Open Access Article
Open Access ArticleEfficient removal of crystal violet dye using EDTA/graphene oxide functionalized corncob: a novel low cost adsorbent†
Huan Wang *,
Xin Lai,
Wei Zhao,
Youning Chen,
Xiaoling Yang,
Xiaohua Meng and
Yuhong Li
*,
Xin Lai,
Wei Zhao,
Youning Chen,
Xiaoling Yang,
Xiaohua Meng and
Yuhong Li
College of Chemistry and Chemical Engineering, Xianyang Normal University, Xianyang 712000, China. E-mail: 237463169@qq.com
First published on 16th July 2019
Abstract
In this study, EDTA functionalized corncob (EDTA-corncob) and EDTA/graphene oxide functionalized corncob (EDTA-GO/corncob) were prepared using disodium ethylenediamine tetraacetic acid and the graphene oxide immersion method. EDTA-corncob and EDTA-GO/corncob were characterized by SEM and FTIR spectroscopy. On this basis, the adsorption properties of EDTA-corncob and EDTA-GO/corncob were studied with crystal violet as the adsorbate. The optimum adsorption conditions were determined by the effect of samples on the adsorption properties of crystal violet at different times, temperatures and pH, and the reusability of the samples was studied. The results showed that adsorption capacity of crystal violet on EDTA-GO/corncob was higher compared with natural corncob and EDTA-corncob. The most suitable pH value of the solution is about 6.0, the adsorption equilibrium time is 200 min. EDTA-GO/corncob can be reused eight times. This study indicated that EDTA-GO/corncob is a reusable adsorbent for rapid, low-cost, and efficient removal of dye from waste water.
1 Introduce
Water pollution is one of the major environmental problems nowadays.1 Dyes are one of the important and basic pollutants in water pollution. With the accelerated process of industrialization development, more and more wastewater containing dyes is discharged from various human activities and causes serious threats to the environment.2–4A wide range of physicochemical techniques, such as electrocoagulation, photocatalytic process, flocculation, ozonation, adsorption, and membrane filtration, have been employed to dispose of the wastewater.5–10 Among these various purification treatments, liquid-phase adsorption is believed to be the most effective way to remove organic dyes from wastewater. A variety of materials, which include chitosan,11 leaves,12 crab shell,13 waste coal gangue,14 peach gum polysaccharide,15 ganoderma lucidum,16 clays,17 and Cucumis sativus peel,18 were prepared or used as adsorbents for removal of dyes from water.
Biomass waste, such as corncob, has great potential to form inexpensive and environmental friendly adsorbents due to the large quantities produced, chemical and mechanical stability, high surface area and structural properties. But generally the adsorption capacity of natural corncob is not ideal. Therefore, many modification methods have been invented to increase the capacity of corncob based adsorbents.19,20 Ma et al. reported that corncob was converted into a novel magnetic adsorbent (MCA) for removal of anionic and cationic dyes.19 Mesoporous activated carbon that was prepared from corncob was used as adsorbent for removal of ammonium from groundwater.20 Hyperbranched polyamide modified corncob was synthesized and used as adsorbent for Cr(VI) with maximum adsorption capacity that could reach up to 131.6 mg g−1.21 EDTA is a kind of complexing agent, EDTA-modified may produce adsorbents with strong metal-complexing property, thus, EDTA-modified materials have been widely used for heavy metal and dyes removal, such as bamboo activated carbon,22 β-cyclodextrin/chitosan,23 electrospun polyacrylonitrile nanofibers.24 Since the graphene was first reported in 2004, it has been widely investigated due to its excellent electrical conductivity, thermal stability, mechanical strength and adsorption capacity.25,26
In this study, in addition to introducing EDTA onto corncob for enhancing adsorption capacity, graphene oxide would be also added. EDTA functionalized corncob (EDTA-corncob) and EDTA/graphene oxide functionalized corncob (EDTA-GO/corncob) were prepared successfully used to remove various dyes from water. Methylene blue, crystal violet, acridine orange, methyl red, acid chrome blue K, rhodamine B and orange IV, as typical anionic and cationic dye, were selected as the pollutants. At meantime, to fully understand the adsorption behavior of EDTA-corncob and EDTA-GO/corncob, studies were carried out under various parameters such as adsorbent dosage, pH, contact time. Adsorption kinetics of EDTA-corncob and EDTA-GO/corncob were investigated by pseudo first order model and pseudo second order model, respectively. Furthermore, the equilibrium data of EDTA-corncob and EDTA-GO/corncob were analyzed by Langmuir, Freundlich and Sips model, respectively. The reusability on adsorption capacity of EDTA-corncob and EDTA-GO/corncob were also tested. Our results demonstrate that EDTA-GO/corncob is a cheap and efficient adsorbent for removal of dye in aqueous media.
