Open Access Article
Open Access ArticleEfficient production of coenzyme Q10 from acid hydrolysate of sweet sorghum juice by Rhodobacter sphaeroides
Y. Wang a,
S. Chena,
J. Liua,
P. Lvb,
D. Caic and
G. Zhao*a
a,
S. Chena,
J. Liua,
P. Lvb,
D. Caic and
G. Zhao*a
aFermentation Engineering Technology Research Center of Heibei Province, College of Bioscience & Bioengineering, Hebei University of Science and Technology, No. 26 Yuxiang Road, Yuhua District, Shijiazhuang 050018, PR China. E-mail: gqzhao18@126.com
bInstitute of Millet Crops, Hebei Academy of Agriculture and Forestry Sciences, Hebei Branch of National Sorghum Improvement Center, Shijiazhuang 050035, PR China
cNational Energy R&D Center for Biorefinery, Beijing University of Chemical Technology, Beijing 100029, People's Republic of China
First published on 18th July 2019
Abstract
In order to achieve efficient bioconversion of biomass-derived sugars, acid hydrolysate of sweet sorghum juice (SSJAH) containing abundant fermentable sugars was used for coenzyme Q10 (CoQ10) fermentation by Rhodobacter sphaeroides CQ-09-1. The synthesis of CoQ10 was facilitated when the initial concentration of total sugar was 80.00 g L−1. And the highest CoQ10 titer was obtained when the pH and temperature were maintained at 7.00 and 30.00 °C, respectively. Moreover, corn steep powder (CSP) was proved to be an efficient nitrogen & salt supplement to SSJAH. Under the optimized conditions, the titer of CoQ10 reached 141.95 mg L−1 in a fed-batch fermentation. The CoQ10 titer reported was about two times higher than that obtained in the previous study using wild strains. This process introduces a potential way to produce CoQ10 using the concept of biorefinery, while making full use of sweet sorghum juice (SSJ).
1 Introduction
Coenzyme Q, which is composed of a benzoquinone group and a varying length side chain of isoprenoid groups, is an essential component of the electron transfer system in the plasma membrane of prokaryotes and the inner mitochondrial membrane of eukaryotes.1 And CoQ10 containing ten isoprenoid units in the side chain has been widely accepted as a potent antioxidative dietary supplement which is related to energy boosting, immune enhancement and ease of hypertension.2,3CoQ10 could be produced by extraction from animal tissues4 or chemical synthesis,5 however, in order to produce biologically potent CoQ10 without optical isomers and reduce the production cost, microbial fermentation has been considered the most viable approach.6,7 Various microorganisms including bacteria,8,9 moulds,10 yeasts11 and genetically engineered Escherichia coli,12 have been reported for CoQ10 production. In particular, photosynthetic bacteria such as Rhodobacter1,13 exhibit excellent ability in bio-synthesis of CoQ10. Recently, in order to achieve the improvement of CoQ10 titer, extensive efforts have been made in designing alternative biosynthetic pathway by metabolic engineering and strain development by natural isolation & chemical mutagenesis.14 However, cheap and alternative substrates for CoQ10 production have been barely studied.
Sweet sorghum (Sorghum bicolor L. Moench) has been considered as an attractive raw material in biorefinery process, and it offers great advantages: greater biomass yield per hectare, high photosynthetic efficiency and wide adaptability to harsh environmental conditions.15 The content of fermentable sugars in sweet sorghum juice (SSJ) from the stalk could reach 160.00–180.00 g L−1.16,17 Furthermore, corn steep powder (CSP) obtained from corn starch processing provides an inexpensive source of proteins, amino acids, minerals, vitamins and trace elements.18 A previous study indicated that CSP could be used as a satisfactory source of nitrogen and metal ions to replace yeast extract (YE) & salts in L-lactic acid fermentation, while resulting in 83.75% reduction in the production cost.19 Thus, this study focused on the cost-efficient production of CoQ10 using cheap and alternative substrates using the concept of biorefinery, and the conditions and modes of the fermentation with R. sphaeroides CQ-09-1 were optimized.
2 Methods
2.1 Raw materials
Sweet sorghum, which was kindly provided by the Institute of Millet Crops of Hebei Academy of Agricultural and Forestry Sciences, was harvested in the national high-tech industrial development zone of Shijiazhuang (114° E, 38° N), October 2018. A three-roller mill (SY-20, Guangzhou Fukang Co., Ltd, China) was used to obtain SSJ, and the husks and leaves were stripped from the stem by hand before juice squeezing. The fresh juice obtained was boiled for 5.00 min to achieve denaturation and precipitation of heteropolymeric proteins, and the suspended solids was removed by the vacuum suction filtration coupled filter paper using diatomite as filter-aids. The clarified SSJ contained 156.60 g L−1 sucrose, 19.60 g L−1 glucose and 19.20 g L−1 fructose. The acid hydrolysis of SSJ was conducted as previously reported,20 and the acid hydrolysate of sweet sorghum juice (SSJAH) which consisted of 102.02 g L−1 glucose and 101.62 g L−1 fructose was stored at −20.00 °C before use.YE and sodium L-glutamate were purchased from Aobox Biotechnology Co., Ltd. (Beijing, China). CSP was provided by Beijing Mannafeed International Group (Beijing, China). Biotin, niacin and thiamine hydrochloride were obtained from Sigma (Shanghai, China). All other chemicals used in this study were of analytical grade and commercially available.
