Open Access Article
Open Access ArticleEffect of counter-ions on the properties and performance of non-conjugated polyelectrolyte interlayers in solar cell and transistor devices†
Ju Hwan Kang a,
Yu Jung Parka,
Myung Joo Chaa,
Yeonjin Yib,
Aeran Songc,
Kwun-Bum Chung
a,
Yu Jung Parka,
Myung Joo Chaa,
Yeonjin Yib,
Aeran Songc,
Kwun-Bum Chung c,
Jung Hwa Seo
c,
Jung Hwa Seo *a and
Bright Walker
*a and
Bright Walker *d
*d
aDepartment of Materials Physics, Dong-A University, 49315, Republic of Korea. E-mail: seojh@dau.ac.kr
bInstitute of Physics and Applied Physics, Yonsei University, Seoul 03722, Republic of Korea
cDivision of Physics and Semiconductor Science, Dongguk University, Seoul, 04620, Republic of Korea
dDepartment of Chemistry, Kyung Hee University, 02453, Seoul, Republic of Korea. E-mail: walker@khu.ac.kr
First published on 2nd July 2019
Abstract
We have investigated a series of non-conjugated polyelectrolytes (NPEs) which are based on a polyethylenimine (PEI) backbone with various counterions, such as Br− I− and BIm4−, as interfacial layers at the electrodes of solar cells and transistor devices to improve the power conversion efficiency (PCE) and device performance. This new series of NPEs with different counterions are capable of forming electric dipoles at NPE/metal electrode interfaces; as a consequence tuning of the energy levels, and work function (WF) of the electrodes is possible. Using this approach, the PCE of organic solar cells could be improved from 1.05% (without NPEs) to 6.77% (with NPEs) while the charge carrier mobility and on/off ratio of FET devices could be improved, showing the broad utility of this type of material. This study provides a novel approach towards investigating the influence of ions on interfacial dipoles and electrode WFs in solution-processed semiconducting devices.
Introduction
Bulk heterojunction (BHJ) organic solar cells (OSCs), which consist of conjugated polymers and a fullerene acceptor may be regarded as next-generation solar cells due to their advantages such as cost-efficiency, printability, light weight, flexibility, semitransparency and the potential for large area fabrication at low cost.1–4 The most important advantage of OSCs is their solution processability, which enables mass production through economical and large-scale processing technologies such as roll-to-roll manufacturing.5–7However, in order to be commercially successful or practical in terms of compatibility with low-cost manufacturing technologies, power conversion efficiencies (PCEs) must be comparable to those of silicon solar cells. Extensive research relating to the development of novel donor and acceptor materials, interfacial materials for better charge-carrier collection, morphology control and processing additives, phase-separation morphology via diversified strategies, have improved PCEs up to 15%.8–18
In order for charges to be extracted efficiently, interfacial materials that minimize energy barriers between semiconducting layers and electrodes are necessary. In this context, organic molecules as well as metal oxides can be used. Currently, the most commonly used oxides include TiO2, and ZnO; alkali metal compounds, such as Cs2CO3, may also be used to control the interfacial properties of junctions formed between electrodes and the photoactive layer.19–22 These inorganic oxides are considered to be excellent interfacial materials due to their superior stability and electrical properties. These interfacial materials facilitate the extraction of n-type charge carriers from the active layer and act as effective cathode interlayers. Interestingly, oxides like TiO2 recently can be used in combination with other materials as well.23 Sn doping of the TiO2 layer has been observed to improve diode characteristics and overall solar cell performance; the PCE of the inverted PTB7-based devices increased from 6.7% to 7.6%.24
ZnO is a semiconducting oxide which has also been used extensively as an electron transport layer (ETLs). In the case of inverted organic solar cells (IOSCs), interfacial ZnO layers deposited on ITO electrodes are designed to reduce ohmic loss which occur during movement of electrons from the active layer to the electrode by adjusting the conduction band energy near the electrode to match that of the photoactive layer. Lim and coworkers developed a ZnO layer with a rippled texture, which was improved significantly when an additional [6,6]-phenyl C61 butyric acid methyl ester (PC61BM) layer was inserted between the ZnO layer and active layer. Using a PTB7-derived copolymer blended with [6,6]-phenyl C71 butyric acid methyl ester (PC71BM) as the active layer in inverted devices, a PCE of up to 7.7% was achieved.25
Conjugated polyelectrolytes (CPEs) are one of the most widely used ETLs in organic semiconductor devices, including solar cells as well as organic light emitting diodes and transistor devices.26–29 The high electron affinity of CPEs can provide excellent energy alignment and facilitate electron transfer and collection at the cathodes of OSCs.30,31 Cao and coworkers, for example, have recently reported a series of tunable n-type CPEs with counterions which allow interfacial engineering of the cathode band structure. This interlayer was applied in various polymer solar cells and yielded up to 10.5% efficiency using an active layer comprising NT812![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM.32
PC71BM.32
Although CPEs have repeatedly been demonstrated to provide excellent control over the band structure at different interfaces in semiconducting devices, the fundamental mechanisms by which they alter electronic band structures of semiconducting devices aren't completely understood. Some general class of materials have proven to be effective, (such as polyfluorenes with pendant quaternized ammonium ions), however, the reasons why these materials work especially well remains somewhat of a mystery, and it still isn't possible to predict what effects new ionic materials will have on semiconductor band structures.