2 Experimental section
2.1 Materials
Flake graphite (99.95%) was supplied by Qingdao Chenyang Graphite Co., Ltd. (Qingdao, China). Corncob was purchased from Deep Processing of Huifeng Straw Agricultural Products (Lianyungang, China). Ethylenediamine tetraacetic acid, were purchased from the National Medicine Group Chemical Reagent Co., Ltd. (Shanghai, China). Acridine orange, methyl red, crystal violet, rhodamine B, orange IV, acidic chrome blue K and methylene blue were purchased from the Aladdin Chemical Reagent Co., Ltd. (Shanghai, China), and the other reagents were of analytical grade.GO was prepared from the flake graphite powder using a modified Hummers–Offeman method.27
2.2 Preparation of adsorbent
10.0 g of corncob powder was immersed in 20% isopropanol solution, stirred for 24 hours, filtered, washed with 20% isopropanol and distilled water to be colorless, dried for 24 hours at 55 °C, then added 0.1 mol L−1 of sodium hydroxide solution to the sample, stirred for 1 hour at room temperature, filtered, then added distilled water to stir for 45 minutes, washed repeatedly to pH 7.0, and filtered. The obtained products were dried at 55 °C for storage.
0.10 g of GO was dispersed in 2.5% EDTA solution under ultrasound. Then the pretreated corncob powder was soaked in 2.5% EDTA/GO solution. After 24 hours, it was filtered and washed with distilled water. The EDTA-GO/corncob sample was dried at 55 °C and stored for reserve.
2.3 Characterization of materials
Fourier infrared spectrometry (FT-IR) of GO, corncob, EDTA-corncob and EDTA-GO/corncob were obtained using a Nicolet iS10 FT-IR spectrophotometer (Thermo Fisher Scientific, USA). Scanning electron microscope of corncob and EDTA-GO/corncob were carried out by an EVO MA10 scanning electron microscope (ZEISS, Germany). Zeta potential of EDTA-corncob and EDTA-GO/corncob were carried out on Zetasizer Nano Series (Malvern, British).2.4 Adsorption experiments
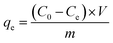 | (1) |
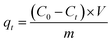 | (2) |
The influence of ionic strength on the adsorption of crystal violet on EDTA/corncob and EDTA-GO/corncob was studied in the same fashion with the adsorption isotherm, except that sodium chloride was varied and the initial concentration of crystal violet was set as 40 mg L−1.
