Open Access Article
Open Access ArticlePreparation of ZnO nanoparticle loaded amidoximated wool fibers as a promising antibiofouling adsorbent for uranium(VI) recovery†
Haichuan Maa,
Fan Zhangb,
Qiaoyu Lia,
Guobing Chena,
Sheng Hub and
Haiming Cheng *ac
*ac
aThe Key Laboratory of Leather Chemistry and Engineering of Ministry of Education, Sichuan University, Chengdu 610065, China
bInstitute of Nuclear Physics and Chemistry, China Academy of Engineering Physics, Mianyang 621900, Sichuan, China
cNational Engineering Laboratory for Clean Technology of Leather Manufacture, Sichuan University, Chengdu 610065, Sichuan, China. E-mail: chenghaiming@scu.edu.cn
First published on 11th June 2019
Abstract
In this study, nano-ZnO loaded amidoxime-functionalized wool fibers (wool-AO@ZnO) were synthesized by radiation-induced copolymerization and in situ co-precipitation as a novel adsorbent with good antibiofouling properties for uranium recovery. The prepared adsorbent was characterized by SEM-EDS and FT-IR spectroscopy. Batch adsorption experiments showed that the prepared wool-AO@ZnO have a good uranium adsorption property at pH 6.0–9.0. Meanwhile, wool-AO@ZnO displayed a good inhibition of aerobic bacteria such as S. aureus and E. coli; of anaerobic bacteria; of sulfate reducing bacteria; of fungus C. albicans and of mildew Aspergillus niger. After four cyclic cultivations with microorganisms, the inhibition rate of wool-AO@ZnO to E. coli and S. aureus still remains at 77.8% and 84.9%, respectively; and a good adsorption capacity for uranium(VI) was maintained. The prepared wool-AO@ZnO could be a promising antibiofouling adsorbent for the recovery of uranium(VI) from seawater.
1. Introduction
Nuclear energy plays an important role in the energy industry. Uranium is the most essential resource for nuclear power. Extraction of uranium from seawater has been paid more and more attention in many countries since the terrestrial uranium supply is limited. Although the uranium concentration in seawater is only approximately 3 ppb, the total amount of uranium in seawater is over 4.5 billion tons, which is around 1000 times larger than the entire proven uranium reserves on land.1Several approaches have been developed for uranium extraction including solvent extraction,2 flotation,3 ion exchange,4 biological collection,5 precipitation6 and adsorption.7 In comparison with other methods, the adsorption technique has been thought of as a promising method due to its ease of operation, advantageous uranium enrichment, and low cost. However, since the complication of the ocean climate and the presence of large excess competing ions in seawater, it is clear that the key roles of the adsorbents in uranium extraction from seawater are their selectivity to uranium ions, extraction efficiency, and its physical stability in the ocean environment. Amidoxime groups based adsorbents have been reported showing good selectivity toward uranium, therefore, various matrixes have been functionalized with amidoxime groups for uranium adsorption including polymeric fibers, hydrogels, metal organic frameworks, and natural biopolymers.8–10 Amidoxime functionalized wool fibers have been developed for recovery of uranium due to its good hydrophilicity and good physical stability.10–13
Recently, the problem of biofouling in decreasing adsorption performance and the corrosion of adsorbent has been noted by Park et al.14 and Hu et al.15 Due to the concentration of uranium in seawater is critical low, the adsorbents were immersed in the seawater usually for several weeks. Therefore, a variety of microorganisms such as aerobic bacteria, anaerobic bacteria and fungus will adhere on the surface of the adsorbents and thus the biofouled surface might occur. Biofouling can decrease the adsorbents stabilities, adsorption efficiency, and elution efficiency. It was noted that the key reason of biofouling is the rapidly binding of the microorganisms on the adsorbent matrix in the ocean environment. Therefore, the development of good antimicrobial adsorbents should be a potential strategy to prevent the biofouling. For instance, Zhang et al.16 prepared a polymeric nonwoven fabric co-functionalized with guanidine and amidoxime, and found that the introduced guanidine groups could inhibit the growth of Escherichia coli (E. coli) on the adsorbent surface. Quaternary ammonium derivatives, guanidine derivatives, and inorganic nanoparticles have been employed on wool fibers for obtaining antibacterial functions.16–18 In the other hand, inorganic nanoparticles such as Ag, ZnO, and TiO2 showed a widely range of antibacterial activity. ZnO have shown excellent antibacterial effect on fabric films and polymer fibers.19–21 In our previous work,11 nano TiO2 loaded amidoximated wool fibers could inhibit the growth of E. coli and Staphylococcus aureus (S. aureus); and maintain good uranium adsorption capacity. The two investigated bacteria are belonging to aerobic bacteria. However, besides aerobic bacteria, anaerobic bacteria and fungi are also found in the ocean. Therefore, the biofouling resistance properties of the promising uranium extraction adsorbents should be evaluated for inhibiting microorganisms, including aerobic bacteria, anaerobic bacteria, and fungi.
