Ren, Cancer Lett., 2017, 408, 102–111 CrossRef CAS PubMed.
- J. Kim, C. Jeong and W. J. Kim, Adv. Drug Deliv. Rev., 2016, 98, 99–112 CrossRef CAS PubMed.
- M. Sandbothe, R. Buurman, N. Reich, L. Greiwe, B. Vajen, E. Gurlevik, V. Schaffer, M. Eilers, F. Kuhnel, A. Vaquero, T. Longerich, S. Roessler, P. Schirmacher, M. P. Manns, T. Illig, B. Schlegelberger and B. Skawran, J. Hepatol., 2017, 66(5), 1012–1021 CrossRef CAS PubMed.
- X. Su, H. Wang, W. Ge, M. Yang, J. Hou, T. Chen, N. Li and X. Cao, Cancer Res., 2015, 75(14), 2875–2885 CrossRef CAS PubMed.
- L. Giunti, R. M. Da, S. Vinci, S. Gelmini, A. L. Iorio, A. M. Buccoliero, S. Cardellicchio, F. Castiglione, L. Genitori and M. M. De, Am. J. Cancer Res., 2015, 5(1), 231–242 CAS.
- H. Li, Y. Mu and J. Lu, Anal. Chem., 2014, 86, 3602–3609 CrossRef CAS PubMed.
- C. C. Harris, J. Natl. Cancer Inst., 1996, 88, 1442–1455 CrossRef CAS PubMed.
- D. Naor, Front. Immunol., 2016, 7, 39 Search PubMed.
- D. M. Kokkinakis, A. G. Brickner, J. M. Kirkwood, X. Liu, J. E. Goldwas-ser, A. Kastrama, C. Sander, D. Bocangel and S. Chada, Mol. Cancer Res., 2006, 4, 575–589 CrossRef CAS PubMed.
- S. P. Chaplot and I. D. Rupenthal, J. Pharm. Pharmacol., 2014, 66, 542 CrossRef CAS PubMed.
- Y. Shan, T. Luo, C. Peng, R. Sheng, A. Cao, X. Cao, M. Shen, R. Guo, H. Tomás and X. Shi, Biomaterials, 2012, 33, 3025–3035 CrossRef CAS PubMed.
- Y. F. Li, H. T. Zhang and L. Xin, J. Cancer Res. Clin. Oncol., 2018, 12, 16 Search PubMed.
- J. Lia, J. Huang, X. Yanga, Y. Yang, K. Quan, N. Xie, Y. Wu, C. Ma and K. Wang, Talanta, 2018, 183, 11–17 CrossRef PubMed.
- Q. Chen, K. Li, S. Wen, H. Liu, C. Peng, H. Cai, M. Shen, G. Zhang and X. Shi, Biomater, 2013, 34, 5200 CrossRef CAS PubMed.
- S. Mansouri, Y. Cuie, F. Winnik, Q. Shi, P. Lavigne, M. Benderdour, E. Beaumont and J. C. Fernandes, Biomater., 2006, 27, 2060 CrossRef CAS PubMed.
- L. S. Mbatha and M. Singh, J. Nanosci. Nanotechnol., 2019, 19, 1959–1970 CrossRef CAS PubMed.
- H. Zhu, W. Liu, Z. Cheng, K. Yao, Y. Yang, B. Xu and G. Su, Int. J. Mol. Sci., 2017, 18, 2029 CrossRef PubMed.
- Y. Lei, L. Tang, Y. Xie, Y. Xianyu, L. Zhang, P. Wang, Y. Hamada, K. Jiang, W. Zheng and X. Jiang, Nat. Commun., 2017, 8, 15130 CrossRef PubMed.
- C. Sardoa, B. Bassib, E. F. Craparoa, C. Scialabbaa, E. Cabrinib, G. Dacarrob, A. D'Agostinob, A. Tagliettib, G. Giammonaa, P. Pallavicinib and G. Cavallaro, Int. J. Pharm., 2017, 519, 113–124 CrossRef PubMed.