2.2 Microorganism and culture media
The R. sphaeroides CQ-09-1, which was derived from CICC 10287 (China Center of Industrial Culture Collection, Beijing, China), was screened by evolutionary engineering and used for CoQ10 production in this study.The medium for agar slant contained 3.00 g L−1 glucose, 8.00 g L−1 YE, 2.00 g L−1 NaCl, 1.30 g L−1 KH2PO4, 0.13 g L−1 MgSO4, 150.00 μg L−1 biotin, 1.00 mg L−1 niacin, 1.00 mg L−1 thiamine hydrochloride and 15.00 g L−1 agar. And the composition of inoculum preparation medium was the same as that of agar slant except the addition of agar.
To investigate the effect sugar concentrations on CoQ10 fermentation, mixed sugar with total sugar concentrations ranging from 40.00–120.00 g L−1 were adopted, and the weight ratio of individual sugars was the same as that of SSJAH. In addition, the media contained 2.00 g L−1 NaCl, 3.00 g L−1 KH2PO4, 6.30 g L−1 MgSO4, 2.00 g L−1 CaCO3, 8.00 g L−1 YE, 3.00 g L−1 sodium L-glutamate, 150.00 μg L−1 biotin and 1.00 mg L−1 niacin.
Also, different nitrogen sources (CSP, ammonium nitrate, ammonium sulfate and urea) were adopted to examine the feasibility of a cheap alternative to YE, and the nitrogen content was the same as that of 8.00 g L−1 YE. Moreover, the concentration optimization of CSP which was selected as the suitable nitrogen source was conducted, and CSP concentrations ranging from 8.00 g L−1 to 16.00 g L−1 were adopted. Besides nitrogen source, the media used also contained 2.00 g L−1 NaCl, 3.00 g L−1 KH2PO4, 6.30 g L−1 MgSO4, 2.00 g L−1 CaCO3, 120.00 g L−1 mixed sugar, 3.00 g L−1 sodium L-glutamate, 150.00 μg L−1 biotin and 1.00 mg L−1 niacin.
The media used for the optimization of temperature & pH contained 2.00 g L−1 NaCl, 3.00 g L−1 KH2PO4, 6.30 g L−1 MgSO4, 2.00 g L−1 CaCO3, 120.00 g L−1 mixed sugar, 12.00 g L−1 CSP, 3.00 g L−1 sodium L-glutamate, 150.00 μg L−1 biotin and 1.00 mg L−1 niacin.
In batch and fed-batch fermentation, the media consisted of SSJAH (containing 120.00 g L−1 total sugar), 2.00 g L−1 NaCl, 3.00 g L−1 KH2PO4, 6.30 g L−1 MgSO4, 2.00 g L−1 CaCO3, 12.00 g L−1 CSP, 3.00 g L−1 sodium L-glutamate, 150.00 μg L−1 biotin and 1.00 mg L−1 niacin. Concentrated SSJAH containing a total sugar concentration of 420.00 g L−1 was fed as supplement of carbon source in fed-batch fermentation.
In particular, the pH of all media was adjusted to 7.00 before use.
2.3 Culture conditions
The strain R. sphaeroides CQ-09-1 was maintained on agar slants, and the stock culture was transferred to fresh medium monthly. After incubation at 30.00 °C for 48.00 h, the stock culture was stored at 4.00 °C. Inoculum preparation was carried out in 250.00 mL conical flasks with a working volume of 100.00 mL, which were incubated at 30.00 °C and 220.00 rpm for 96.00 h on a rotary shaker, and the pH was maintained at 7.00. The seed culture was then inoculated into fermentation medium with an inoculum volume of 10.00% (v/v).The optimization of total sugar concentration, nitrogen source, temperature and pH were conducted in 250.00 mL conical flasks with a working volume of 100.00 mL. In particular, different temperatures (from 28.00 °C to 34.00 °C) and pH levels (from 6.80 to 7.00) were adopted. And other conditions were the same as those of inoculum preparation.
In order to confirm the optimized conditions obtained and to further achieve improvement in CoQ10 titer, batch and fed-batch fermentations were conducted in dark condition, and a 5 L fermentor (SGB-5L, Changzhou Sungod Bio-technology & Engineering Equipment Co., Ltd., Jiangsu, China) was used. The pH and agitation rate were maintained at 7.00 and 400.00 rpm, respectively, and an aeration rate of 0.50 vvm with a temperature of 30.00 °C was adopted.