Non-conjugated polyelectrolytes (NPEs, also known simply as polyelectrolytes), do not possess the conjugated polymer backbones which are present in CPEs; this greatly reduces the amount of synthetic effort required to prepare them compared to CPEs. Additionally, NPEs make an excellent platform to study the purely ionic effects of interfacial electrolytic materials without introducing an additional semiconducting material (i.e. the conjugated backbone in CPEs) which may convolute the effects of the ionic functionalities.
NPEs are also generally water soluble and can be deposited from aqueous solutions, making them an environmentally friendly approach to control the work function (WF) of electrode such as ITO.33 NPEs such as polyethyleneimine (PEI) and polyallylamine (PAA) have been used as interfacial layers on ITO, creating a polarized surface with a reduced WF and allowing ITO to function effectively as a cathode. In this context, NPEs are able to align the lowest unoccupied molecular orbital band (LUMO) level of the active layer with the Fermi level at the cathode to create an ohmic contact which allows efficient extraction of electrons from the photoactive layer. An efficiency of 6.3% was reported for inverted devices based on a PTB7 active layer, comparable to results using CPE interfacial layers.36 Although it appears that the ionic character of NPEs allows them to have similar effects on the band structure of semiconducting devices as CPEs, the range of NPE materials studied so far has remained very limited and further studies are necessary to understand how different ionic functionalities in NPEs affect the band structure of semiconducting devices.
In this contribution, we've prepared a series of NPEs with different counterions to investigate the effect of counter-ion identity on the band structure and properties of semiconducting devices including solar cells based on P3HT and PTB7 active layers as well as transistor devices based on Indium Zinc Oxide (IZO). The influence of NPEs on the WF of electrodes and band structures of semiconducting layers was investigated by ultraviolet photoelectron spectroscopy (UPS). Finally, NPEs were introduced as interfacial layers between the ITO substrate (cathode) and the photoactive layer in solar cell devices, and between the transport channel and source – drain electrodes to investigate the influence of the anion identity on solar cell and transistor parameters.
Experimental details
Non-conjugated polyelectrolytes (PEIH+Br−, PEIH+I−, PEIH+BIm4−) were synthesized following previously reported procedures as described in the ESI (Fig. S1 and S2†).34,35 Patterned ITO substrates (1.5 × 1.5 cm, 7 Ω sq−1) were cleaned by ultra-sonication in deionized water, acetone and isopropanol (IPA) for 20 minutes each. The cleaned ITO substrates were then treated with ozone using a UV–ozone cleaner to remove trace organic residues remaining on the surface, followed by drying in an oven at 100 °C immediately prior to use. NPE layers were deposited by spin coating NPE solutions based on the PEI backbone with variable concentration (0.1–0.005%). The counterions included bromide (PEIH+Br−), iodide (PEIH+I−), and tetrakis(imidazolyl) borate (PEIH+BIm4−); these layers were deposited by spin-coating solutions in methanol at 2000 rpm.Active layers comprised BHJ mixtures of PTB7 (1-Material) and PC71BM (Aldrich) and were used as received from the vendors. Active layers were deposited by spin coating a mixed solution of PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) in chlorobenzene (concentration of 10 mg mL−1) containing 3% DIO additive at 1300 rpm for 60 s on top of the NPE treated ITO substrates to obtain BHJ films with thicknesses of approximately 80 nm. Samples were then brought under vacuum (about 10−7 Torr), and MoO3 (8 nm) and Ag electrodes (100 nm) were deposited on top of the BHJ layers by thermal evaporation.
1.5) in chlorobenzene (concentration of 10 mg mL−1) containing 3% DIO additive at 1300 rpm for 60 s on top of the NPE treated ITO substrates to obtain BHJ films with thicknesses of approximately 80 nm. Samples were then brought under vacuum (about 10−7 Torr), and MoO3 (8 nm) and Ag electrodes (100 nm) were deposited on top of the BHJ layers by thermal evaporation.