- R. Goyal, C. H. Kapadia, J. R. Melamed, R. S. Riley and E. S. Day, Cell. Mol. Bioeng., 2018, 11(5), 383–396 CrossRef CAS PubMed.
- A. Verma and K. Mehta, Curr. Cancer Drug Targets, 2007, 7, 559–565 CrossRef CAS PubMed.
- J. Wang, M. Thomas, P. Lin, J. X. Cheng, D. E. Matei and A. Wei, Bioconjugate Chem., 2019, 30(2), 443–453 CrossRef CAS PubMed.
- S. M. Mantovani and B. S. Moore, J. Am. Chem. Soc., 2013, 135, 18032–18035 CrossRef CAS PubMed.
- V. Nandwana, I. Samuel, G. Cooke and V. M. Rotello, Accounts Chem. Res., 2012, 46, 1000–1009 CrossRef PubMed.
- A. Sau, S. Sanyal, K. Bera, S. Sen, A. K. Mitra, U. Pal, P. K. Chakraborty, S. Ganguly, B. Satpati, C. Das and S. Basu, ACS Appl. Mater. Interfaces, 2018, 10(5), 4582–4589 CrossRef CAS PubMed.
- C. Bang and T. Thum, Int. J. Biochem. Cell Biol., 2012, 44, 2060–2064 CrossRef CAS PubMed.
- C. Roma-Rodrigues, A. R. Fernandes and P. V. Baptista, BioMed Res. Int., 2014, 2014, 1–10 CrossRef PubMed.
- J. Zhang, S. Li, L. Li, M. Li, C. Guo, J. Yao and S. Mi, J. Proteomics Bioinf., 2015, 13, 17–24 CAS.
- B. N. Hannafon and W. Q. Ding, Int. J. Mol. Sci., 2013, 14, 14240–14269 CrossRef PubMed.
- C. Roma-Rodrigues, F. Pereira, A. P. A. de Matos, M. Fernandes, P. V. Baptista and A. R. Fernandes, Nanomed. Nanotechnol. Biol. Med., 2017, 13(4), 1389–1398 CrossRef CAS PubMed.
- X. Chen, Q. Zhang, J. Li, M. Yang, N. Zhao and F. J. s Xu, ACS Nano, 2018, 12(6), 5646–5656 CrossRef CAS PubMed.
- J. H. Choi, H. J. Hwang, S. W. Shin, J. W. Choi, S. H. Um and B. K. Oh, Nanoscale, 2015, 7, 9229–9237 RSC.
- B. K. Wang, X. F. Yu, J. H. Wang, Z. B. Li, P. H. Li, H. Wang, L. Song, P. K. Chu and C. Li, Biomaterials, 2016, 78, 27–39 CrossRef CAS PubMed.
- H. J. Kim, A. Kim, K. Miyata and K. Kataoka, Adv. Drug Deliv. Rev., 2016, 104, 61–77 CrossRef CAS PubMed.
- K. J. F. Carnevale and G. F. Strouse, Bioconjugate Chem., 2018, 29(10), 3429–3440 CrossRef CAS PubMed.
- Z. Zhang, L. Wang, J. Wang, X. Jiang, X. Li, Z. Hu, Y. Ji, X. Wu and C. Chen, Adv. Mater., 2012, 24, 1418–1423 CrossRef CAS PubMed.
- J. Niua, Y. Chua, Y. F. Huang, Y. S. Chong, Z. H. Jiang, Z. W. Mao, L. H. Penga and J. Q. Gao, ACS Appl. Mater. Interfaces, 2017, 9(11), 9388–9401 CrossRef PubMed.
- J. R. Baker Jr, Hematol Am Soc Hematol Educ Program, 2009, 1, 708–719 CrossRef PubMed.
- J. Deng, N. Li, K. Mai, C. Yang, L. Yan and L. M. Zhang, J. Mater. Chem., 2011, 21, 5273–5281 RSC.
- V. M. Platt and F. C. Szoka, Mol. Pharm., 2008, 5, 474–486 CrossRef CAS PubMed.