2.4 Analytical methods
Three parallel replicates were conducted in all fermentations, and deviations from the mean are given in tables or provided as error bars in figures. The cell density was measured by a spectrophotometer (UV-1100, Beijing Eternal Cause Instrument Co., Ltd, China) at 600.00 nm,13 and a calibration curve which indicated the relationship between OD600 and dry cell weight (DCW) (1.00 OD600 = 0.61 g DCW L−1) was adopted. Dry cells were obtained by centrifugation and lyophilization in this study. Monosaccharides in fermentation broth were determined by high-performance liquid chromatography (HPLC) equipped with a Sugar-Pak 1 Column (Waters Ltd., USA) at 80.00 °C. A refractive index (RI) detector was used and deionized water was adopted as mobile-phase (0.50 mL min−1).21 To compare the amounts of amino acids in YE, CSP and SSJAH, an amino acid analyzer (Sykam S433D, German) equipped with a cation separation column (LCA K06/Na 1.6 mm × 150 mm; Sykam GmbH, Eresing, German) was used to determine the amounts of amino acids. The amounts of metal ions and phosphorus were measured by inductively coupled plasma-optical emission spectrometer (ICP-OES) (PerkinElmer Optima 8300, USA).The extraction and determination of CoQ10 were according to methods previously reported.22 The cells of R. sphaeroides CQ-09-1 were ruptured at 75.00 °C for 15.00 min, and 200.00 μL HCl (pH 2.00) was added into 1.00 mL fermentation broth. A centrifugation (5000.00 rpm, 10.00 min) was further conducted to collect solid phase, and an solution (ethyl acetate/ethanol = 5/3, v/v) with an amount of 4.00 mL was used for CoQ10 extraction. The mixture, which was vortexed vigorously for 15.00 min, was kept in dark and incubated for 1.00 h at room temperature. Then, the supernatant obtained by centrifugation (8000.00 rpm, 5.00 min) was filtered through a 0.22 μm filter and further analyzed by HPLC equipped with a Synergi Max-RP 80A column (Phenomenex, USA) at 35.00 °C. A UV detector at 275.00 nm was used and a mixture of methanol/ethanol (3/7, v/v) was used as the mobile phase (0.80 mL min−1).
3 Results and discussion
3.1 Optimization of the total sugar concentration in SSJAH
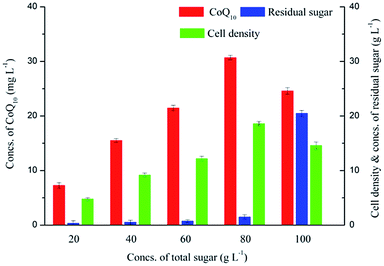
Various carbon sources including glucose, fructose, xylose, acetate and malate have been reported in fermentation with R. sphaeroides.23–25 SSJAH used in this study mainly contained glucose and fructose, and the effects of total sugar concentrations on CoQ10 fermentation were investigated to avoid the carbon catabolite repression (CCR). As shown in Fig. 1, when the initial concentration of total sugar increased from 20.00 g L−1 to 80.00 g L−1, the CoQ10 titer was correspondingly enhanced from 7.28 mg L−1 to 30.70 mg L−1. However, when the initial concentration of total sugar reached 100.00 g L−1, a CoQ10 titer of 24.60 mg L−1, which decreased 19.87% of that obtained at 80.00 g L−1 total sugar, was achieved. The tendency of cell density was similar to that of CoQ10 titer, and the a top value of 18.60 g L−1 was obtained with 80.00 g L−1 total sugar. Moreover, the residual sugar concentrations were kept at relative low levels when the total sugar concentration ranged from 20.00 g L−1 to 80.00 g L−1. And a significant increase in residual sugar concentration was observed with a total sugar concentration of 100.00 g L−1. The results indicated that a total sugar concentration of 80.00 g L−1 should be adopted with high CoQ10 titer and cell density while controlling the residual sugar concentration at a relative low level.3.2 The feasibility of a cheap alternative to YE in CoQ10 fermentation
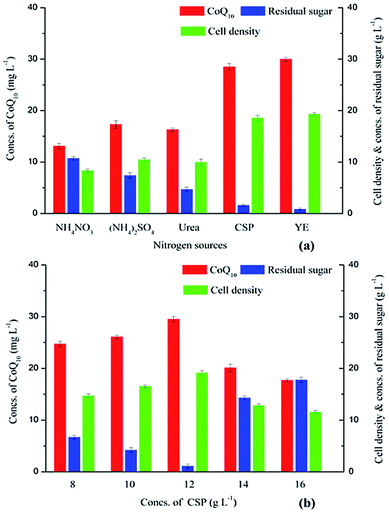