Herein, a novel adsorbent wool-AO@ZnO was prepared by loading ZnO nanoparticles on amidoxime functionalized wool fibers by in situ co-precipitation and radiation-induced graft copolymerization. ZnO nanoparticles were used for the enhancement of antibacterial properties to amidoxime-functionalized wool fibers. The uranium adsorption property and antibacterial characteristics such as inhibiting of aerobic bacteria (Gram-negative and Gram-positive), anaerobic bacteria, and fungi were evaluated.
2. Experimental
2.1. Reagents and materials
Merino wool fibers were purchased from Qige Textile Company (Tongxiang, China). Acrylonitrile and methacrylate were purchased from Kelong Chemicals (Chengdu, China). Zinc oxide (ZnO) (size 30 ± 10 nm) was purchased from Aladdin (Shanghai China). The stock solution of uranium (1000 mg L−1) was prepared by dissolving UO2(NO3)2·6H2O in deionized water. Uranium(VI) standard solution at 1000 ppm concentration was supplied by National Analysis and Testing Center (Beijing China). All other regents were analytical grade and used without further purification.2.2. Preparation of wool-AO@ZnO10–12
Pristine wool fibers were degreased by acetone. After degreasing, the wool fibers were immersed into 1000 mL of 1% NaOH (m/v) and treated at 50 °C for 10 min to open the cuticle of the wool fibers, which were then taken out and washed with deionized water till the pH value of the solution was at neutral. Then the wool fibers were dried to constant weight at 40 °C.Co-irradiation grafting method was used for preparation of nano-ZnO particles loaded amidoxime functionalized wool fibers (wool-AO@ZnO). Briefly, 1.0 g of the pretreated wool fibers were mixed with 7 mL of acrylonitrile, 3 mL of methacrylic acid, and 3 mL of deionized water, and a certain amount of ZnO nanoparticles (5–20 mg) in a weighing bottle, which then was sealed with an aluminum foil. The sealed bottle was then radiated with 200 kGy irradiation dose under electron beam (dose rate 15 kGy m−1, beam current 12 kW). Afterwards, the wool fibers were taken out and washed with acetone and deionized water three times, respectively; to remove homopolymers (polyacrylonitrile) and un-reacted monomers. The product finally dried at 40 °C to constant weight that designated as wool-AN@ZnO.
To obtain amidoxime groups, 1.0 g of wool-AN@ZnO was placed into 50 mL of neutralized NH2OH·HCl methanol solution (methanol: water = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pH 7.0). The mixture was refluxed at 70 °C for 5 h, after which the wool fibers was washed with methanol thoroughly and dried at 40 °C. The obtained adsorbent was designated as wool-AO@ZnO. As controls, wool-AN and wool-AO fibers without adding ZnO nanoparticles were prepared following the same procedures as described above.