Current density–voltage (J–V) measurements were collected using a Keithley 2635 source measure unit, carried out inside a nitrogen filled glove-box using a high quality optical fiber to guide the light from a xenon arc lamp to the solar cell devices. Solar cell devices were illuminated with an intensity of 100 mW cm−2, which was calibrated using a standard silicon reference cell immediately prior to testing. External quantum efficiency (EQE) measurements were carried out using a QEX7 system manufactured by PV Measurements, Inc.
For field effect transistors (FETs) 10 nm IZO channel layers were deposited by sputtering onto cleaned Si/SiO2 substrates. The films were annealed at 250 °C for an hour in air. PEIH+BIm4− solutions were prepared in methanol at different concentrations (0.005%, 0.01%, 0.05% and 0.1%) to control the film thickness. PEIH+BIm4− films were spin coated at a spin rate of 2000 rpm for 60 s. Finally, about 70 nm thick Au source and drain electrodes were deposited under vacuum by thermal evaporation on top of the channel layerusing a shadow mask. The channel length (L) and width (W) were 40 and 2500 μm, respectively. Electrical characterization of transistor devices was performed in an N2-atmosphere glove box using a Keithley SCS-4200 semiconductor parameter analyser. For each condition, 40 devices were fabricated and tested. Average values are reported with uncertainty intervals (±) corresponding to one standard deviations for each parameter measured.
Atomic force microscopy (AFM) images were obtained using a Veeco Multimode microscope operating in tapping mode. X-ray photoelectron spectroscopy (XPS) and UPS experiments were carried out using a Thermo Fischer Scientific ESCALAB 250XI.
Results and discussion
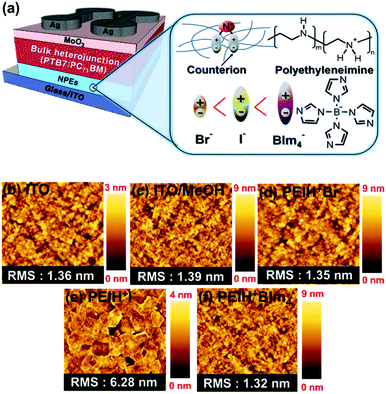
Our primary interest in these NPE materials was to understand their impact on the characteristics and performance of semiconducting devices. To investigate their effect on photovoltaic properties, IOSCs were prepared with NPEs layers deposited at the semiconductor/cathode interface, as depicted in Fig. 1. Fig. 1 shows a schematic device diagram of the IOSC architecture used in this study as well as the chemical structures of the NPEs employed. These NPEs comprise protonated (positively charged) branched PEI backbones (which lack π-conjugation), and bromide (PEIH+Br−), iodide (PEIH+I−) or tetrakis (imidazoly) borate (PEIH+BIm4−) counterions.AFM was used to characterize the surface morphology and roughness of the interlayers; topographic images (2 μm × 2 μm) of each cathode interlayer are shown in Fig. 1b–f. Each sample was fabricated by spin coating NPE films onto ITO substrates using the same conditions used to fabricate solar cell devices. Pristine ITO substrates (Fig. 1b) exhibited homogenous surfaces with a root mean square (RMS) roughness of 1.36 nm. Fig. 1c shows an AFM image of a methanol treated ITO surface. The film showed a low RMS of 1.39 nm, similar to the pristine ITO surface. Fig. 1d shows the topography of a PEIH+Br− (12 nm) film deposited on ITO. The RMS roughness of the surface was 1.35 nm for the PEIH+Br− layer, indicating increased roughness compared to the pristine ITO substrate. Fig. 1e shows that PEIH+I− (6.4 nm) deposited on ITO comprised large agglomerates, with an RMS roughness of 6.28 nm, which is the roughest surface among the different interlayers. The morphology of a PEIH+BIm4− (7.1 nm) layer deposited on ITO is shown in Fig. 1f. This film exhibited an RMS roughness of 1.41 nm, which was the lowest among the NPE counterions. The surface of the thin film was smooth and showed good morphological characteristics for OPV devices.
Fig. 2 shows the J–V characteristics of inverted BHJ devices with various cathode interfacial layers (PEIH+Br−, PEIH+I−, and PEIH+BIm4−) under AM 1.5G illumination with an intensity of 100 mW cm−2. Photovoltaic parameters of these devices are summarized in Table 1. Device data corresponding to poly 3-hexyl thiophene active layers can be found in the ESI (Fig. S3†). The control device without any interlayer exhibited a low PCE of 1.05% with a short-circuit current density (JSC) of 15.71 mA cm−2, an open-circuit voltage (VOC) of 0.211 V and a fill factor (FF) of 29.4%, these characteristics indicate that bare ITO is a poor n-type contact. Fig. 2a shows the J–V characteristics of the devices with PEIH+Br− layer, which under optimal processing conditions exhibited a PCE of 5.96%, including a VOC of 0.703 V, a JSC of 15.70 mA cm−2 and FF of 54.2% at a film thickness of 4.8 nm. Fig. 2b shows the J–V characteristics of devices using PEIH+I−. Optimal processing conditions for this NPE constituted relatively thin layers; these devices exhibited a PCE of 4.95% including a VOC of 0.635 V, JSC of 15.31 mA cm−2 and FF of 51.5% at a thickness of 6.4 nm.