- W. Wang, S. Huang, J. Yuan, X. Xu, H. Li, Z. Lv, W. Yu, S. Duan and Y. Hu, Mol. Pharm., 2018, 15(9), 3767–3776 CrossRef CAS PubMed.
- O. Boussif, F. Lezoualc'h, M. A. Zanta, M. D. Mergny, D. Scherman, B. Demeneix and J. P. Behr, Proc. Natl. Acad. Sci. U. S. A., 995, 92, 7297–7301 CrossRef PubMed.
- U. Lungwitz, M. Breunig, T. Blunk and A. Gö pferich, Eur. J. Pharm. Biopharm., 2005, 60, 247–266 CrossRef CAS PubMed.
- A. Li, J. Qiu, B. Zhou, B. Xu, Z. Xiong, X. Hao, X. Shi and X. Cao, Arabian J. Chem., 2018 DOI:10.1016/j.arabjc.2018.06.009.
- D. Wang and R. N. DuBois, Oncogene, 2010, 29, 781 CrossRef CAS PubMed.
- W. K. K. Wu, J. J. Y. Sung, C. W. Lee, J. Yu and C. H. Cho, Cancer Lett., 2010, 295, 7–16 CrossRef CAS PubMed.
- X. Z. Yang, J. Z. Du, S. Dou, C. Q. Mao, H. Y. Long and J. Wang, ACS Nano, 2012, 6, 771 CrossRef CAS PubMed.
- L. Zhang, W. Zheng, R. Tang, N. Wang, W. Zhang and X. Jiang, Biomaterials, 2016, 104, 269 CrossRef CAS PubMed.
- P. Wang, L. Zhang, Y. Xie, N. Wang, R. Tang, W. Zheng and X. Jiang, Adv. Sci., 2017, 4, 1700175 CrossRef PubMed.
- Y. G. Assaraf, C. P. Leamon and J. A. Reddy, Drug Resist. Updates, 2014, 17, 89 CrossRef PubMed.
- J. Akinyelu and M. Singh, J. Nanosci. Nanotechnol., 2018, 18(7), 4478–4486 CrossRef CAS PubMed.
- M. F. Press, G. Sauter, L. Bernstein, I. E. Villalobos, M. Mirlacher, J. Y. Zhou, R. Wardeh, Y. T. Li, R. Guzman, Y. Ma, J. Sullivan-Halley, A. Santiago, J. M. Park, A. Riva and D. J. Slamon, Clin. Cancer Res., 2005, 11, 6598–6607 CrossRef CAS PubMed.
- A. Berchuck, R. Whitaker, G. Olt, J. T. Soper, D. L. Clarke-Pearson, A. Kamel, R. C. Bast Jr and S. Yin, Cancer Res., 1990, 50, 4087–4091 CAS.
- R. Kotcherlakotaa, D. J. Srinivasanb, S. Mukherjeea, M. M. Haroonb, G. H. Darb, U. Venkatramanb, C. R. Patra and V. Gopal, J. Mater. Chem. B, 2017, 5, 7082–7098 RSC.
- A. A. Bapat, G. Hostetter and D. D. Von Hoff, Nat. Rev. Cancer, 2011, 11, 695–707 CrossRef CAS PubMed.
- G. O. Ceyhan, I. E. Demir, U. Rauch, F. Bergmann, M. W. Müller, M. W. Büchler, H. Friess and K. H. Schäfer, Am. J. Gastroenterol., 2009, 104, 2555–2565 CrossRef PubMed.
- P. D. Zamore, T. Tuschl, P. A. Sharp and D. P. Bartel, Cell, 2000, 101, 25–33 CrossRef CAS PubMed.
- S. M. Hammond, E. Bernstein, D. Beach and G. J. Hannon, Nature, 2000, 404, 293–296 CrossRef CAS PubMed.
- L. A. MacFarlane and P. R. Murphy, Curr. Genom., 2010, 11, 537–561 CrossRef CAS PubMed.