1, pH 7.0). The mixture was refluxed at 70 °C for 5 h, after which the wool fibers was washed with methanol thoroughly and dried at 40 °C. The obtained adsorbent was designated as wool-AO@ZnO. As controls, wool-AN and wool-AO fibers without adding ZnO nanoparticles were prepared following the same procedures as described above.
2.3. Characterization
The FT-IR spectra of pristine wool fibers, wool-AN@ZnO and wool-AO@ZnO were recorded on a Nicolet iS10 FT-IR spectrophotometer (Thermo Scientific, USA) by the KBr pellet method in a range from 4000 to 600 cm−1. The morphologies of the fibers were observed by a JSM-F7500 Scanning electron microscope (SEM) (Japan Electron Optical Laboratory Co., LTD, Japan). The elemental compositions of the samples were measured by a 51-XMX0019 X-Max Energy-dispersive X-ray spectroscopy (EDXS) (Oxford Instruments, UK). The nitrogen adsorption isotherms of the samples were detected by a Tristar 3000 surface area and porosity analyzer (Micromeritics, USA) at 77 K. The surface area of samples was calculated by Barrett–Emmett–Teller (BET) equation. All tests were run in triplicate.2.4. Batch adsorption experiments
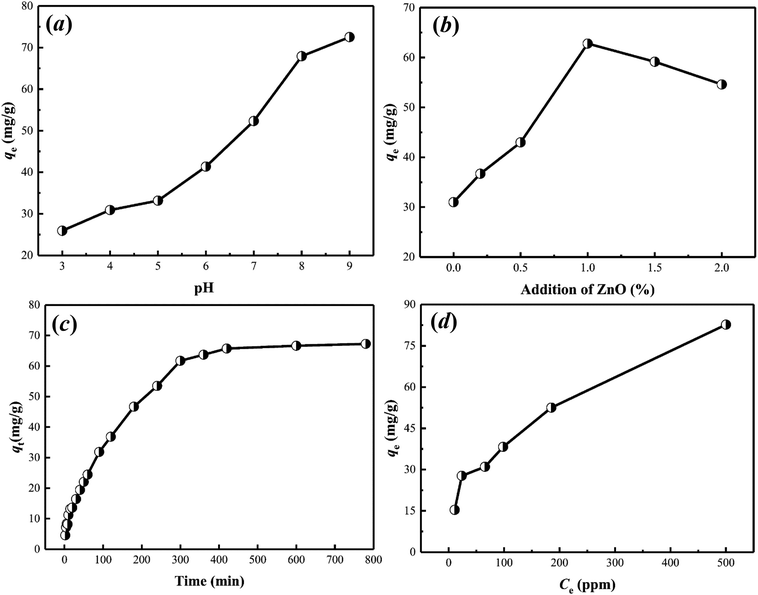
Batch experiment was applied for studying uranium(VI) adsorption by wool-AO@ZnO. Briefly, 10.0 mg of wool-AO@ZnO absorbent and 20 mL of a set concentration of uranium(VI) solution were placed in a 50 mL plastic flask, shaking at a WS-300R thermostat shaker (Wiggens, Germany) with 100 rpm for 24 h at 25 °C. While studying the effect of initial pH on the adsorption, the pH value of the solution varied from 3.0 to 9.0, which was adjusted by 0.1 M NaOH and 0.1 M HCl. All adsorption experiments were conducted with an initial uranium(VI) concentration of 50 ppm for 24 h at 25 °C. The kinetic studies were performed at pH 8.0 with temperature varying from 20–40 °C. The isotherm studies were performed with the initial uranium(VI) concentrations varying from 10–500 ppm. The concentration of uranium(VI) before and after adsorption was determined by a 2300 DV inductively coupled plasma atomic emission spectrometry (ICP-AES) (PerkinElmer, USA).The amount of the adsorption to uranium (mg g−1) was calculated by eqn (1):
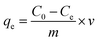 | (1) |
2.5. Antibacterial evaluation
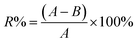 | (2) |
To investigate the antibacterial activity of wool-AO@ZnO under prolonged bacterial environment, 15 mg of wool-AO@ZnO was applied to follow the experiments of successive uranium(VI) adsorption.