Nitrogen source significantly affects the growth of R. sphaeroides.26 Two inorganic nitrogen sources and three organic nitrogen sources were used in this study as shown in Fig. 2. In particular, the nitrogen contents of various nitrogen sources were kept at the same level which was equal to 8.00 g L−1 YE. For inorganic nitrogen sources, ammonium sulfate was more suitable than ammonium nitrate for CoQ10 fermentation with R. sphaeroides CQ-09-1, and the CoQ10 titer and cell density with ammonium sulfate reached 17.30 mg L−1 and 10.48 g L−1, respectively, which were higher than those obtained with ammonium nitrate (13.10 mg L−1 and 8.37 g L−1). Moreover, considerable amounts of residual sugar were observed (10.70 g L−1 and 7.40 g L−1) when ammonium nitrate and ammonium sulfate were used. For organic nitrogen sources, the CoQ10 titer and cell density with urea were 16.30 mg L−1 and 9.96 g L−1, respectively. And CoQ10 titer and cell density obtained with CSP reached 28.50 mg L−1 and 18.56 g L−1, respectively, which were 95.00% and 96.02% of those provided by YE (30.00 mg L−1 and 19.33 g L−1). Moreover, the residual sugar concentration was 1.60 g L−1 with CSP, which was only 34.04% of that with urea (4.70 g L−1), and YE provided a residual sugar concentration of 0.85 g L−1. The results indicated CSP could replace YE without a significant drop in product titer and cell density, and the residual sugar concentration was kept at a relative low level.In fact, CSP has been used as an effective nutrition supplement in CoQ10 production by Agrobacterium tumefaciens.27 As shown in Fig. 2b, the effect of CSP concentration on CoQ10 production was further studied. The CoQ10 titer increased from 24.70 mg L−1 to 29.50 mg L−1 when the CSP concentration was enhanced from 8.00 g L−1 to 12.00 g L−1. However, the CoQ10 titers obtained with 14.00 g L−1 CSP and 16.00 g L−1 CSP were only 68.14% and 60.00% of that provided by 12.00 g L−1 CSP. The cell density trend was similar to that of product titer, and a top value of 19.13 g L−1 was obtained with 12.00 g L−1 CSP. As expected, when the CSP concentration was enhanced from 8.00 g L−1 to 16.00 g L−1, the residual sugar first decreased from 6.70 g L−1 to 1.10 g L−1, then increased to 17.80 g L−1, and the bottom value was achieved with 12.00 g L−1 CSP. It indicated that the suitable CSP concentration for CoQ10 fermentation was 12.00 g L−1. The negative effect provided by high concentration of CSP may be the excess organic salts, which needs further research.
Constituents of metal ions and phosphorus of SSJAH (containing a total sugar concentration of 80.00 g L−1), CSP and YE were determined in this study. As shown in Table 1, except for Na+, the concentrations of K+, Ca2+, Mg2+, Mn2+ and P in CSP solution were all higher than those in YE solution. The lack of Na+ could be supplemented by the addition of NaCl and sodium L-glutamate in the fermentation medium. Moreover, the contents of K+, Ca2+, Mg2+ and Mn2+ in SSJAH solution approached or exceed the levels of those in YE solution. As shown in Table 2, amino acids composition of 8.00 g L−1 YE, 12.00 g L−1 CSP and SSJAH solution (containing a total sugar concentration of 80.00 g L−1) were analyzed. Only the contents of Glu, Gly, Tyr, Trp, Lys, Arg and Gln in CSP solution were significantly lower than those in YE solution, and all other amino acids contents in CSP solution were at or above the levels of those in YE solution. Fortunately, the contents of Glu (204.16 μmol L−1), Gly (78.88 μmol L−1), Tyr (97.92 μmol L−1), Trp (50.24 μmol L−1), Lys (80.16 μmol L−1), Arg (178.88 μmol L−1) and Gln (1400.32 μmol L−1) in CSP solution could be an efficient supplement when CSP and SSJAH were used together for the CoQ10 fermentation. It is worth to mention that the total amino acid content in the combination of CSP and SSJAH solution reached 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 730.08 μmol L−1 which was 1.57 times higher than that in YE solution. Thus, it indicated that CSP can serve as an efficient nitrogen & salts supplement to SSJAH in the replacement of YE.