- Z. W. Wu, C. T. Chien, C. Y. Liu, J. Y. Yan and S. Y. Lin, J. Drug Target., 2012, 20, 551–560 CrossRef CAS PubMed.
- K. A. Avery-Kiejda, S. G. Braye, A. Mathe, J. F. Forbes and R. J. Scott, BMC Cancer, 2014, 14, 51 CrossRef PubMed.
- L. Li, L. Yuan, J. Luo, J. Gao, J. Guo and X. Xie, Clin. Exp. Med., 2013, 13, 109–117 CrossRef CAS PubMed.
- D. Ben-Shushan, E. Markovsky, H. Gibori, G. Tiram, A. Scomparin and R. Satchi-Fainaro, Drug Delivery Transl. Res., 2014, 4, 38–49 CrossRef CAS PubMed.
- H. Dong, J. Lei, L. Ding, Y. Wen, H. Ju and X. Zhang, Chem. Rev., 2013, 113(8), 6207–6233 CrossRef CAS PubMed.
- D. Sidransky, Science, 1997, 278(5340), 1054–1058 CrossRef CAS PubMed.
- D. P. Bartel, Cell, 2004, 116(2), 281–297 CrossRef CAS PubMed.
- A. Shenoy and R. H. Blelloch, Nat. Rev. Mol. Cell Biol., 2014, 15(9), 565–576 CrossRef CAS PubMed.
- M. Jin, T. Zhang, C. Liu, M. A. Badeaux, B. Liu, R. Liu, C. Jeter, X. Chen, A. V. Vlassov and D. G. Tang, Cancer Res., 2014, 74(15), 4183–4195 CrossRef CAS PubMed.
- G. A. Calin and C. M. Croce, Nat. Rev. Cancer, 2006, 6(11), 857–866 CrossRef CAS PubMed.
- B. J. Reinhart, F. J. Slack, M. Basson, A. E. Pasquinelli, J. C. Bettinger, A. E. Rougvie, H. R. Horvitz and G. Ruvkun, Nature, 2000, 403(6772), 901–906 CrossRef CAS PubMed.
- B. Li, P. Chen, Y. Chang, J. Qi, H. Fu and H. Guo, Biochem. Biophys. Res. Commun., 2016, 478(2), 739–745 CrossRef CAS PubMed.
- H. Lee, S. Han, C. S. Kwon and D. Lee, Protein Cell, 2016, 7(2), 100–113 CrossRef CAS PubMed.
- D. J. Li, Y. Chen and Z. Liu, Chem. Soc. Rev., 2015, 44(22), 8097–8123 RSC.
- D. Sturm, S. Bender, D. T. W. Jones, P. Lichter, J. Grill, O. Becher, C. Hawkins, J. Majewski, C. Jones, J. F. Costello, A. Iavarone, K. Aldape, C. W. Brennan, N. Jabado and S. M. Pfister, Nat. Rev. Cancer, 2014, 14, 92–107 CrossRef CAS PubMed.
- L. Galluzzi, O. Kepp, M. G. Vander Heiden and G. Kroemer, Nat. Rev. Drug Discov., 2013, 12, 829–846 CrossRef CAS PubMed.
- J. Yue, T. J. Feliciano, W. Li, A. Lee and T. W. Odom, Bioconjugate Chem., 2017, 28(6), 1791–1800 CrossRef CAS PubMed.
- B. Du, X. Gu, X. Han, G. Ding, Y. Wang, D. Li, E. Wang and J. Wang, ChemMedChem, 2017, 12, 1768–1775 CrossRef CAS PubMed.
- K. Zagorovsky, L. Y. T. Chou and W. C. M. Chan, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 13600–13605 CrossRef CAS PubMed.
- L. Y. Chou, K. Zagorovsky and W. C. Chan, Nat. Nanotechnol., 2014, 9, 148–155 CrossRef CAS PubMed.
- R. Prado-Gotor and E. Grueso, Phys. Chem., 2011, 3, 1479–1489 Search PubMed.