3. Results and discussion
3.1. Characteristics of wool-AO@ZnO
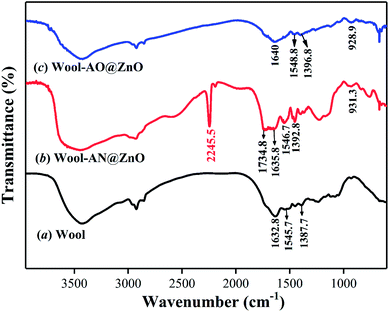
The FT-IR spectra of pristine wool, wool-AN@ZnO and wool-AO@ZnO were shown in Fig. 1. The peak around 3300 cm−1 belongs to –OH and –NH2 groups of wool matrix. The peaks at 1632.8, 1545.7, and 1387.7 cm−1 are assigned to stretching vibration of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (amide I), bending vibration of –N–H (amide II), and stretching vibration of C–N (amide III), respectively.13 The FT-IR profile of wool-AN@ZnO showed a new peak at 2245.5 cm−1 which ascribes to stretching vibration of –C
O (amide I), bending vibration of –N–H (amide II), and stretching vibration of C–N (amide III), respectively.13 The FT-IR profile of wool-AN@ZnO showed a new peak at 2245.5 cm−1 which ascribes to stretching vibration of –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N. Meanwhile, the stretching vibration peak (1734.8 cm−1) attributed to C
N. Meanwhile, the stretching vibration peak (1734.8 cm−1) attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and the broad strong peak at 932.9 cm−1 belongs to the bending vibration of –OH of carboxylic acid, indicating that acrylonitrile and methacrylic acid was successfully grafted onto wool fibers. After amidoximation, the disappearance of the peak at 2245.5 cm−1 and a new observed peak at 929.9 cm−1 belongs to the stretching of N–O band of amidoxime group.10 The results suggested that the amidoximation reaction was success.
O, and the broad strong peak at 932.9 cm−1 belongs to the bending vibration of –OH of carboxylic acid, indicating that acrylonitrile and methacrylic acid was successfully grafted onto wool fibers. After amidoximation, the disappearance of the peak at 2245.5 cm−1 and a new observed peak at 929.9 cm−1 belongs to the stretching of N–O band of amidoxime group.10 The results suggested that the amidoximation reaction was success.
SEM and EDS profiles of pristine wool and wool-AO@ZnO were shown in Fig. 2. A clear and smooth scale layer could be observed on the SEM profiles of pristine wool (Fig. 2a), illustrating that the structure of wool was relatively complete before co-irradiation reaction. While after co-irradiation the exterior structure of wool-AO was damaged seriously (Fig. 2b) and the surface of wool-AO became rough since the polymers were grafted on. Moreover, the introduction of ZnO nanoparticles imparted the surface of wool fibers uniformly covered with a size of 20–40 nm nanoparticles (Fig. 2d). The EDS profiles of wool-AO and wool-AO@ZnO characterized the elemental composition on the fiber surface. In comparison with wool-AO, element Zn was observed on the surface of wool-AO@ZnO (Fig. 2f), which further confirming that the ZnO nanoparticles were successfully loaded onto the wool fibers.