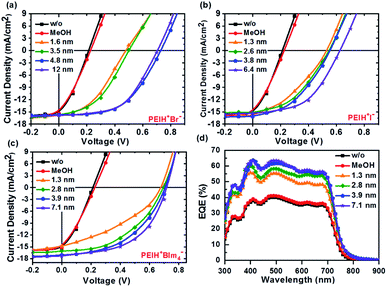 | ||
Fig. 2 Current density–voltage curves of PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM solar cells with the NPE ETL layers (a) PEIH+Br−, (b) PEIH+I−, (c) PEIH+BIm4−, and (d) EQE spectra of the devices corresponding to (c). PC71BM solar cells with the NPE ETL layers (a) PEIH+Br−, (b) PEIH+I−, (c) PEIH+BIm4−, and (d) EQE spectra of the devices corresponding to (c). | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM solar cells with different NPE thicknesses
PC71BM solar cells with different NPE thicknesses
| Device | Concentration (%) | Thickness (nm) | JSC (mA cm−2) | JSC, EQE (mA cm−2) | VOC (V) | FF (%) | PCE (%) | |
|---|---|---|---|---|---|---|---|---|
| Average | Best | |||||||
| w/o | — | — | 15.71 ± 0.08 | 12.12 | 0.211 ± 0.008 | 29.4 ± 3.1 | 0.89 ± 0.31 | 1.05 |
| MeOH treatment | — | — | 15.88 ± 0.07 | 12.21 | 0.226 ± 0.006 | 31.5 ± 2.8 | 0.93 ± 0.29 | 1.09 |
| PEIH+Br− | 0.005 | 1.6 | 15.64 ± 0.05 | — | 0.471 ± 0.009 | 42.6 ± 0.8 | 2.87 ± 0.17 | 3.09 |
| 0.01 | 3.5 | 15.79 ± 0.09 | — | 0.497 ± 0.011 | 44.2 ± 0.9 | 3.12 ± 0.15 | 3.45 | |
| 0.05 | 4.8 | 15.70 ± 0.13 | — | 0.703 ± 0.003 | 54.2 ± 1.3 | 5.73 ± 0.15 | 5.96 | |
| 0.1 | 12 | 15.81 ± 0.08 | — | 0.695 ± 0.005 | 52.2 ± 0.9 | 5.45 ± 0.14 | 5.71 | |
| PEIH+I− | 0.005 | 1.3 | 16.10 ± 0.05 | — | 0.523 ± 0.005 | 32.4 ± 2.7 | 2.45 ± 0.16 | 2.69 |
| 0.01 | 2.6 | 15.25 ± 0.09 | — | 0.541 ± 0.009 | 38.7 ± 2.8 | 3.01 ± 0.10 | 3.13 | |
| 0.05 | 3.8 | 16.14 ± 0.07 | — | 0.544 ± 0.006 | 43.2 ± 2.8 | 3.48 ± 0.27 | 3.77 | |
| 0.1 | 6.4 | 15.31 ± 0.52 | — | 0.635 ± 0.003 | 51.5 ± 2.2 | 4.72 ± 0.21 | 4.95 | |
| PEIH+BIm4− | 0.005 | 1.3 | 14.84 ± 0.11 | 14.23 | 0.697 ± 0.002 | 31.6 ± 1.0 | 3.02 ± 0.11 | 3.2 |
| 0.01 | 2.8 | 16.03 ± 0.05 | 15.89 | 0.706 ± 0.006 | 46.7 ± 0.7 | 5.11 ± 0.09 | 5.2 | |
| 0.05 | 3.9 | 17.04 ± 0.08 | 16.94 | 0.708 ± 0.004 | 50.2 ± 1.1 | 5.87 ± 0.12 | 6.03 | |
| 0.1 | 7.1 | 17.34 ± 0.17 | 17.09 | 0.710 ± 0.003 | 55.2 ± 0.9 | 6.59 ± 0.09 | 6.77 | |
When PEIH+BIm4− was used as the interfacial layer, the PCE was observed to increase with increasing interlayer thickness. As shown in Fig. 2c, the J–V characteristics of the device with a PEIH+BIm4− thickness of 7.1 nm showed the highest performance, with a significant increase in PCE (6.77%) compared to control devices, with a VOC of 0.710 V, JSC of 17.34 mA cm−2 and FF of 55.2%.
When the NPE thickness became sufficiently thick (>10 nm), “quantum tunneling” for electron transfer from the photoactive BHJ layer to the ITO cathode was inhibited due to the wide bandgap and absence of a conjugated backbone in the NPE layer, which causes it to act as an electrical insulator and impose a large energy barrier.37,38 EQE spectra of various devices are shown in Fig. 2d The EQE spectra for all devices show similar spectral features, consistent with the identical active layers used in each device. However, the EQE of the devices with NPE ETLs show similar spectral shape with higher quantum efficiencies throughout the spectra. This is consistent with the larger JSC observed in devices with NPE layers and the negligible absorption/reflection of the ultra-thin NPE layers. Although devices without NPEs have similar optical properties as the NPE devices, the reduced EQE in this condition is consistent with the lower built-in potential across the active layer, which reduces the efficiency with which charge carriers are extracted.38
Since the VOC of BHJ solar cell is usually determined by the difference in LUMO of acceptor and highest occupied molecular orbital band (HOMO) of the donor, as well as the WF difference between anode and cathode,39,40 the increase in VOC can be attributed to a decrease in the WF of the ITO cathode after NPE deposition. This effective reduction in WF is mediated by an interfacial dipole effect caused by the NPE layer. Upon depositing a thin NPE film, the ionic functionalities in the NPE orient themselves relative to the film surface in such a way that permanent electrical dipole is formed. This surface dipole reduces the energy which is required to remove an electron from the film surface and thus reduces the effective WF, it is an effect which has been well documented.41,42
Additionally, the NPE layer may block back-diffusion of holes, a process which leads to carrier recombination at the cathode in devices without NPE layers. This reduction in effective WF is mediated by an interfacial dipole effect caused by the NPE layers. Upon depositing thin NPE films, the ionic functionalities in the NPEs orient themselves relative to the film surface in such a way that permanent electric dipoles are formed. This surface dipole reduces the energy which is required to remove an electron from the film surface and thus reduces the effective WF, an effect which has been well documented.33,36
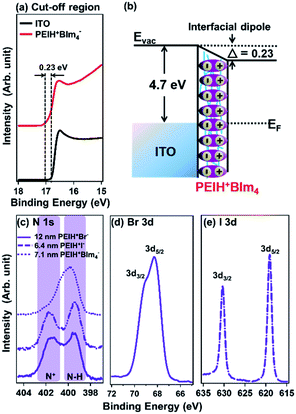
The electronic structures of the interlayers were further investigated by UPS. Fig. 3a shows the secondary electron cut-off regions of photoelectron spectra of various interlayers on ITO substrates. The secondary electron cut-off allows accurate determination of the WF of interlayers; by measuring the shift in the edge of these spectra, relative to the substrate (ITO) it is possible to accurately quantify changes in WF and the magnitude of the interfacial dipole effect caused by each interlayer. Our UPS measurements indicate a WF for pristine ITO to be 4.70 eV, which decreased to 4.47 eV after the application of a PEIH+BIm4− ETL, indicating that the ETL facilitated electron extraction relative to the pristine film, with a corresponding interfacial dipole (Δ) of 0.23 eV. It is well-known that the effective WF of electrodes can be adjusted by introducing a thin layer of NPE, due to the formation of interfacial dipoles.43 Fig. 3b shows a schematic diagram of the interfacial dipole created by a PEIH+BIm4−, which lowers the WF of the cathode by 0.23 eV. Although the magnitude of the dipole is smaller than non-conjugated polyelectrolytes described in previous reports using other active layers, the observed trends in data are the same, showing a decrease in electrode WF and reduced electron injection barrier.34 The magnitude of the interfacial dipole and corresponding effects on device performance follow the size of the anion, where the interfacial dipole created by PEIH+BIm4− as the greatest and decreased for I− while the dipole for Br− was close to zero. This trend is consistent with trends observed by Do et al. using viologen based cationic polyelectrolytes with variously sized anions.
In order to investigate the chemical properties of the different counterions, XPS spectra were collected. Fig. 3c shows the XPS spectra of PEIH+Br− and PEIH+I− films and indicate that the N 1s core level occurs at 403.3 eV in PEIH+Br− and PEIH+I−, which is consistent with quaternized N+.44,45 Fig. 3d also shows that the Br 3d emission line at 68.3 eV for the thin PEIH+Br− film, corresponding to the anionic bonding state of Br.46 Also, Fig. 3e shows the I 3d emission lines at 619.2 eV and 630.8 eV comes from the ionic and covalent bond of PEIH+I− films respectively. XPS was additionally used to calculate film thickness from attenuation of the substrate (Au 4f) signal, detailed data can be found in the ESI (Fig. S5†).