730.08 μmol L−1 which was 1.57 times higher than that in YE solution. Thus, it indicated that CSP can serve as an efficient nitrogen & salts supplement to SSJAH in the replacement of YE.
| Constituents (mg L−1) | SSJAH (containing a total sugar concentration of 80.00 g L−1) | YE solution (containing 8.00 g L−1 YE) | CSP solution (containing 12.00 g L−1 CSP) |
|---|---|---|---|
| K | 103.52 ± 5.16 | 130.74 ± 3.36 | 317.19 ± 6.62 |
| Na | 7.72 ± 1.21 | 139.48 ± 2.82 | 27.70 ± 2.24 |
| Ca | 57.16 ± 2.26 | 14.28 ± 1.15 | 29.09 ± 1.95 |
| Mg | 106.44 ± 2.52 | 1.28 ± 0.36 | 73.25 ± 0.62 |
| Mn | 0.20 ± 0.03 | — | 0.29 ± 0.04 |
| P | 12.68 ± 0.61 | 109.80 ± 4.46 | 239.34 ± 7.46 |
| Amino acid (μmol L−1) | YEa | CSPb | SSJAHc |
|---|---|---|---|
| a 8.00 g L−1 YE solution.b 12.00 g L−1 CSP solution.c SSJAH containing a total sugar concentration of 80.00 g L−1. | |||
| Asp | 236.32 ± 0.52 | 161.53 ± 0.76 | 693.42 ± 0.36 |
| Thr | 345.13 ± 0.38 | 271.95 ± 0.91 | 260.11 ± 0.55 |
| Ser | 267.36 ± 0.82 | 200.18 ± 1.11 | 440.32 ± 1.21 |
| Glu | 568.00 ± 1.55 | 190.82 ± 0.61 | 204.16 ± 0.96 |
| Gly | 1769.70 ± 2.26 | 529.29 ± 0.96 | 78.88 ± 0.82 |
| Ala | 980.98 ± 1.82 | 1991.54 ± 2.11 | 190.88 ± 1.51 |
| Val | 466.89 ± 0.72 | 833.54 ± 1.85 | 324.16 ± 1.26 |
| Met | 88.50 ± 2.24 | 189.85 ± 1.36 | 44.96 ± 0.78 |
| Ile | 358.72 ± 0.61 | 430.57 ± 1.45 | 112.64 ± 0.61 |
| Leu | 737.12 ± 1.54 | 1326.96 ± 1.16 | 124.48 ± 0.83 |
| Tyr | 328.65 ± 2.06 | 121.92 ± 0.91 | 97.92 ± 0.65 |
| Phe | 384.82 ± 3.16 | 446.19 ± 0.74 | 67.20 ± 1.11 |
| His | 164.65 ± 0.92 | 115.46 ± 0.51 | 127.04 ± 0.92 |
| Trp | 84.90 ± 0.81 | 13.69 ± 1.17 | 50.24 ± 0.53 |
| Lys | 608.17 ± 1.46 | 271.47 ± 2.01 | 80.16 ± 0.97 |
| Arg | 524.06 ± 1.81 | 226.56 ± 1.65 | 178.88 ± 1.24 |
| Pro | 175.33 ± 0.96 | 834.72 ± 0.62 | 98.08 ± 0.77 |
| Gln | 24.90 ± 1.51 | — | 1400.32 ± 2.22 |
| Total amino acid | 8114.19 ± 25.15 | 8156.24 ± 19.89 | 4573.84 ± 17.30 |
3.3 The effects of temperature and pH on CoQ10 fermentation
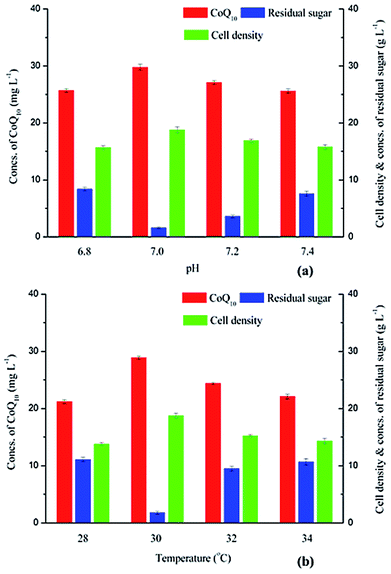
As a facultative microorganism, R. sphaeroides can be cultivated by photoheterotrophy, photoautotrophy, chemoheterotrophy and fermentation.28 And the effects of pH and temperature on CoQ10 production using R. sphaeroides were investigated under fermentation conditions in this study.CoQ10 fermentations using R. sphaeroides CQ-09-1 were conducted at different pHs, and the effects of pH on CoQ10 titer, residual sugar and cell density were shown in Fig. 3a. The CoQ10 titer first increased from 25.70 mg L−1 to 29.80 mg L−1, then decreased to 25.60 mg L−1, when the pH level was enhanced from 6.80 to 7.40, and the top value of CoQ10 titer (29.80 mg L−1) was obtained at pH 7.00. The change of cell density was similar to that of product titer, and the highest cell density (18.80 g L−1) was also achieved when the pH was maintained at 7.00. Jeong et al.29 has reported that the CoQ10 production was growth-associated, possibly as a primary metabolite, and the product titer increased as the cells accumulated. The concentration of residual sugar decreased from 8.40 g L−1 to 1.60 g L−1 when pH was increased from 6.80 to 7.00. However, the residual sugar concentration reached 3.60 g L−1 and 7.60 g L−1, when the pHs were maintained at 7.20 and 7.40, respectively. The results indicated that the pH of 7.00 was the optimized value for CoQ10 production under fermentation conditions.