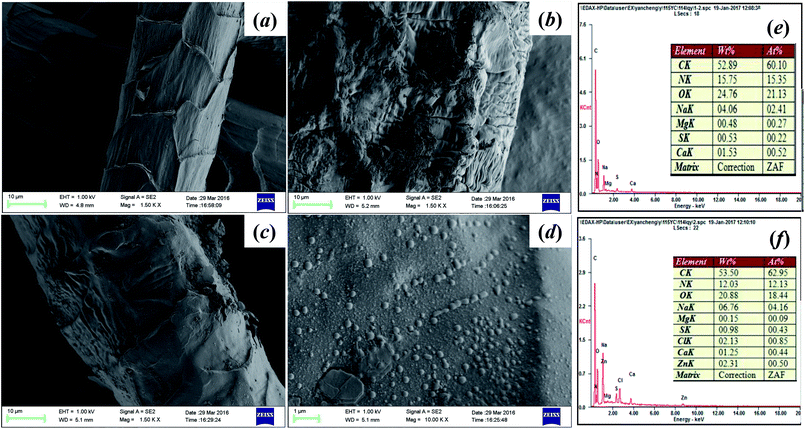 | ||
| Fig. 2 The SEM and EDS profiles of wool samples. (a) Pristine wool; (b) wool-AO; (c and d) wool-AO@ZnO; (e) EDS profiles of wool-AO; (f) EDS profiles of wool-AO@ZnO. | ||
3.2. Uranium(VI) adsorption experiments
Kinetic models like pseudo-first-order, pseudo-second-order, and Elovich models31 were applied for fitting the experimental data (Fig. S1†). The calculated parameters are listed in Table 1. The results indicate that the kinetic behavior of uranium(VI) adsorption on wool-AO@ZnO is fitted well with pseudo-second-order model, with the correlation coefficient (R2) value of 0.99. The result implies that chemical adsorption was responsible for the adsorption of uranium(VI) by wool-AO@ZnO, which is similar to our previous report.11
| Pseudo-first order | Pseudo-second order | Elovich | ||||||
|---|---|---|---|---|---|---|---|---|
| k1 | qe | R2 | k2 | qe | R2 | α | β | R2 |
| 12.9482 | 31.0270 | 0.8952 | 0.0002 | 75.5858 | 0.9964 | 3.0538 | 0.0802 | 0.9208 |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| kL (L g−1) | qm (mg g−1) | R2 | kF | n | R2 |
| 0.0095 | 95.6022 | 0.9286 | 6.2864 | 2.5560 | 0.8796 |
3.3. Antimicrobial evaluation
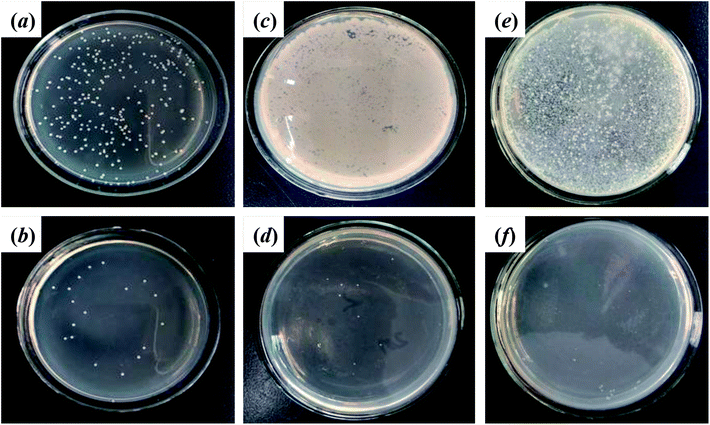
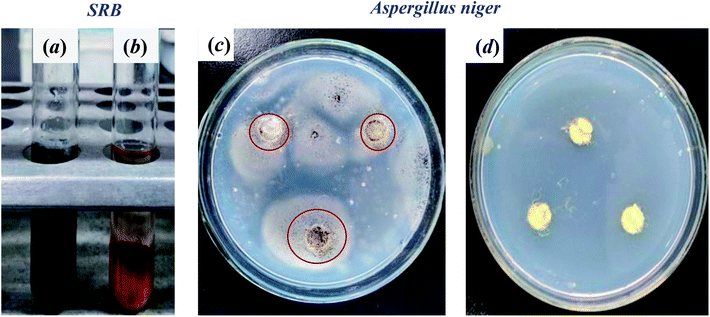
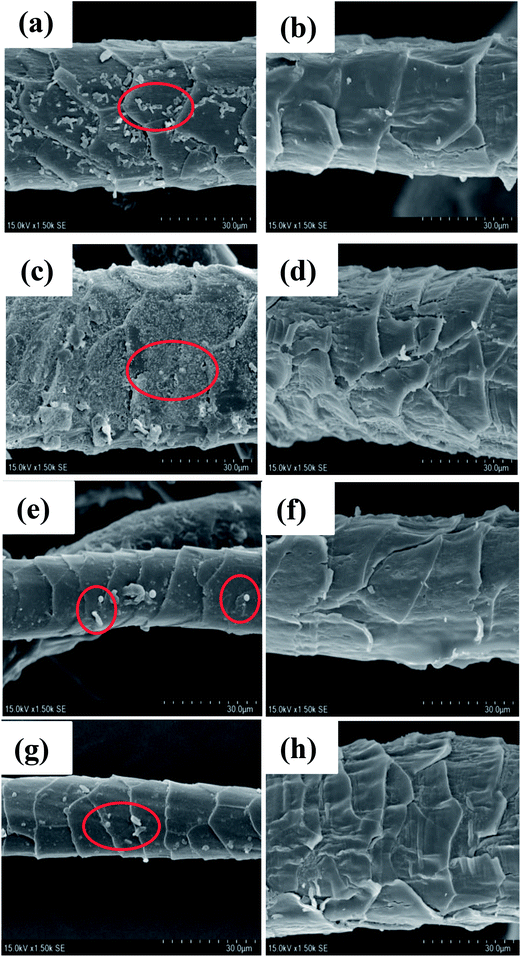
After 24 h cultivation with E. coli, S. aureus, SRB, and C. albicans, the SEM profiles clearly showed that much more microorganisms were attached on the pristine wool fibers than on wool-AO@ZnO (Fig. 6). It suggests that the introduction of ZnO nanoparticles can impart the wool fibers with good antibacterial properties.
3.4. Antibacterial assessment by cyclic cultivation
The antibacterial properties of wool-AO@ZnO and its uranium(VI) adsorption were evaluated in 4 consecutive cyclic cultivations with bacteria. New living bacterial solutions were replaced after each measurement cycle. It can be seen that the inhibition rate of wool-AO@ZnO decreased after cultivation with E. coli and S. aureus (Fig. 7). After 4 cyclic cultivations, the inhibition rate decreased to 77.8% (E. coli) and 84.9% (S. aureus). The difference of their cell structure may cause the inhibition rate of S. aureus was higher than that of E. coli.39 After four cyclic cultivations, uranium(VI) adsorption of wool-AO@ZnO still maintained at 46.5 mg g−1 (E. coli) and 57.6 mg g−1 (S. aureus). The results indicated that the wool-AO@ZnO has a good reusability property (Table 3).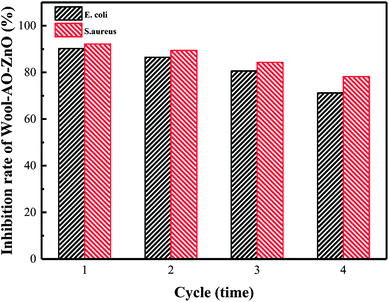 | ||
| Fig. 7 Antibacterial properties of wool-AO@ZnO after cyclic cultivation and uranium adsorption and desorption. | ||
| Sample | E. coli (mg g−1) | S. aureus (mg g−1) |
|---|---|---|
| Wool-AO | 17.7 | 15 |
| Wool-AO-@ZnO | 46.5 | 57.6 |
4. Conclusions
To sum up, amidoxime-functionalized wool fibers loaded with nano-ZnO was successfully prepared by in situ coprecipitation and radiation-induced copolymerization. The introduction of ZnO nanoparticles not only made the adsorbent an increase of uranium adsorption capacity but also imparted the adsorbent a good antimicrobial property. The adsorption process followed the Langmuir isotherm and psuedo-second-order kinetic. The maximum uranium adsorption capacity of wool-AO@ZnO was 96.2 mg g−1 from the Langmuir isotherm. Wool-AO@ZnO showed excellent antimicrobial properties to aerobic bacteria, anaerobic bacteria, and fungi. The inhibition of the bacteria S. aureus and E. coli growth could be up to 99.9%, and the inhibition of C. albicans could be at 92.6%. The inhibition of anaerobic bacteria SRB could be up to 95%. After 4 cycles' cultivation, the inhibition rate of E. coli and S. aureus can still keep the initial inhibition rate at 77.8% and 84.9%, while still maintained good adsorption capacity for uranium(VI). Therefore, the prepared wool-AO@ZnO showed a good prospect for extraction of uranium(VI) from seawater where multiple species of microorganisms exist.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully thank to the financial support of the National Key Technology R&D Program of China (No. 2017YFB0308400) and the Opening Project of Key Laboratory of Leather Chemistry and Engineering, (Sichuan University), Ministry of Education, China (No. 20826041C4159).References
- V. Jegatheesan, B. K. Pramanik, J. Chen, D. Navaratna, C. Y. Chang and L. Shu, Bioresour. Technol., 2016, 204, 202–212 CrossRef CAS PubMed.
- P. G. Barbano and L. Rigali, Anal. Chim. Acta, 1978, 96, 199–201 CrossRef CAS.
- J. C. B. S. Amaral and C. A. Morais, Miner. Eng., 2010, 23, 498–503 CrossRef CAS.
- T. V. Molchanova, L. I. Vodolazov, V. A. Peganov and V. G. Litvinenko, At. Energy, 2001, 90, 208–212 CrossRef CAS.
- Q. Li, Y. Liu, X. Cao, C. Pang, Y. Wang, Z. Zhang, Y. Liu and M. Hua, J. Radioanal. Nucl. Chem., 2012, 293, 67–73 CrossRef CAS.
- Y. A. El-Nadi and J. A. Daoud, J. Nucl. Radiochem. Sci., 2004, 5, 11–15 CrossRef CAS.
- P. A. Brown, S. A. Gill and S. J. Allen, Water Res., 2000, 34, 3907–3916 CrossRef CAS.
- B. F. Parker, Z. Zhang, L. Rao and J. Arnold, Dalton Trans., 2018, 47, 639–644 RSC.
- J. Qian, S. Zhang, Y. Zhou, P. Dong and D. Hua, RSC Adv., 2015, 5, 4153–4161 RSC.
- Z. Yin, J. Xiong, M. Chen, S. Hu and H. Cheng, J. Radioanal. Nucl. Chem., 2016, 307, 1471–1479 CrossRef CAS.
- F. Zhang, M. Chen, S. Hu and H. Cheng, J. Radioanal. Nucl. Chem., 2017, 314, 1927–1937 CrossRef CAS.
- J. Wen, Q. Li, H. Li, M. Chen, S. Hu and H. Cheng, Ind. Eng. Chem. Res., 2018, 57, 1826–1833 CrossRef CAS.
- M. Monier, N. Nawar and D. A. Abdel-Latif, J. Hazard. Mater., 2010, 184, 118–125 CrossRef CAS PubMed.
- J. Park, G. A. Gill, J. E. Strivens, L. J. Kuo, R. T. Jeters, A. Avila, J. R. Wood, N. J. Schlafer, C. J. Janke and E. A. Miller, Ind. Eng. Chem. Res., 2016, 55, 4328–4338 CrossRef CAS.
- J. Hu, H. Ma, Z. Xing, X. Liu, L. Xu, R. Li, C. Lin, M. Wang, J. Li and G. Wu, Ind. Eng. Chem. Res., 2015, 55, 4118–4124 CrossRef.
- H. Zhang, L. Zhang, X. Han, L. Kuang and D. Hua, Ind. Eng. Chem. Res., 2018, 57, 1662–1670 CrossRef CAS.