- E. Grueso, P. Perez-Tejeda, R. Prado-Gotor and C. Cerrillos, J. Phys. Chem. C, 2014, 4416–4428 CrossRef CAS.
- E. Grueso, P. Perez-Tejeda, R. M. Giráldez-Pérez, R. Prado-Gotor and F. Muriel-Delgado, J. Colloid Interface Sci., 2018, 529, 65–76 CrossRef CAS PubMed.
- J. B. Wolinsky, Y. L. Colson and M. W. Grinstaff, J. Controlled Release, 2012, 159, 14–26 CrossRef CAS PubMed.
- M. Ihle, M. Trautmann, H. Kuenstlinger, S. Huss, C. Heydt, J. Fassunke, E. Wardelmann, S. Bauer, H. Schildhaus, R. Buettner and S. Merkelbach-Bruse, Mol. Oncol., 2015, 9, 1421–1433 CrossRef CAS PubMed.
- S. Rietwyk and D. Peer, ACS Nano, 2017, 11, 7572–7586 CrossRef CAS PubMed.
- C. Zhang, Y. Yong, L. Song, X. Dong, X. Zhang, X. Liu, Z. Gu, Y. Zhao and Z. Hu, Adv. Healthcare Mater., 2016, 5, 2776–2787 CrossRef CAS PubMed.
- S. Kang, K. Kang, H. Huh, H. Kim, S. J. Chang, T. Jung Park, Ki S. Chang, D.-H. Min and H. Jang, ACS Appl. Mater. Interfaces, 2017, 9(40), 35268–35278 CrossRef CAS PubMed.
- Z. Zhou, W. Zheng, J. Kong, Y. Liu, P. Huang, S. Zhou, Z. Chen, J. Shi and X. Chen, Nanoscale, 2017, 9, 6846–6853 RSC.
- W. Fan, N. Lu, C. Xu, Y. Liu, J. Lin, S. Wang, Z. Shen, Z. Yang, J. Qu, T. Wang, S. Chen, P. Huang and X. Chen, ACS Nano, 2017, 11, 5864–5872 CrossRef CAS PubMed.
- J. Shi, M. Sun, X. Sun and H. Zhang, J. Mater. Chem. B, 2016, 4, 7845–7851 RSC.
- R. Yuvakkumar, V. Elango, V. Rajendran and N. Kannan, Digest Journal of Nanomaterials and Biostructures, 2011, 6, 1771 Search PubMed.
- X. Zhao, J. Zhong, C. Wei, C. W. Lin and T. Ding, Front. Microbiol., 2017, 8, 580 Search PubMed.
- X. Zhao, C. W. Lin, J. Wang and D. H. Oh, J. Microbiol. Biotechnol., 2014, 24, 297–312 CrossRef CAS PubMed.
- X. Zhao and C. W. Lin, RSC Adv., 2017, 7, 48554–48560 RSC.
- S. Qian, M. Lin, W. Ji, H. Yuan, Y. Zhang, Z. Jing, J. Zhao, J. F. Masson and W. Peng, ACS Sens, 2018, 3(5), 929–935 CrossRef CAS PubMed.
- L. He, M. D. Musick, S. R. Nicewarner, F. G. Salinas, S. J. Benkovic, M. J. Natan and C. D. Keating, J. Am. Chem. Soc., 2000, 122(38), 9071–9077 CrossRef CAS.
- S. Szunerits, J. Spadavecchia and R. Boukherroub, Rev. Anal. Chem., 2014, 33, 153 CAS.
- B. Xu, A. Li, X. Hao, R. Guo, X. Shi and X. Cao, RSC Adv., 2018, 8, 1265 RSC.
- Y. Li, H. Wang, K. Wang, Q. Hu, Q. Yao, Y. Shen, G. Yu and G. Tang, Small, 2017, 13, 1602697 CrossRef PubMed.
- C. Ma, L. Shi, Y. Huang, L. Shen, H. Peng, X. Zhu and G. Zhou, Biomater. Sci., 2017, 5, 494–501 RSC.
| This journal is © The Royal Society of Chemistry 2019 |