In order to investigate the effects of temperature on CoQ10 production, different temperatures ranging from 28.00 °C to 34.00 °C were adopted for R. sphaeroides CQ-09-1 cultivation. As shown in Fig. 3b, the CoQ10 titer increased from 21.20 mg L−1 to 28.90 mg L−1 when the temperature was enhanced from 28.00 °C to 30.00 °C. However, the CoQ10 titers decreased to 24.40 mg L−1 and 22.10 mg L−1, when the temperatures reached 32.00 °C and 34.00 °C, respectively. As expected, the change of cell density was similar to that of product titer, and the top value (18.81 g L−1) was obtained at 30.00 °C. For the concentration of residual sugar, a bottom value of 1.80 g L−1 was obtained at 30.00 °C. And the residual sugar concentrations were 11.10 g L−1, 9.50 g L−1 and 10.70 g L−1, when the temperatures were maintained at 28.00 °C, 32.00 °C and 34.00 °C, respectively. Thus, the temperature was maintained at 30.00 °C in the following fermentations conducted in fermentor to achieve further improvement of CoQ10 titer.
3.4 Batch and fed-batch fermentation with R. sphaeroides CQ-09-1
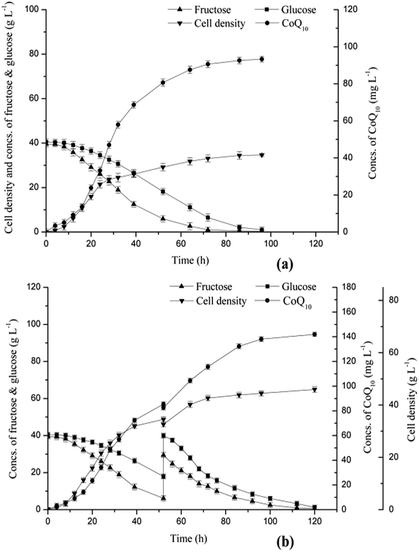
A 5 L fermentor with an initial broth volume of 2.00 L were adopted for batch and fed-batch fermentations. As shown in Fig. 4a, the CoQ10 titer increased as the cells of R. sphaeroides CQ-09-1 accumulated. The CoQ10 titer was rapidly enhanced to 58.00 mg L−1 within 32.00 h, and the cell density reached 24.50 g L−1 at 32.00 h. The rising tendency of product titer slowed down with the slow growth of cell density after 32.00 h. A final product titer of 93.30 mg L−1 was obtained at 96.00 h, and the final cell density was 34.67 g L−1. It is worth to mention that, although the concentrations of sugars were at the same level (40.00 g L−1), fructose and glucose were sequentially metabolized by R. sphaeroides CQ-09-1, and a lag phase as long as 12.00 h in glucose metabolism was observed. The fructose was exhausted at 72.00 h, and the residual glucose concentration declined to 1.01 g L−1 at 96.00 h.A logistic growth model combined with the Luedeking–Piret equation has been reported to model the batch production of CoQ10 in the cultivation of R. sphaeroides, and the results indicated CoQ10 production was a primary metabolite.30 A longer cell growing stage would facilitate the accumulation of biomass, which would lead to a higher product titer. Thus, a fed-batch fermentation was conducted to further improve the titer of CoQ10. As shown in Fig. 4b, from 0.00 h to 52.00 h, the CoQ10 titer reached 85.48 mg L−1, and the cell density accumulated to 34.52 g L−1. Because of the difference of utilization efficiency in glucose and fructose, the residual sugar concentrations of glucose and fructose were 17.96 g L−1 and 6.15 g L−1, respectively, at 52.00 h. The supplement medium containing 420.00 g L−1 total sugar was added to the fermentation broth, and the concentrations of glucose and fructose reached 39.98 g L−1 and 29.50 g L−1, respectively. From 50.00 h to 120.00 h, a CoQ10 titer of 141.95 mg L−1 was finally obtained, and the cell density reached 45.98 g L−1. Interestingly, a simultaneous utilization of glucose and fructose was observed after 50.00 h. It was speculated that the cells preferring to utilize glucose survived and reproduced in the fermentation medium, and an evolution of strain CQ-09-1 could be achieved, which caused an enhanced capability of using glucose.