- M. Ranjbar-Mohammadi, M. Arami, H. Bahrami, F. Mazaheri and N. M. Mahmoodi, Colloids Surf., B, 2010, 76, 397–403 CrossRef CAS PubMed.
- P. Zhu and G. Sun, J. Appl. Polym. Sci., 2010, 93, 1037–1041 CrossRef.
- J. L. Castro-Mayorga, M. J. Fabra, A. M. Pourrahimi, R. T. Olsson and J. M. Lagaron, Food Bioprod. Process., 2017, 101, 32–44 CrossRef CAS.
- M. Wang, M. Zhang, L. Pang, C. Yang, Y. Zhang, J. Hu and G. Wu, J. Colloid Interface Sci., 2019, 537, 91–100 CrossRef CAS PubMed.
- H. Rodriguez-Tobias, G. Morales and D. Grande, Mater. Chem. Phys., 2016, 182, 324–331 CrossRef CAS.
- C. C. Okoro, Liq. Fuels Technol., 2015, 33, 1366–1372 CAS.
- E. Guibal, C. Roulph and P. L. Cloirec, Water Res., 1992, 26, 1139–1145 CrossRef CAS.
- Z. Niu, Q. Fan, W. Wang, J. Xu, L. Chen and W. Wu, Appl. Radiat. Isot., 2009, 67, 1582–1590 CrossRef CAS PubMed.
- W. P. Li, X. Y. Han, X. Y. Wang, Y. Q. Wang, W. X. Wang, H. Xu, T. S. Tan, W. S. Wu and H. X. Zhang, Chem. Eng. J., 2015, 279, 735–746 CrossRef CAS.
- A. Degen and M. Kosec, J. Eur. Ceram. Soc., 2000, 20, 667–673 CrossRef CAS.
- H. R. Shakur, K. R. E. Saraee, M. R. Abdi and G. Azimi, J. Mater. Sci., 2016, 51, 1–14 CrossRef.
- Ü. H. Kaynar, M. Ayvacıklı, S. Ç. Kaynar and Ü. Hiçsönmez, J. Radioanal. Nucl. Chem., 2014, 299, 1469–1477 CrossRef.
- M. Khajeh and E. Jahanbin, Chemom. Intell. Lab. Syst., 2014, 135, 70–75 CrossRef CAS.
- W. Li, L. D. Troyer, S. S. Lee, J. Wu, C. Kim, B. J. Lafferty, J. G. Catalano and J. D. Fortner, ACS Appl. Mater. Interfaces, 2017, 9, 13163–13172 CrossRef CAS PubMed.
- M. Solgy, M. Taghizadeh and D. Ghoddocynejad, Ann. Nucl. Energy, 2015, 75, 132–138 CrossRef CAS.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- H. Freundlich, J. Phys. Chem., 1906, 57, 1100–1107 Search PubMed.
- R. Brayner, R. Ferrari-Iliou, N. Brivois, S. Djediat, M. F. Benedetti and F. Fiévet, Nano Lett., 2006, 6, 866–870 CrossRef CAS PubMed.
- A. Sirelkhatim, Nano-Micro Lett., 2015, 7, 219–242 CrossRef CAS PubMed.
- Y. Wang, Q. Zhang, C. L. Zhang and L. Ping, Food Chem., 2012, 132, 419–427 CrossRef CAS PubMed.
- M. M. Hassan, ChemistrySelect, 2017, 2, 504–512 CrossRef CAS.
- D. Yu, W. Tian, B. Sun, Y. Li and W. Tian, Mater. Lett., 2015, 151, 1–4 CrossRef CAS.
- E. V. Skorb, L. I. Antonouskaya, N. A. Belyasova, D. G. Shchukin, H. Möhwald and D. V. Sviridov, Appl. Catal., B, 2008, 84, 94–99 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Adsorption isotherms and adsorption kinetics fitting data. See DOI: 10.1039/c9ra03777b |
| This journal is © The Royal Society of Chemistry 2019 |