3 Results and discussion
3.1 Characterization of materials
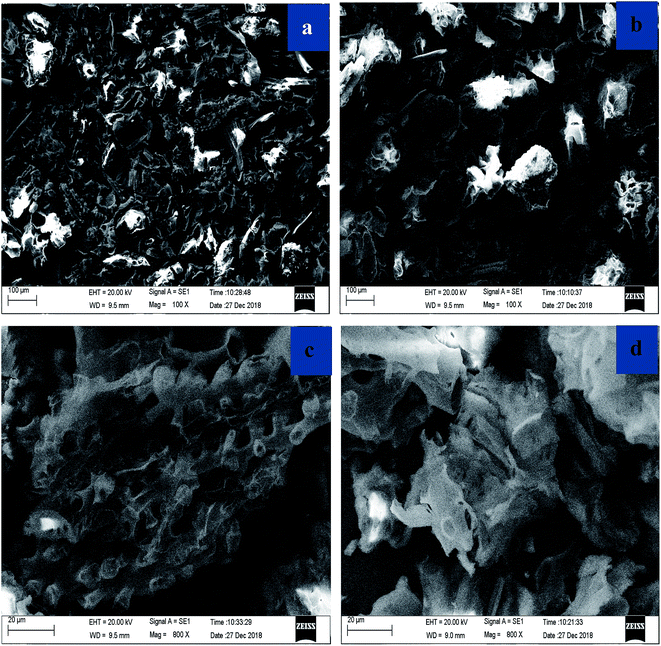
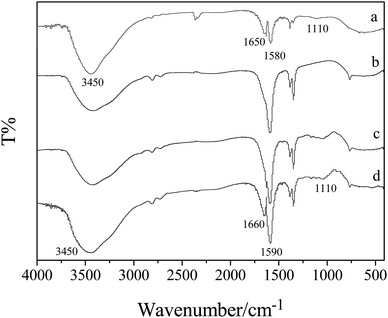
Scanning electron microscope (SEM) for corncob and EDTA-GO/corncob is shown in Fig. 1. The structure of corncob (Fig. 1a and c) and EDTA-GO/corncob (Fig. 1b and d) were clearly revealed by SEM investigations. The SEM micrograph of corncob shows porous surface structure (Fig. 1a and c). Comparing to the SEM images of corncob, EDTA-GO/corncob is mainly lamellar structure.The FTIR spectra of GO, corncob, EDTA-corncob and EDTA-GO/corncob are shown in Fig. 2. The characteristic peaks for GO appear at 1110, 1580, 1650 and 3450 cm−1 which correspond to stretching vibrations of C–O–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –OH bonds, respectively (Fig. 2a). The EDTA-GO/corncob and EDTA-corncob presented peaks for N–H (νN–H 3450 cm−1), C
O and –OH bonds, respectively (Fig. 2a). The EDTA-GO/corncob and EDTA-corncob presented peaks for N–H (νN–H 3450 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (νC
O (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 1660 cm−1), N–H (δN–H, 1590 cm−1) and C–O–C (νC–O–C, 1110 cm−1), all these functional groups are expected from EDTA and GO (Fig. 2c and d). These peaks at 1110 and 1660 cm−1 are not present in the spectra of unmodified corncob (Fig. 2b), indicating that EDTA and GO are attached successfully on the surface of EDTA-GO/corncob.
O, 1660 cm−1), N–H (δN–H, 1590 cm−1) and C–O–C (νC–O–C, 1110 cm−1), all these functional groups are expected from EDTA and GO (Fig. 2c and d). These peaks at 1110 and 1660 cm−1 are not present in the spectra of unmodified corncob (Fig. 2b), indicating that EDTA and GO are attached successfully on the surface of EDTA-GO/corncob.
3.2 Adsorption experiment
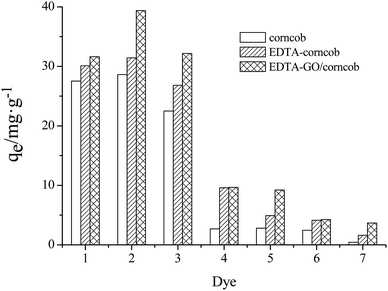 | ||
| Fig. 3 Comparisons of adsorption capacity (pH = 7.0) (1) methylene blue; (2) crystal violet; (3) acridine orange; (4) methyl red; (5) acid chrome blue K; (6) rhodamine B; (7) orange IV. | ||
In order to explain the adsorption mechanism, the adsorption kinetic data of crystal violet on EDTA-corncob and EDTA-GO/corncob were treated with pseudo-first-order and pseudo-second-order models, as shown in Fig. S1 and S2 (ESI†), and the experimental data was listed in Table 1.