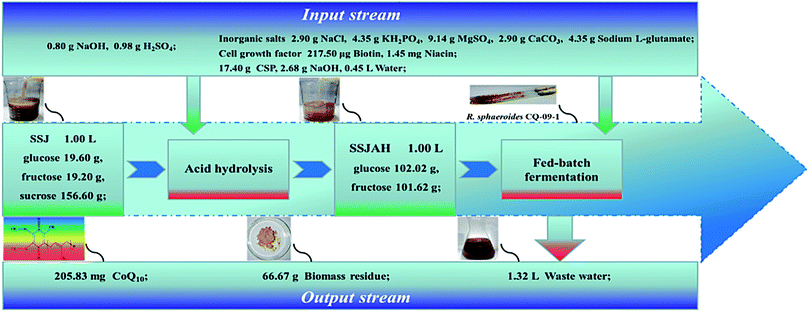
Comparing with the industrial price of yeast extract (YE) ($ 9.2 kg−1), the cost of industrial corn steep liquor is only $ 0.18 kg−1.31 In addition, the yield of fermentable sugars obtained from sweet sorghum could reach 7.6 Mg ha−1 which was higher than that of corn (5.7 Mg ha−1).32 It indicated the raw material cost could be reduced by 54.06% using SSJ and CSP for CoQ10 production. Fig. 5 briefly showed a mass balance of the experimental process, and a CoQ10 yield of 205.83 mg L−1 SSJ was obtained by fed-batch fermentation, which indicated the feasibility of this process for cost-efficient CoQ10 production from abundant biomass-derived sugars. The comparison of CoQ10 production based on different carbon sources using wild strains was shown in Table 3, and the CoQ10 concentration, maximum cell density and overall productivity in this work were all higher than the results obtained in previously literatures. However, to achieve the commercially production of CoQ10,14 further improvement of fermentation technology or metabolic regulation of R. sphaeroides CQ-09-1 will be necessary.
| Microorganism | Carbon source | Fermentation type | CoQ10 concentration (mg L−1) | Maximum cell density (g L−1) | Overall productivity (mg L−1 h−1) | Reference |
|---|---|---|---|---|---|---|
| R. sphaeroides BCRC 13100 | Molasses | Fed-batch | 45.65 | 10.14 | 0.420 | Yen and Shih1 |
| R. rubrum ATCC 25852 | Malic acid | Batch | 10.81 | 1.71 | 0.113 | Tian et al.33 |
| P. dinitrificans NRRL B-3785 | Glycerol | Batch | 14.12 | 9.65 | 0.147 | Bule and Singhal34 |
| Rhodotorula glutinis | Glucose | Fed-batch | 60.10 | 53 | 0.501 | Balakumaran and Meenakshisundaram35 |
| R. sphaeroides CQ-09-1 | SSJAH | Fed-batch | 141.95 | 45.98 | 1.183 | This work |
4 Conclusions
A cost-efficient way to produce CoQ10 from abundant SSJ was introduced in this study. An optimization of total sugar concentration, nitrogen source, cultivation temperature and pH was conducted. In particular, CSP, which was used as a nitrogen & salt supplement to SSJAH, was proved to be an efficient alternative to YE. Based on SSJ with the optimized conditions, the CoQ10 titer reached 93.30 mg L−1 within 96.00 h in a batch fermentation. To further improve the product titer, a fed-batch fermentation was carried out, and a CoQ10 titer of 141.95 mg L−1 was obtained. Totally, the product yield reached 205.83 mg L−1 SSJ, and the simultaneous utilization of glucose and fructose was achieved in the fed-batch fermentation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to acknowledge the establishment of the Fermentation Engineering Technology Research Center of Heibei Province. This work was supported by the Science and Technology Research Fund for Youth of Colleges and Universities in Hebei Province Science (Grant No. QN2019115) and the Postdoctoral Science Foundation of China (Grant No. 2017M610037; 2018T110036).Notes and references
- H. W. Yen and T. Y. Shih, Bioprocess Biosyst. Eng., 2009, 394, 711 CrossRef.
- L. Ernster and G. Dallner, BBA, Mol. Basis Dis., 1995, 1271, 195 CrossRef.
- F. M. Sohet, A. M. Neyrinck, B. D. Pachikian, F. C. de Backer, L. B. Bindels, P. Niklowitz, T. Menke, P. D. Cani and N. M. Delzenne, Biochem. Pharmacol., 2009, 78, 1391 CrossRef CAS.
- L. Serge, S. Nathalie and B. Piotr, Eur. J. Lipid Sci. Technol., 2009, 111, 135 CrossRef.
- I. Ehud and E. Doron, Pure Appl. Chem., 1998, 60, 89 Search PubMed.
- J. H. Choi, Y. W. Ryu and J. H. Seo, Appl. Microbiol. Biotechnol., 2005, 68, 9 CrossRef CAS.
- P. C. Corinne, M. B. Adam and J. J. M. Vincent, Trends Biotechnol., 2007, 25, 514 CrossRef.