In order to confirm the effect of NPE layer at the interface, we investigated field effect transistor (FET) devices based on IZO which is inorganic n-type semiconductor without and with a variety of thickness of PEIH+BIm4− layers. (1.3, 2.8, 3.9 and 7.1 nm) Fig. 4 shows the transfer characteristics of n-type FETs. FET performance of data is summarized in Table 2 and output characteristics are in shown in the ESI (Fig. S4†). IZO FET without NPE layer exhibited the electron mobility (μe) of 7.20 cm2 V−1 s−1. When using the thin PEIH+BIm4− layer (1.3 nm), the μe was slightly decreased to 5.12 cm2 V−1 s−1. The μes were gradually increased 12.5 and 32.9 cm2 V−1 s−1 for devices using PEIH+Bim4− processed with thicknesses of 2.8 and 3.9 nm, respectively. Specially, in case of using 3.9 nm-thick NPE layer, IZO FET show the optimized μe of 32.9 cm2 V−1 s−1 with high on–off ratio (Ion/off) of ∼108. While, too thicker PEIH+Bim4− layer such as 7.1 nm led to the low μe of 1.33 cm2 V−1 s−1 and Ion/off of ∼103.
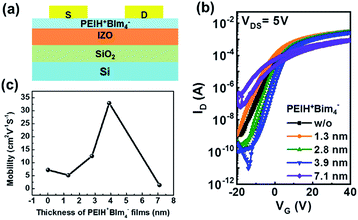 | ||
| Fig. 4 Transfer characteristics of n-type transistors without and with PEIH+BIm4− as a function of the film thicknesses. (1.3, 2.8, 3.9 and 7.1 nm). | ||
| ETL | Thickness (nm) | μe (cm2 V−1 s−1) | Ion/off | Vth (V) | SS (V dec−1) |
|---|---|---|---|---|---|
| w/o | — | 7.20 ± 3.84 | 4.17(±2.09) × 105 | −4.38(±1.34) | 3.46 ± 0.31 |
| PEIH+BIm4− | 1.3 | 5.12 ± 2.34 | 4.15(±2.01) × 105 | −6.10(±0.92) | 3.35 ± 0.24 |
| 2.8 | 12.5 ± 2.02 | 2.97(±1.26) × 105 | −1.97(±0.63) | 2.50 ± 0.19 | |
| 3.9 | 32.9 ± 0.93 | 8.29(±3.10) × 107 | −4.29(±0.52) | 2.61 ± 0.21 | |
| 7.1 | 1.33 ± 1.34 | 8.48(±2.31) × 106 | −6.80(±1.25) | 5.87 ± 0.45 |
Conclusions
We investigated IOSC devices using solution-processed organic interfacial layers and compared their characteristics. All of the NPE cathode interlayers were effectively used with PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM active layers, demonstrating a universal increase in performance and suggesting broad applicability for this type of interlayer.
PC71BM active layers, demonstrating a universal increase in performance and suggesting broad applicability for this type of interlayer.
NPE films were also found to improve the performance of thin-film transistor devices via a decrease in the WF of the source and drain electrodes. UPS of PEIH+BIm4− films confirmed a decrease in WF as a result of deposition on the ITO interface. The observed interfacial dipole and change in Fermi energy are consistent with n-type doping at the interface.
The RMS roughness values of PEIH+BIm4−, PEIH+Br− and PEIH+I− films were found to be 1.41, 3.35 and 6.28 nm respectively. Such modification of film morphologies can affect the performance of OSCs; particularly, the PEIH+I− films are fairly rough for application in OPV devices, and the decreased RMS roughness value upon application of the NPE layers corresponds well with the improved device performance.
These results demonstrate that solution-processed, IOSCs can be success-fully realized using a variety of organic cathode interlayers, and that each interlayer has unique properties which may be optimal for specific applications or active materials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Research Foundation of Korea (2017R1C1B1010627 and 2017R1A2B2012971) and through the Kyung Hee University Research Equipment Support Project (KHU-20181297) This work was supported by Busan Institute of Science and Technology Evaluation and Planning (BISTEP) grant (2019 Busan Open Lab. Program) funded by the Korea government (Ministry of Trade, Industry 5 and Energy) and Busan metropolitan city. This research was also supported by the National Research Foundation of Korea (NRF) Grant funded by the Ministry of Science and ICT for First-Mover Program for Accelerating Disruptive Technology Development (NRF-2018M3C1B9088457).Notes and references
- L. Lu, T. Zheng, Q. Wu, A. M. Schneider, D. Zhao and L. Yu, Chem. Rev., 2015, 115, 12666–12731 CrossRef CAS PubMed.
- M. Kaltenbrunner, M. S. White, E. D. Głowacki, T. Sekitani, T. Someya, N. S. Sariciftci and S. Bauer, Nat. Commun., 2012, 3, 770 CrossRef PubMed.
- X. Ouyang, R. Peng, L. Ai, X. Zhang and Z. Ge, Nat. Photonics, 2015, 9, 520 CrossRef CAS.
- R. R. Søndergaard, M. Hösel and F. C. Krebs, J. Polym. Sci., Part B: Polym. Phys., 2013, 51, 16–34 CrossRef.
- A. J. Nozik, M. C. Beard, J. M. Luther, M. Law, R. J. Ellingson and J. C. Johnson, Chem. Rev., 2010, 110, 6873–6890 CrossRef CAS PubMed.
- H. Youn, H. J. Park and L. J. Guo, Small, 2015, 11, 2228–2246 CrossRef CAS PubMed.
- Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591 CrossRef.
- B. Kan, Q. Zhang, M. Li, X. Wan, W. Ni, G. Long, Y. Wang, X. Yang, H. Feng and Y. Chen, J. Am. Chem. Soc., 2014, 136, 15529–15532 CrossRef CAS PubMed.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed.
- A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629–634 CrossRef CAS PubMed.
- W. Ma, C. Yang, X. Gong, K. Lee and A. J. Heeger, Adv. Funct. Mater., 2005, 15, 1617–1622 CrossRef CAS.
- Z. Zheng, O. M. Awartani, B. Gautam, D. Liu, Y. Qin, W. Li, A. Bataller, K. Gundogdu, H. Ade and J. Hou, Adv. Mater., 2017, 29, 1604241 CrossRef PubMed.
- P. W. Liang, C. Y. Liao, C. C. Chueh, F. Zuo, S. T. Williams, X. K. Xin, J. Lin and A. K. Y. Jen, Adv. Mater., 2014, 26, 3748–3754 CrossRef CAS PubMed.
- D. Baran, R. S. Ashraf, D. A. Hanifi, M. Abdelsamie, N. Gasparini, J. A. Röhr, S. Holliday, A. Wadsworth, S. Lockett and M. Neophytou, Nat. Mater., 2017, 16, 363 CrossRef CAS PubMed.
- W. Zhao, D. Qian, S. Zhang, S. Li, O. Inganäs, F. Gao and J. Hou, Adv. Mater., 2016, 28, 4734–4739 CrossRef CAS PubMed.
- W. Zhao, S. Li, S. Zhang, X. Liu and J. Hou, Adv. Mater., 2017, 29, 1604059 CrossRef PubMed.
- Q. An, F. Zhang, J. Zhang, W. Tang, Z. Deng and B. Hu, Energy Environ. Sci., 2016, 9, 281–322 RSC.
- X. Che, Y. Li, Y. Qu and S. R. Forrest, Nat. Energy, 2018, 3, 422 CrossRef CAS.
- T. Kuwabara, K. Yano, T. Yamaguchi, T. Taima, K. Takahashi, D. Son and K. Marumoto, J. Phys. Chem. C, 2015, 119, 5274–5280 CrossRef CAS.
- V. Vohra, K. Kawashima, T. Kakara, T. Koganezawa, I. Osaka, K. Takimiya and H. Murata, Nat. Photonics, 2015, 9, 403 CrossRef CAS.
- Y. Sun, J. H. Seo, C. J. Takacs, J. Seifter and A. J. Heeger, Adv. Mater., 2011, 23, 1679–1683 CrossRef CAS PubMed.
- Z.-S. Ma, Q.-K. Wang, C. Li, Y.-Q. Li, D.-D. Zhang, W. Liu, P. Wang and J.-X. Tang, Opt. Commun., 2015, 356, 541–545 CrossRef CAS.
- O. Örnek, Z. A. Kösemen, S. Öztürk, B. Canımkubey, Ş. Fındık, M. Erkovan and A. Kösemen, Surf. Interfaces, 2017, 9, 64–69 CrossRef.
- M. Thambidurai, J. Y. Kim, H.-j. Song, Y. Ko, N. Muthukumarasamy, D. Velauthapillai, V. W. Bergmann, S. A. Weber and C. Lee, J. Mater. Chem. A, 2014, 2, 11426–11431 RSC.
- S. Cho, K.-D. Kim, J. Heo, J. Y. Lee, G. Cha, B. Y. Seo, Y. D. Kim, Y. S. Kim, S.-Y. Choi and D. C. Lim, Sci. Rep., 2014, 4, 4306 CrossRef PubMed.
- C. V. Hoven, A. Garcia, G. C. Bazan and T. Q. Nguyen, Adv. Mater., 2008, 20, 3793–3810 CrossRef CAS.
- W. Lee, J. H. Seo and H. Y. Woo, Polymer, 2013, 54, 5104–5121 CrossRef CAS.
- A. Duarte, K.-Y. Pu, B. Liu and G. C. Bazan, Chem. Mater., 2010, 23, 501–515 CrossRef.
- S. Lee, T. L. Nguyen, S. Y. Lee, C. H. Jang, B. R. Lee, E. D. Jung, S. Y. Park, Y. J. Yoon, J. Y. Kim, H. Y. Woo and M. H. Song, Adv. Mater., 2018, 30, 1706034 CrossRef PubMed.
- M. Al Kobaisi, S. V. Bhosale, K. Latham, A. M. Raynor and S. V. Bhosale, Chem. Rev., 2016, 116, 11685–11796 CrossRef PubMed.
- X. Guo, A. Facchetti and T. J. Marks, Chem. Rev., 2014, 114, 8943–9021 CrossRef CAS PubMed.
- Z. Chen, Z. Hu, Z. Wu, X. Liu, Y. Jin, M. Xiao, F. Huang and Y. Cao, J. Mater. Chem. A, 2017, 5, 19447–19455 RSC.
- Y. Zhou, C. Fuentes-Hernandez, J. Shim, J. Meyer, A. J. Giordano, H. Li, P. Winget, T. Papadopoulos, H. Cheun and J. Kim, Science, 2012, 336, 327–332 CrossRef CAS PubMed.
- H. Kang, S. Hong, J. Lee and K. Lee, Adv. Mater., 2012, 24, 3005 CrossRef CAS PubMed.
- H.-B. Kim, Y. J. Yoon, J. Jeong, J. Heo, H. Jang, J. H. Seo, B. Walker and J. Y. Kim, Energy Environ. Sci., 2017, 10, 1950–1957 RSC.
- Y. J. Park, M. J. Cha, Y. J. Yoon, S. Cho, J. Y. Kim, J. H. Seo and B. Walker, Adv. Electron. Mater., 2017, 3, 1700184 CrossRef.
- C. Han, Y. Cheng, L. Chen, L. Qian, Z. Yang, W. Xue, T. Zhang, Y. Yang and W. Cao, ACS Appl. Mater. Interfaces, 2016, 8, 3301–3307 CrossRef CAS PubMed.
- X. Ouyang, R. Peng, L. Ai, X. Zhang and Z. Ge, Nat. Photonics, 2015, 9, 520 CrossRef CAS.
- D. Baran, S. Erten-Ela, A. Kratzer, T. Ameri, C. J. Brabec and A. Hirsch, RSC Adv., 2015, 5, 64724 RSC.
- C. J. Brabec, A. Cravino, D. Meissner, N. S. Sariciftci, T. Fromherz, M. T. Rispens, L. Sanchez and J. C. Hummelen, Adv. Funct. Mater., 2001, 11, 374–380 CrossRef CAS.
- T. T. Do, H. S. Hong, Y. E. Ha, J. Park, Y.-C. Kang and J. H. Kim, ACS Appl. Mater. Interfaces, 2015, 7, 3335–3341 CrossRef CAS PubMed.
- L. Yan, Y. Song, Y. Zhou, B. Song and Y. Li, Org. Electron., 2015, 17, 94–101 CrossRef CAS.
- J. H. Seo, R. Yang, J. Z. Brzezinski, B. Walker, G. C. Bazan and T. Q. Nguyen, Adv. Mater., 2009, 21, 1006–1011 CrossRef CAS.
- R. Hauert, A. Glisenti, S. Metin, J. Goitia, J. Kaufman, P. Van Loosdrecht, A. Kellock, P. Hoffmann, R. White and B. Hermsmeier, Thin Solid Films, 1995, 268, 22–29 CrossRef CAS.
- L. Köhler, D. Gourdin, R. Sporken, K. Grigorov, J. Riga and R. Caudano, Polym. Int., 1998, 47, 474–478 CrossRef.
- S. A. Al-Bataineh, L. G. Britcher and H. J. Griesser, Surf. Interface Anal., 2006, 38, 1512–1518 CrossRef CAS.
Footnote |
† Electronic supplementary information (ESI) available: Synthesis of NPEs, device data for P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM solar cells, output characteristics of IZO FETs, Au 4f XPS spectra, AFM images and water contact angle measurements of NPE films. See DOI: 10.1039/c9ra04299g PC61BM solar cells, output characteristics of IZO FETs, Au 4f XPS spectra, AFM images and water contact angle measurements of NPE films. See DOI: 10.1039/c9ra04299g |
| This journal is © The Royal Society of Chemistry 2019 |