| Adsorbent | qeqex/mg g−1 | Pseudo-first-order kinetics model | Pseudo-second-order kinetics model | ||||
|---|---|---|---|---|---|---|---|
| K1 (×10 min−1) | qe/mg g−1 | R | K2 (×102 g mg−1 min−1) | qe/mg g−1 | R | ||
| EDTA/corncob | 88.33 | 0.1497 | 67.74 | 0.9231 | 0.05535 | 88.50 | 0.9949 |
| EDTA-GO/cornob | 95.87 | 0.1566 | 71.12 | 0.9421 | 0.05171 | 95.24 | 0.9981 |
The pseudo first-order kinetics model (eqn (3)) and the pseudo-second-order kinetics model (eqn (4)) can be expressed as follows:
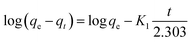 | (3) |
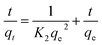 | (4) |
As listed in Table 1, the adsorption kinetics of EDTA-corncob and EDTA-GO/corncob were nicely described by pseudo-second-order model (R = 0.99) and the calculated qe was very close to the experimental qe. This indicated that chemisorption or chemical bonding between active sites of EDTA-corncob and EDTA-GO/corncob and crystal violet might dominate the adsorption process.
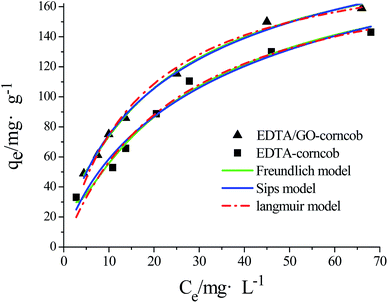
The experimental adsorption equilibrium results on EDTA-corncob and EDTA-GO/corncob were fitted by the Langmuir model (eqn (5) and (6)), Freundlich isotherm model (eqn (7)) and Sips model (eqn (8)). The Langmuir model base on three assumptions:28 (1) the adsorption of molecule is a monolayer adsorption; (2) the adsorption of adsorbent surface is uniform; (3) there is no interaction among adsorbed molecules. The Freundlich model assumes that the adsorption surface is heterogeneous, that interactions between adsorbed molecules can occur, and that multilayer adsorption is possible. Sips model is an improved Langmuir model.
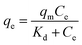 | (5) |
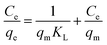 | (6) |
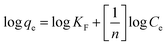 | (7) |
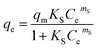 | (8) |
The Freundlich model provided a slightly better fit for the adsorption data of crystal violet onto EDTA-corncob and EDTA-GO/corncob (Table 2) than Langmuir model and Sips model. Therefore, the adsorption of crystal violet onto EDTA-corncob and EDTA-GO/corncob is heterogeneous adsorptions.
| Adsorbent | Langmuir model | Freundlich model | Sips model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qm/mg g−1 | Kd | R | KF mg−1 g−1 | n | R | KS | mS | R | |
| EDTA/corncob | 185.2 | 20.81 | 0.9494 | 18.08 | 1.943 | 0.9808 | 0.01798 | 0.6117 | 0.9538 |
| EDTA-GO/cornob | 203.9 | 16.54 | 0.9929 | 22.91 | 1.985 | 0.9982 | 0.03865 | 0.5864 | 0.9981 |
A comparison of the maximum adsorption capacities (qm) of EDTA-corncob and EDTA-GO/corncob for crystal violet are listed in Table 3 with literature values of qm of other adsorbents. It can be seen that the maximum adsorption capacities of EDTA-corncob and EDTA-GO/corncob for crystal violet are higher than that of other materials which might be attributed to the higher specific surface area of graphene and the EDTA groups.
| Adsorbent | Dye | qm/mg g−1 | Ref. |
|---|---|---|---|
| AC-AgNPLs | Crystal violet | 87.2 | 30 |
| Chitosan/nanodiopside nanocomposite | Crystal violet | 104.66 | 31 |
| Gum Arabic-cl-poly(acrylamide) nanohydrogel | Crystal violet | 90.90 | 32 |
| ZVI-GAM | Crystal violet | 172.41 | 33 |
| Chitin nanowhiskers | Crystal violet | 39.56 | 34 |
| Zinc oxide nanorods loaded on activated carbon | Crystal violet | 113.64 | 35 |
| Surfactant modified magnetic nanoadsorbent | Crystal violet | 166.67 | 36 |
| NH2-MIL-125(Ti) modified MOF | Crystal violet | 129.87 | 37 |
| EDTA/corncob | Crystal violet | 185.2 | This work |
| EDTA-GO/cornob | Crystal violet | 203.9 | This work |
3.3 Adsorption mechanism
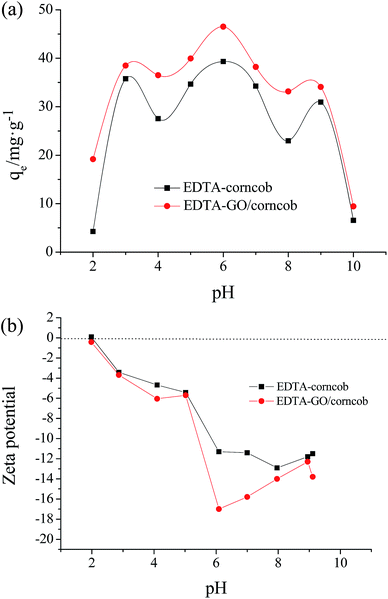
The pH of solution affects the surface charge of the adsorbents as well as the degree of ionization of pollutants. Change of pH value affects the adsorptive process through dissociation of functional groups on the adsorbent surface active site.38The impact of initial pH in the range of 2.0–10.0 on crystal violet adsorption by the EDTA/corncob and EDTA-GO/corncob are shown in Fig. 7a. The highest adsorption capacities of crystal violet on EDTA/corncob and EDTA-GO/corncob were detected at pH of 6.0. The adsorption of crystal violet on EDTA/corncob and EDTA-GO/corncob are pH dependent, the adsorption capacity of crystal violet on EDTA/corncob and EDTA-GO/corncob increases with the increase of pH (pH < 6.0), but the adsorption capacity of crystal violet on EDTA/corncob and EDTA-GO/corncob decreases with the increase of pH (pH > 6.0).
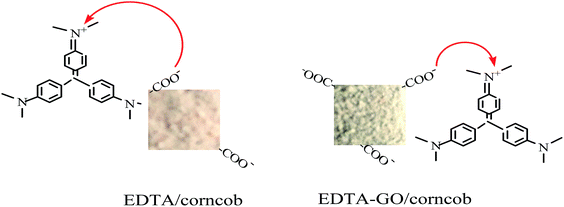
The results may be attributed to the electrostatic attraction between the anions on the adsorbent and the cations on the dye. Fig. 7b showed the zeta potentials of EDTA/corncob and EDTA-GO/corncob. As shown in Fig. 7b, the isoelectric point (pHzpc) of EDTA/corncob and EDTA-GO/corncob are 2.05 and 1.98, respectively. At low pH (pH > 2.05), the surface of EDTA/corncob is negative charge because of the –COO− groups on the surface of EDTA/corncob. At low pH (pH > 1.98), the surface of EDTA-GO/corncob is negative charge, which is attributed to more –COO− groups. The negative charge on the surface of EDTA/corncob and EDTA-GO/corncob increases from pH 2.0 to 6.0 and then decreases with the increase of pH, and reaches the maximum at pH 6.0. The surface of acridine orange is positive charge. At pH around 6.0, the electrostatic interaction between crystal violet and EDTA/corncob or EDTA-GO/corncob is strongest, so the adsorption capacity of crystal violet on the absorbents reaches the maximum.
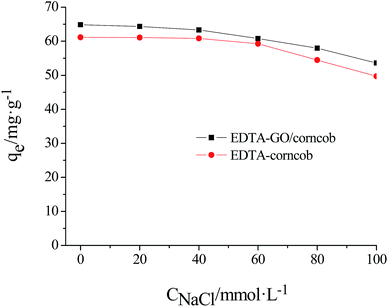
The effect of ionic strength on the adsorption of crystal violet on EDTA/corncob or EDTA-GO/corncob were studied by carried out a series of experiments at NaCl solutions (0.00, 0.02, 0.04, 0.06, 0.08, 0.10 mol L−1), and solution pH was adjusted to 6.0 before adsorption. The results are shown in Fig. 8. It is observed that the adsorption capacity of crystal violet on EDTA/corncob or EDTA-GO/corncob under the condition of 0.10 M NaCl remains 81.31% and 82.63% that of without addition of NaCl. The adsorption processes of crystal violet on EDTA/corncob or EDTA-GO/corncob are dependent on NaCl concentrations, which showed that electrostatic attraction dominates the adsorption process. The interaction mechanisms of crystal violet adsorption with EDTA/corncob and EDTA-GO/corncob were showed in Fig. 9.
3.4 Adsorbent recycling
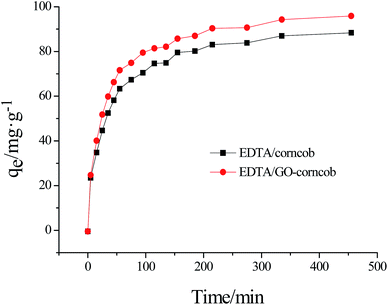
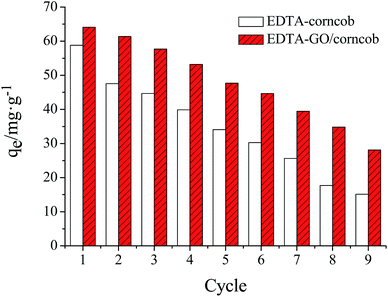
Taking into account the practical application, the adsorption capacity and the reusability property are two key parameters to evaluate an adsorbent. An ideal adsorbent should not only possess higher adsorption capability, but also show better reusability property, which will significantly reduce the overall cost for the adsorbents.39Reusability of EDTA/corncob or EDTA-GO/corncob is shown in Fig. 10. The crystal violet solutions with the concentration of 40 mg L−1 were prepared and EDTA/corncob or EDTA-GO/corncob was recycled for nine times to investigate the adsorption cycle number of EDTA/corncob or EDTA-GO/corncob. At the first adsorption equilibrium, the maximum adsorption capacity of EDTA/corncob or EDTA-GO/corncob was 58.8 and 64.1 mg g−1, respectively. With the increase of cycle number, the adsorption capacity showed a trend of decreasing. The adsorption capacity of EDTA/corncob was 30.3 mg g−1 at the sixth adsorption, while the maximum adsorption capacity of EDTA-GO/corncob is 28.1 mg g−1 at the ninth adsorption. This result showed that EDTA/corncob can be reused for five times and EDTA-GO/corncob can be reused for eight times.
4 Conclusions
In summary, EDTA/corncob and EDTA-GO/corncob were prepared successful and could be used as an efficient and recyclable adsorbent for crystal violet. The adsorption capacity of crystal violet on EDTA-GO/corncob is higher than EDTA/corncob. The most suitable pH of adsorption of crystal violet on EDTA-GO/corncob was around 6.0. The adsorption equilibration time was 200 minutes. The adsorption kinetics fit well with pseudo-second-order model and the adsorption isotherm fit well with Freundlich isotherm model. Adsorption mechanism of crystal violet on EDTA/corncob and EDTA-GO/corncob were electrostatic attraction. EDTA/corncob can be reused for five times and EDTA-GO/corncob can be reused for eight times. The facile regeneration of EDTA-GO/corncob definitely could reduce the operating cost. We believe that EDTA-GO/corncob will have potentially wide application in the removal of dye pollutants from aquatic systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Nation Natural Science Foundation of China (No. 21703189), Xianyang Science and Technology Research Project of China (No. 2018k02-20), Shaanxi Province Innovation and Entrepreneurship Project for College Students of China in 2017 (No. 2502) and Xianyang Normal University Innovation and Entrepreneurship Project for College Students of China in 2017 (No. 2017070).References
- M. G. Dosskey, Environ. Manage., 2001, 28, 577–598 CrossRef CAS PubMed.
- A. J. Jafari, B. Kakavandi, R. R. Kalantary, H. Gharibi, A. Asadi, A. Azari, A. A. Babaei and A. Takdastan, Korean J. Chem. Eng., 2016, 33(10), 2878–2890 CrossRef CAS.
- K. Shakir, A. F. Elkafrawy, H. F. G. Shokry, G. E. Beheir and M. Refaat, Water Res., 2010, 44(5), 1449–1461 CrossRef CAS.
- H. Zhang, Q. Luan, H. Tang, F. Huang, M. Zheng, Q. Deng, X. Xiang, C. Yang, J. Shi, C. Zheng and Q. Zhou, Cellulose, 2017, 24(2), 903–914 CrossRef CAS.
- J. Núñez, M. Yeber, N. Cisternas, R. Thibaut, P. Medina and C. Carrasco, J. Hazard. Mater., 2019, 371, 705–711 CrossRef.
- K. Sirirerkratana, P. Kemacheevakul and S. Chuangchote, J. Cleaner Prod., 2019, 215, 123–130 CrossRef CAS.
- Q. Y. Yue, B. Y. Gao, Y. Wang, H. Zhang, X. Sun, S. G. Wang and R. R. Gu, J. Hazard. Mater., 2008, 152(1), 221–227 CrossRef CAS.
- J. Liang, X. A. Ning, J. Sun, J. Song, Y. Hong and H. Cai, J. Cleaner Prod., 2018, 204, 12–19 CrossRef CAS.
- I. Chaari, E. Fakhfakh, M. Medhioub and F. Jamoussi, J. Mol. Struct., 2019, 1179, 672–677 CrossRef CAS.
- N. Nikooe and E. Saljoughi, Appl. Surf. Sci., 2017, 413, 41–49 CrossRef CAS.
- J. Zhao, Z. Zou, R. Ren, X. Sui, Z. Mao, H. Xu, Y. Zhong, L. Zhang and B. Wang, Eur. Polym. J., 2018, 108, 212–218 CrossRef CAS.
- N. A. H. M. Zaidi, L. B. L. Lim and A. Usman, Environmental Technology & Innovation, 2019, 13, 211–223 Search PubMed.
- L. Dai, W. Zhu, L. He, F. Tan, N. Zhu, Q. Zhou, M. He and G. Hu, Bioresour. Technol., 2018, 267, 510–516 CrossRef CAS.
- L. Zhou, H. Zhou, Y. Hu, S. Yan and J. Yang, J. Environ. Manage., 2019, 234, 245–252 CrossRef CAS.
- Y. Song, J. Tan, G. Wang and L. Zhou, Carbohydr. Polym., 2018, 199, 178–185 CrossRef CAS.
- J. Wu, T. Zhang, C. Chen, L. Feng, X. Su, L. Zhou, Y. Chen, A. Xia and X. Wang, Bioresour. Technol., 2018, 266, 134–138 CrossRef CAS.
- N. Abidi, J. Duplay, A. Jada, E. Errais, M. Ghazi, K. Semhi and M. Trabelsi-Ayadi, C. R. Chim., 2019, 22(2–3), 113–125 CrossRef CAS.
- S. Shakoor and A. Nasar, Groundwater for Sustainable Development, 2017, 5, 152–159 CrossRef.
- H. Ma, J. B. Li, W. W. Liu, M. Miao, B. J. Cheng and S. W. Zhu, Bioresour. Technol., 2015, 190, 13–20 CrossRef CAS.
- M. T. Vu, H. P. Chao, T. V. Trinh, T. T. Le, C. C. Lin and H. N. Tran, J. Cleaner Prod., 2018, 180, 560–570 CrossRef CAS.
- H. Lin, S. Han, Y. Dong and Y. He, Appl. Surf. Sci., 2017, 412, 152–159 CrossRef CAS.
- D. Lv, Y. Liu, J. Zhou, K. Yang, Z. Lou, S. A. Baig and X. Xu, Appl. Surf. Sci., 2018, 428, 648–658 CrossRef CAS.
- D. Wu, L. Hu, Y. Wang, Q. Wei, L. Yan, T. Yan, Y. Li and B. Du, J. Colloid Interface Sci., 2018, 523, 56–64 CrossRef CAS.
- E. F. C. Chaúque, L. N. Dlamini, A. A. Adelodun, C. J. Greyling and J. C. Ngila, Physics and Chemistry of the Earth, Parts A/B/C, 2017, 100, 201–211 CrossRef.
- Y. Yang, S. Song and Z. Zhao, Colloids Surf., A, 2017, 513, 315–324 CrossRef CAS.
- M. Lv, L. Yan, C. Liu, C. Su, Q. Zhou, X. Zhang, Y. Lan, Y. Zheng, L. Lai, X. Liu and Z. Ye, Chem. Eng. J., 2018, 349, 791–799 CrossRef CAS.
- L. Sun, H. Yu and B. Fugetsu, J. Hazard. Mater., 2012, 203–204, 101–110 CrossRef CAS.
- X. Xin, W. Si, Z. Yao, R. Feng, B. Du, L. Yan and Q. Wei, J. Colloid Interface Sci., 2011, 359, 499–504 CrossRef CAS.
- G. McKay, A. Mesdaghinia, S. Nasseri, M. Hadi and M. S. Aminabad, Chem. Eng. J., 2014, 251, 236–247 CrossRef CAS.
- A. H. AbdEl-Salam, H. A. Ewais and A. S. Basaleh, J. Mol. Liq., 2017, 248, 833–841 CrossRef CAS.
- S. G. Nasab, A. Semnani, A. Teimouri, M. J. Yazd, T. M. Isfahani and S. Habibollahi, Int. J. Biol. Macromol., 2019, 124, 429–443 CrossRef CAS.
- G. Sharma, A. Kumar, M. Naushad, A. García-Peñas, A. H. Al-Muhtaseb, A. A. Ghfar, V. Sharma, T. Ahamad and F. J. Stadler, Carbohydr. Polym., 2018, 202, 444–453 CrossRef CAS.
- J. Liu, Y. Wang, Y. Fang, T. Mwamulima, S. Song and C. Peng, J. Mol. Liq., 2018, 250, 468–476 CrossRef CAS.
- S. Gopi, A. Pius and S. Thomas, Journal of Water Process Engineering, 2016, 14, 1–8 CrossRef.
- E. A. Dil, M. Ghaedi, A. Ghaedi, A. Asfaram, M. Jamshidi and M. K. Purkait, J. Taiwan Inst. Chem. Eng., 2016, 59, 210–220 CrossRef CAS.
- C. Muthukumaran, S. V. Murugaiyan and M. Thirumarimurugan, J. Taiwan Inst. Chem. Eng., 2016, 63, 354–362 CrossRef CAS.
- G. Wen and Z. G. Guo, Colloids Surf., A, 2018, 541, 58–67 CrossRef CAS.
- C. Yang, L. Lei, P. Zhou, Z. Zhang and Z. Lei, J. Colloid Interface Sci., 2015, 443, 97–104 CrossRef CAS PubMed.
- H. T. Xing, J. H. Chen, X. Sun, Y. H. Huang, Z. B. Su, S. R. Hu, W. Weng, S. X. Li, H. X. Guo, W. B. Wu, Y. S. He, F. M. Li and Y. Huang, Chem. Eng. J., 2015, 263, 280–289 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1 The pseudo first-order kinetics model. Fig. S2 The pseudo second-order kinetics model. See DOI: 10.1039/c9ra04003j |
| This journal is © The Royal Society of Chemistry 2019 |