- Y. Natori and T. Nagasaki, Agric. Biol. Chem., 1981, 45, 2175 CAS.
- H. Yoshida, Y. Kotani and K. Ochiai, J. Gen. Appl. Microbiol., 1998, 44, 19 CrossRef CAS.
- K. Kroll, E. Shekhova, D. J. Mattern, A. Thywissen, I. D. Jacobsen, M. Strassburger, T. Heinekamp, E. Shelest, A. A. Brakhage and O. Kniemeyer, Mol. Microbiol., 2016, 101, 92 CrossRef CAS.
- M. Kawamukai, Biotechnol. Appl. Biochem., 2009, 53, 217 CrossRef CAS.
- H. S. Zahiri, S. H. Yoon, J. D. Keasling, S. H. Lee, S. W. Kim, S. C. Yoon and Y. C. Shina, Metab. Eng., 2008, 8, 406 CrossRef PubMed.
- J. Zhang, D. Gao, J. Cai, H. Liu and Z. Qi, Biochem. Eng. J., 2018, 135, 98 CrossRef CAS.
- S. Shukla and K. Dubey, 3 Biotech, 2018, 8, 249 CrossRef.
- E. M. Rubin, Nature, 2008, 454, 841 CrossRef CAS PubMed.
- L. Laopaiboon, S. Nuanpeng, P. Srinophakun, P. Klanrit and P. Laopaiboon, Bioresour. Technol., 2009, 100, 4176 CrossRef CAS.
- C. V. Ratnavathi, S. K. Chakravarhy, V. V. Komala, U. D. Chavan and J. V. Patil, Sugar Tech, 2011, 13, 399 CrossRef CAS.
- A. C. Silva, P. M. Guimaraes, J. A. Teixeira and L. Domingues, J. Ind. Microbiol. Biotechnol., 2010, 37, 973 CrossRef CAS.
- Y. Wang, C. Chen, D. Cai, Z. Wang, P. Qin and T. Tan, Bioresour. Technol., 2016, 218, 1098 CrossRef CAS.
- Y. Wang, J. Chang, D. Cai, Z. Wang, P. Qin and T. Tan, J. Chem. Technol. Biotechnol., 2017, 92, 1848 CrossRef CAS.
- H. Ye, Y. Jin, S. Lin, M. Liu, Y. Yang, M. Zhang, P. Zhao and G. Jones, Int. J. Biol. Macromol., 2012, 50, 1315 CrossRef CAS PubMed.
- W. Lu, L. Ye, H. Xu, W. Xie, J. Gu and H. Yu, Biotechnol. Bioeng., 2014, 111, 761 CrossRef CAS PubMed.
- G. Kars, U. Gündüz, M. Yücel, G. Rakhely, K. L. Kovacs and İ. Eroğlu, Int. J. Hydrogen Energy, 2009, 34, 2184 CrossRef CAS.
- T. Liu, L. Zhu, W. Wei and Z. Zhou, Int. J. Hydrogen Energy, 2014, 39, 4215 CrossRef CAS.
- W. Pattanamanee, W. Choorit, D. Kantachote and Y. Chisti, Int. J. Hydrogen Energy, 2012, 37, 15855 CrossRef CAS.
- L. Hakobyan, L. Gabrielyan and A. Trchounian, Int. J. Hydrogen Energy, 2012, 37, 6519 CrossRef CAS.
- S. J. Ha, S. Y. Kim, J. H. Seo, D. K. Oh and J. K. Lee, Appl. Microbiol. Biotechnol., 2007, 74, 974 CrossRef CAS PubMed.
- H. Koku, I. Eroğlu, U. Gündüz, M. Yücel and L. Türker, Int. J. Hydrogen Energy, 2002, 27, 1315 CrossRef CAS.
- S. K. Jeong, V. T. Dao, N. Kien and J. K. Kim, J. Fish. Sci. Technol., 2008, 11, 219 CAS.
- H. W. Yen, C. Y. Feng and J. L. Kang, Appl. Biochem. Biotechnol., 2010, 160, 1441 CrossRef CAS.
- P. Maddipati, H. K. Atiyeh, D. D Bellmer and R. L. Huhnke, Bioresour. Technol., 2011, 102, 6494 CrossRef CAS.
- T. H. Regassa and C. S. Wortmann, Biomass Bioenergy, 2014, 64, 348 CrossRef CAS.
- Y. Tian, T. Yue, Y. Yuan, P. K. Soma and Y. M. Lo, Biochem. Eng. J., 2010, 51, 160 CrossRef CAS.
- M. V. Bule and R. S. Singhal, Food Sci. Biotechnol., 2011, 20, 607 CrossRef CAS.
- P. A. Balakumaran and S. Meenakshisundaram, Prep. Biochem. Biotechnol., 2015, 45, 398 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |