Open Access Article
Open Access ArticleA catalyst- and solvent-free protocol for the sustainable synthesis of fused 4H-pyran derivatives†
Md. Musawwer Khan *a,
Sumbulunnisan Shareefa,
Saigala and
Subash C. Sahoo
*a,
Sumbulunnisan Shareefa,
Saigala and
Subash C. Sahoo b
b
aDepartment of Chemistry, Aligarh Muslim University, Aligarh, 202002, India. E-mail: musawwer@gmail.com
bDepartment of Chemistry, Center of Advanced Studies in Chemistry, Panjab University, Chandigarh-160014, India
First published on 22nd August 2019
Abstract
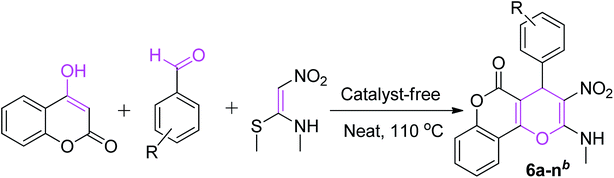
An efficient and cost-effective method was developed for the synthesis of two kinds of fused 4H-pyran derivatives, namely, dihydropyrano[2,3-c]pyrazole 4 and pyrano[3,2-c]chromenone 6. The reactions of 3-methyl-1-phenyl-5-pyrazolone/4-hydroxycoumarin with aromatic aldehydes and (E)-N-methyl-1-(methylthio)-2-nitroethenamine (NMSM), involving the Knoevenagel, Michael-addition, O-cyclization and elimination reactions under thermal heating, afforded the desired products. The synthesized compounds were characterized by standard spectroscopic techniques. Further, the structures of pyrazole-fused 4H-pyran 4a and coumarin-fused 4H-pyran 6b were confirmed by single-crystal XRD analysis. The short reaction time, good-to-excellent yields, elimination of the use of expensive, metallic and toxic catalysts or hazardous organic solvents and high atom-economy are some noteworthy features of this protocol.
Introduction
Recent advances in science and technology are focusing on the development of approaches that prevent pollution and minimize the usage of non-renewable resources. Recently, green techniques and processes, which obey green chemistry principles have gained significant ecological and economic interest.1 Green chemistry not only deals with processes that reduce the generation of hazardous chemicals but also introduces modern synthetic approaches, wherein synthetic chemists construct compounds more ecologically and proficiently.2In this context, catalyst- and solvent-free multi-component syntheses have appeared as the most powerful strategy in contemporary situations, as they fulfill maximum principles of green chemistry.3 Multicomponent domino reactions (MDRs) are convergent organic reactions, in which three or more reactant molecules with multiple bond-forming tendencies and high atom-economy combine in one pot without the isolation of undesirable intermediates.4 Therefore, MDR strategies have been explored to access functionalized organic compounds under green-chemistry conditions and are preferred over stepwise synthetic strategies.5
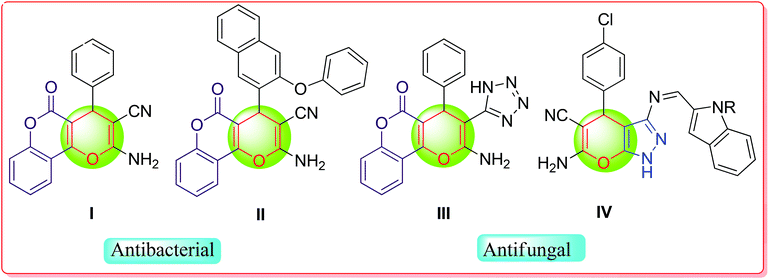
Fused heterocycles represent a dynamic class of organic compounds with notable medicinal and agrochemical properties.6 Among the fused heterocycles, pyrazole- and coumarin-fused oxygen-containing heterocyclic systems, particularly 4H-pyran derivatives, are omnipresent in several natural and unnatural bioactive molecules with promising properties both in pharmacological and materials sciences.7 Pyrazole-fused 4H-pyran derivatives have shown several biological activities such as antimalarial,8 antibacterial,9 antimicrobial,10 anti-inflammatory,11 anti-tubercular,12 hypoglycemic and vasodilator activities.13 On the other hand, coumarin-fused 4H-pyrans also exhibit several activities like antioxidant,14 antifungal15 and anticancer.16 Fig. 1 provides a glimpse of fused 4H-pyran-based biologically and medicinally active compounds. For example, compounds I and II have antibacterial characteristics,17 whereas, compounds III and IV possess antifungal activity.18
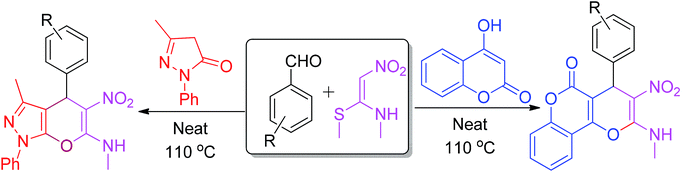
Recently, (E)-N-methyl-1-(methylthio)-2-nitroethenamine (NMSM) was explored as a fascinating building block for the construction of diverse heterocyclic compounds.19 The starting material NMSM has a polarizable ethylene skeleton, bearing electrophilic and nucleophilic centers at the two ends, as well as electron-withdrawing (–NO2), electron-donating (CH3NH–) and good leaving groups (methylsulfanyl), where each of them plays an important role in the formation of products.
A literature scan revealed that the synthesis of pyrazole-fused 4H-pyran was performed previously using InCl3 (ref. 20) and piperidine,21 whereas coumarin-fused 4H-pyran scaffolds were prepared using catalysts such as STA,22 6,6′-thiobis(methylene)-β-cyclodextrin dimer23 and piperidine.24 Regardless of the adequacy of these reported methods, each exhibits one or more shortcomings, such as the required usage of catalysts20–24 or solvents20,24 and prolonged reaction time.20,24 Therefore, there still remains a scope of improvement of the synthetic strategy to access such important fused 4H-pyrans. In continuation of our effort to develop green and sustainable methodologies for the synthesis of heterocycles by using NMSM and other building blocks,25,26 we herein intended to utilize the distinctive reactivity of NMSM and aromatic aldehydes with 3-methyl-1-phenyl-5-pyrazolone/4-hydroxycoumarin for the synthesis of fused 4H-pyran, as depicted in Scheme 1.
Results and discussion
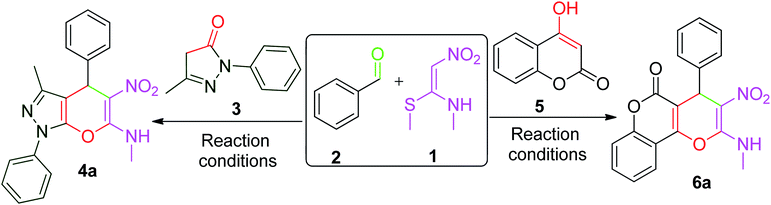
For the present study, a model reaction was initially conducted between NMSM 1 (1.0 mmol), benzaldehyde 2 (1.0 mmol) and 3-methyl-1-phenyl-5-pyrazolone 3 (1.0 mmol) in the presence of 10 mol% of ZnO nano-catalyst under refluxing ethanol (4 mL) (Table 1, entry 1). The progress of the reaction was monitored by thin layer chromatography (TLC). After the completion of the reaction, the product 4a was isolated in 60% yield by simple filtration. The structure of the compound 4a was confirmed by its melting point, IR, 1H and 13C NMR spectroscopies, and the data matched well with the reported data.20–24 Encouraged by these results, we proceeded to optimize the reaction conditions. For this process, the model reaction was executed in the presence of different acid and base catalysts (10 mol%) in EtOH (4 mL) as well as without catalyst in EtOH with reflux (Table 1, entries 2–6). Unfortunately, we did not observe any improvement in the results. Therefore, the original reaction was performed without any catalyst or solvent at 80 °C (Table 1, entry 7). Amusingly, the reaction afforded better results in terms of reaction time and yield.| Entry | Catalyst (mol%) | Solvent | Temp. | 4a | 6a | ||
|---|---|---|---|---|---|---|---|
| Time | Yieldb (%) | Time | Yieldb (%) | ||||
| a Reaction conditions: 3-methyl-1-phenyl-5-pyrazolone/4-hydroxy coumarin (1 mmol), benzaldehyde (1 mmol) and NMSM (1 mmol).b Isolated yields.c Optimized reaction conditions. | |||||||
| 1 | Nano ZnO (10 mol%) | EtOH | Reflux | 7 h | 60 | 5 h | 65 |
| 2 | DMAP (10 mol%) | EtOH | Reflux | 10 h | 49 | 9 h | 51 |
| 3 | Et3N (10 mol%) | EtOH | Reflux | 11 h | 43 | 12 h | 40 |
| 4 | I2 (10 mol%) | EtOH | Reflux | 6 h | 55 | 4 h | 61 |
| 5 | AcOH (10 mol%) | EtOH | Reflux | 5 h | 50 | 3 h | 53 |
| 6 | No catalyst | EtOH | Reflux | 4 h | 34 | 2 h | 55 |
| 7 | No catalyst | Neat | 80 °C | 2 h | 63 | 1 h | 66 |
| 8 | No catalyst | Neat | 90 °C | 1 h | 70 | 55 min | 73 |
| 9 | No catalyst | Neat | 100 °C | 50 min | 78 | 40 min | 82 |
| 10c | No catalyst | Neat | 110 °C | 45 min | 87 | 30 min | 91 |
| 11 | No catalyst | Neat | 120 °C | 45 min | 87 | 30 min | 91 |
| 12 | No catalyst | Neat | 130 °C | 40 min | 84 | 30 min | 89 |
In addition, the above reaction was executed at different temperatures ranging from 90 to 130 °C under catalyst- and solvent-free conditions (Table 1, entries 8–12) to assess the effect of temperature. We observed that maximum yields were obtained at 110 °C within a short time period, and no further increase in the yield occurred with an increase in the temperature. Thus, the solvent-free and catalyst-free reaction at 110 °C emerged as the optimized condition for the present protocol for the synthesis of compound 4a.
Similar optimal conditions were found for the synthesis of compound 6a by performing a series of reactions between 4-hydroxycoumarine, benzaldehyde and NMSM, which are also summarized in Table 1.
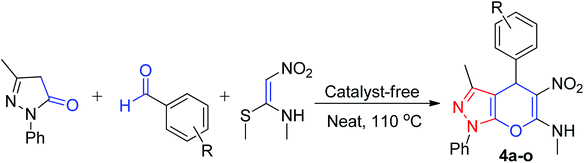
After determining the optimized conditions, we focused on establishing the substrate scope. A reaction was performed between compound 3, 4-chlorobenzaldehyde and NMSM under the optimized reaction conditions, which gave the desired product 4b with 85% yield (Table 2).
| Entry | Ar | Time (min) | Yieldb (%) | Product | mp (°C) | Reported mp (°C) | Ref. |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 3-methyl-1-phenyl-5-pyrazolone (1 mmol), aryl aldehydes (1 mmol), NMSM (1 mmol) under neat conditions at 110 °C.b Isolated yields. | |||||||
| 1 | C6H5 | 45 | 87 | 4a | 206–208 | 208–209 | 21 |
| 2 | 4-Cl-C6H5 | 40 | 85 | 4b | 206–208 | 210–211 | 20 |
| 3 | 4-Br-C6H5 | 45 | 86 | 4c | 208–209 | 228–229 | 20 |
| 4 | 4-F-C6H5 | 40 | 86 | 4d | 202–204 | 223–224 | 20 |
| 5 | 4-Me-C6H5 | 50 | 81 | 4e | 216–218 | 219–220 | 21 |
| 6 | 4-Et-C6H5 | 50 | 79 | 4f | 210–212 | — | — |
| 7 | 4-NO2-C6H5 | 40 | 89 | 4g | 217–218 | 217–218 | 21 |
| 8 | 4-OMe-C6H5 | 45 | 87 | 4h | 210–212 | 220–221 | 20 |
| 9 | 3-Cl-C6H5 | 50 | 85 | 4i | 254–256 | 256–258 | 20 |
| 10 | 2-Cl-C6H5 | 55 | 83 | 4j | 244–245 | 244–245 | 21 |
| 11 | 3-Br-C6H5 | 55 | 83 | 4k | 238–240 | 238–239 | 20 |
| 12 | 3-NO2-C6H5 | 45 | 86 | 4l | 227–228 | 228–229 | 20 |
| 13 | 3,4-OMe-C6H5 | 40 | 90 | 4m | 216–217 | — | — |
| 14 | 3,4,5-OMe-C6H5 | 45 | 91 | 4n | 218–220 | 218–219 | 20 |
| 15 | Pyrid-3-yl | 50 | 83 | 4o | 220–221 | — | — |
Next, using the optimized reaction conditions, reactions were performed with other aromatic aldehydes having substituents such as 4-Br, 4-F, 4-Me, 4-Et, 4-NO2, 4-OMe, 3-Cl, 2-Cl, 3-Br, 3-NO2, 3,4-(OMe)2, and 3,4,5-(OMe)3 with 3-methyl-1-phenyl-5-pyrazolone and NMSM. The reactions afforded corresponding products 4c–4n with good-to-excellent yields. Similarly, this reaction was also performed with a hetero-aromatic aldehyde, i.e. pyridine-3-carbaldehyde, which afforded the expected product 4o in 83% yield.
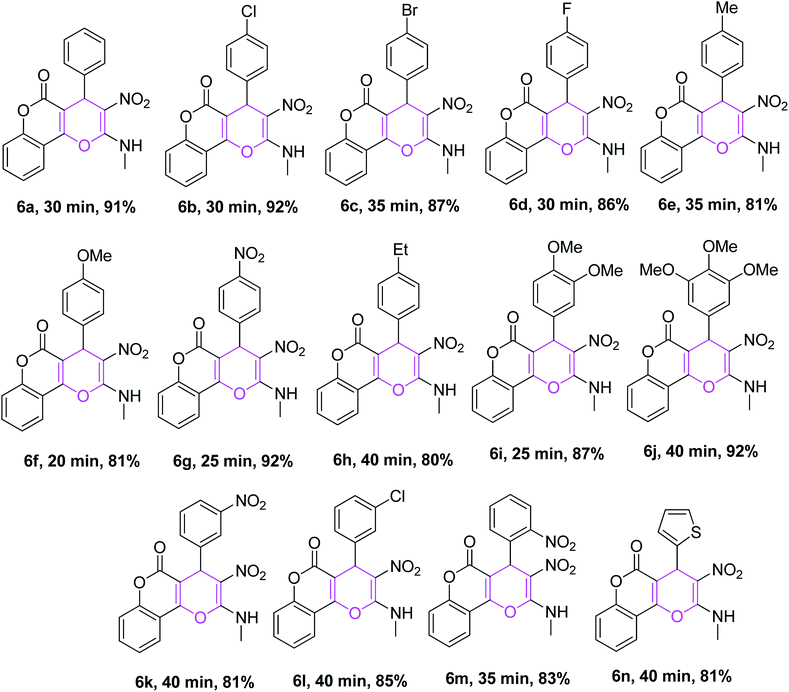
Further, to investigate the generality and applicability of the above protocol, we executed the reaction by replacing reactant 3 with 4-hydroxycoumarin and 5 using aromatic aldehydes tethered to substituents like 4-Cl, 4-Br, 4-F, 4-Me, 4-OMe, 4-NO2, 4-Et, 3,4-diOMe, 3,4,5-triOMe, 3-NO2, 3-Cl, 2-NO2 and NMSM under similar optimized conditions, which afforded the desired coumarin-fused 4H-pyrans 6b–m in good yields, as shown in Table 3. Aldehydes possessing both electron-withdrawing groups as well as electron-donating groups afforded products smoothly. The reaction of 4-hydroxycoumarin was also performed with thiophene-2-carbaldehyde and NMSM, which furnished the product 6n in good yield.
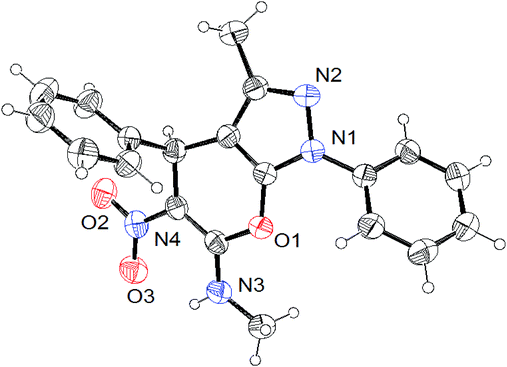
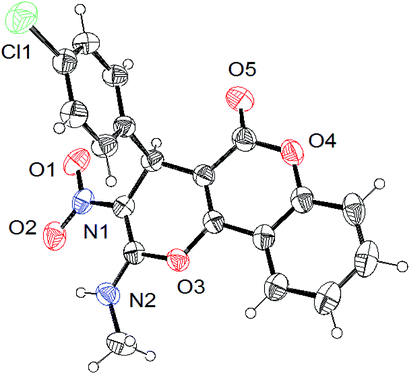
All the synthesized compounds were characterized by spectroscopic techniques including IR, 1H and 13C NMR spectroscopies and elemental analysis; the resulting data for several of the reported compounds are well-matched with the literature.20–24 Further, the structure and stereochemistry of the compounds 4a (CCDC 1901104) and 6b (CCDC 1901105) were unambiguously confirmed by single-crystal X-ray diffraction analysis, and the ORTEP diagrams are shown in Fig. 2 and 3, respectively.
 | ||
| Fig. 2 ORTEP representation of compound 4a (CCDC 1901104). | ||
 | ||
| Fig. 3 ORTEP representation of compound 6b (CCDC 1901105). | ||
Single crystals of the compounds 4a and 6b were mounted on a glass fiber and utilized for XRD data collection. Light yellow and white-colored crystals of compounds 4a and 6b, respectively, were grown by the slow evaporation of CH3CN solvent for X-ray analysis. The data were obtained from a Super Nova, single source at offset/far and HyPix3000 diffractometer using graphite-monochromated Mo(Kα) radiation (λ = 0.71073 Å) at 293 K.
The crystallized compound of 4a has a monoclinic crystal system, with the space group P21/c and the unit cell dimensions a = 10.7875(3) Å, b = 20.8262(4) Å, c = 8.0335(2) Å. On the other hand, the crystallized compound of 6b also has a monoclinic crystal system with the space group P21/c and unit cell dimensions a = 8.5412(2) Å, b = 13.1791(3) Å, c = 14.8906(3) Å.
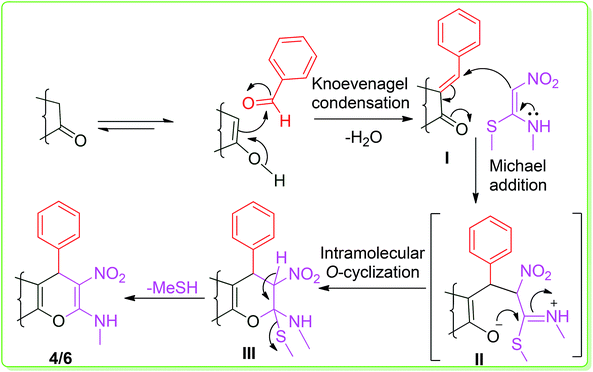
Finally, the formation of the products can be rationalized via a domino reaction involving Knoevenagel condensation, Michael-addition, O-cyclization and elimination mechanisms, as illustrated in Scheme 2.
The first step is a simple Knoevenagel condensation of an aromatic aldehyde with a 1,3-dinuleophilic oxygen-containing compound (4-hydroxycoumarin or 3-methyl-1-phenyl-5-pyrazolone) to generate an intermediate I. The intermediate I undergoes a Michael-addition reaction with NMSM to form species II. Then, species II affords the desired product 4/6 by an intra-molecular O-cyclization followed by the elimination of –MeSH via species III.
Conclusion
In summary, we have successfully developed a practical and green method for the synthesis of highly functionalized pyrazole- and coumarin-fused 4H-pyrans by the reaction of NMSM or aryl aldehydes with 3-methyl-1-phenyl-5-pyrazolone or 4-hydroxycoumarin, respectively, under thermal heating and neat conditions. The presented protocol is characterized by important features such as a short reaction time, good-to-excellent yields, elimination of the use of toxic catalysts or solvents, easy isolation of compounds without traditional column purification and applicability to a broad range of substrates.Experimental
General
4-Hydroxycoumarin, 3-methyl-1-phenyl-5-pyrazolone, NMSM and all the aldehydes were procured from Sigma-Aldrich. All the solvents were obtained from Merck and Otto Chemie. All the reactions were completed in a REMI 2MLH thermo-mechanical stirrer. TLC analysis was carried out using silica gel GF-254 from SRL (Alfa Aesar). Melting points were obtained on a Stuart digital melting point apparatus (SMP10) and are uncorrected. IR spectra were performed with potassium bromide (KBr) pellets on a PerkinElmer 10.4.00 IR spectrophotometer. 1H and 13C NMR spectral analyses were performed on Bruker (Avance-II 400 MHz), Varian-AS400 NMR, and Bruker Bio Spin GmbH spectrometers using tetramethylsilane (TMS) as the internal standard and DMSO-d6 or CDCl3 as the solvent. Crystal data were collected with a Super Nova, single source at offset/far and HyPix3000 diffractometer (CCD) using graphite monochromated MoKα radiation (λ = 0.71073 Å) at 296 K. HRMS spectra were recorded on a high-resolution mass spectrometer XEVO G2-XS QTOF.Typical procedure for the preparation of functionalized fused 4H-pyrans (4 and 6)
A dried 5 mL round-bottom flask was equipped with a Teflon-coated magnet and charged with a combination of 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one/4-hydroxycoumarin (1 mmol), aromatic aldehyde (1 mmol), and NMSM (1 mmol). The mixture of all the reagents was heated at 110 °C with stirring for a specified time under neat conditions. The progress of the reactions was monitored by TLC. After completion of the reaction as indicated by TLC, the resulting precipitate was cooled, and 2 mL of ethanol was added and stirred for 5 min. Next, the precipitate was filtered and washed with 3 mL cold ethanol. Purification of the crude product was done by recrystallization from hot acetonitrile to yield the pure products.Characterization data of selected compounds
Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. Shareef and Saigal are grateful to CSIR, New Delhi for their research fellowship. The authors would like to thanks SAIF-PU, Panjab University, Chandigarh and SAIF, IIT Roorkee, IIT Ropar for providing the spectral data such as 1H and 13C NMR, HRMS, elemental analysis and SC Sahoo thanks DST-FIST for the single crystal XRD facility at Panjab University Chandigarh. We are also obliged to the Chairman, Department of Chemistry, AMU, Aligarh for providing general facility to execute this task.Notes and references
- (a) R. B. Carvalho and S. V. Joshi, Green Chem., 2019, 21, 1921 RSC; (b) Y. Gu and F. Jerôme, Green Chem., 2010, 12, 1127 RSC; (c) P. J. Dunn, Chem. Soc. Rev., 2012, 41, 1452 RSC.
- (a) P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press Inc., 1st edn, New York, 1998 Search PubMed; (b) M. M. Kirchhoff, Environ. Sci. Technol., 2003, 37, 5349 CrossRef CAS; (c) I. T. Horvath and P. T. Anastas, Chem. Rev., 2007, 107, 2169 CrossRef CAS PubMed.
- (a) M. S. Singh and S. Chowdhury, RSC Adv., 2012, 2, 4547 RSC; (b) A. Kumar and S. A. Sharma, Green Chem., 2011, 13, 2017 RSC.
- (a) M. M. Khan, S. Khan, Saigal and S. Iqbal, RSC Adv., 2016, 6, 42045 RSC; (b) A. T. Khan, D. K. Das and M. M. Khan, Tetrahedron Lett., 2011, 52, 4539 CrossRef CAS; (c) R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958 RSC; (d) A. T. Khan, M. M. Khan, D. K. Das and M. Lal, J. Heterocycl. Chem., 2012, 49, 1362 CrossRef CAS.
- (a) H. G. O. Alvim, J. R. Correa, J. A. F. Assumpcao, W. A. da Silva, M. O. Rodrigues, J. L. de Macedo, M. Fioramonte, F. C. Gozzo, C. C. Gatt and B. A. D. Neto, J. Org. Chem., 2018, 83, 4044 CrossRef CAS; (b) H. G. O. Alvim, D. L. J. Pinheiro, V. H. Carvalho-Silva, M. Fioramonte, F. C. Gozzo, W. A. da Silva, G. W. Amarante and B. A. D. Neto, J. Org. Chem., 2018, 83, 12143 CrossRef CAS; (c) L. Zeng, B. Huang, Y. Shen and S. Cui, Org. Lett., 2018, 20, 3460 CrossRef CAS PubMed.
- (a) P. N. Kalaria, S. C. Karad and D. K. Raval, Eur. J. Med. Chem., 2018, 158, 917 CrossRef CAS PubMed; (b) A. K. Mourad, F. K. Mohamed and A. Y. Soliman, Heterocycles, 2018, 96, 50 CrossRef CAS; (c) Saigal, S. Shareef, H. Rahman and M. M. Khan, Curr. Org. Chem., 2019, 23, 1045 CrossRef.
- (a) S. C. Huang, P. L. Wu and T. S. Wu, Phytochemistry, 1997, 44, 179 CrossRef CAS; (b) C. R. Su, S. F. Yeh and C. M. Liu, Bioorg. Med. Chem., 2009, 17, 6137 CrossRef CAS; (c) D. K. Yadav and M. A. Quraishi, Ind. Eng. Chem. Res., 2012, 51, 8194 CrossRef CAS.
- (a) M. Klein, P. Diner, D. Dorin-Semblat, C. Doerig and M. Grotli, Org. Biomol. Chem., 2009, 7, 3421 RSC; (b) S. M. Deshmukh and D. P. Hiwarale, Der Pharma Chem., 2017, 9, 109 CAS.
- (a) B. S. Dawane, O. S. Yemul, S. S. Chobe, G. G. Mandawad, R. D. Kamble, A. V. Shinde, V. S. Kale, A. O. Hurne, M. A. Pawde, M. P. Kale, N. P. Desai, R. R. Salgare, M. B. Patil, S. N. Mundhe and S. R. Chavan, Der Pharma Chem., 2011, 3, 300 CAS; (b) S. Ahadi, Z. Yasaei and A. Bazgir, J. Heterocycl. Chem., 2010, 47, 1090 CrossRef CAS.
- (a) M. T. Flavin, J. D. Rizzo, A. Khilevich, A. Kucherenko, A. K. Sheinkman, V. Vilaychack, L. Lin, W. Chen, E. M. Greenwood, T. Pengsuparp, J. M. Pezzuto, S. H. Hughes, T. M. Flavin, M. Cibulski, W. A. Boulanger, R. L. Shone and Z. Q. Xu, J. Med. Chem., 1996, 39, 1303 CrossRef CAS PubMed; (b) V. F. De Andrade-Neto, M. O. Goulart, J. F. Da Silva Filho, M. J. Da Silva, M. D. C. Pinto, A. V. Pinto, M. G. Zalis, L. H. Carvalho and A. U. Krettli, Bioorg. Med. Chem. Lett., 2004, 14, 1145 CrossRef CAS PubMed.
- A. Kumar, R. A. Maurya, S. A. Sharma, P. Ahmad, A. B. Singh, G. Bhatia and A. K. Srivastava, Bioorg. Med. Chem. Lett., 2009, 19, 6447 CrossRef CAS PubMed.
- S. J. Vaghasiya, D. K. Dodiya, A. R. Trivedi and V. H. Shah, ARKIVOC, 2008,(xii), 1 Search PubMed.
- Y. L. Zhang, B. Z. Chen, K. Q. Zheng, M. L. Xu, X. H. Lei and X. B. Yaoxue, Chem. Abstr., 1982, 96, 135383 Search PubMed.
- E. Melliou, P. Magiatis, S. Mitaku, A.-L. Skaltsounis, E. Chinou and I. Chinou, J. Nat. Prod., 2005, 68, 78 CrossRef CAS PubMed.
- (a) M. A. Al-Haiza, M. S. Mostafa and M. Y. El-Kady, Molecules, 2003, 8, 275 CrossRef CAS; (b) P. C. B. Page, L. F. Appleby, D. Day, Y. Chan, B. R. Buckley, S. M. Allim and M. J. Mckenzie, Org. Lett., 2009, 11, 1991 CrossRef PubMed.
- A. Lacy and R. O' Kennedy, Curr. Pharm. Des., 2004, 10, 3797 CrossRef CAS PubMed.
- (a) P. N. Kalaria, S. P. Satasia and D. K. Raval, New J. Chem., 2014, 38, 1512 RSC; (b) D. C. Mungra, M. P. Patel, D. P. Rajani and R. G. Patel, Eur. J. Med. Chem., 2011, 46, 4192 CrossRef CAS PubMed.
- S. A. El-Assiery, G. H. Sayed and A. Fouda, Acta Pharm., 2004, 54, 143 CAS.
- (a) Saigal, S. Khan, H. Rahman, Shafiullah and M. M. Khan, RSC Adv., 2019, 9, 14477 RSC; (b) F. Rahimi, H. Hosseini, M. Bayat and Y. T. Jeong, Tetrahedron, 2018, 59, 818 CrossRef CAS; (c) A. M. Jadhav, Y. I. Kim, K. T. Lim and Y. T. Jeong, Tetrahedron, 2018, 59, 554 CrossRef CAS; (d) A. Chaudhary, J. M. Khurana, G. Khanna and M. Saroha, ChemistrySelect, 2018, 3, 6334 CrossRef CAS; (e) B. Balachandra and S. Shanmugam, ChemistrySelect, 2018, 3, 2037 CrossRef CAS.
- D. N. Survase, H. V. Chavan, S. B. Dongare, S. D. Ganapure and V. B. Helavi, Iran. Chem. Commun., 2017, 5, 105 CAS.
- K. Jayabal and T. P. Paramasivan, Tetrahedron Lett., 2014, 55, 2010 CrossRef CAS.
- A. M. Jadhav, S. K. Krishnammagari, J. T. Kim and Y. T. Jeong, Tetrahedron, 2017, 73, 5163 CrossRef CAS.
- V. V. Shinde, D. Jeong, E. Cho and S. Jung, Tetrahedron, 2018, 74, 194 CrossRef CAS.
- J. Kamalraja, D. Muralidharan and P. T. Perumal, Synlett, 2012, 23, 2894 CrossRef CAS.
- (a) M. M. Khan, B. Saigal, S. Shareef, S. Khan and S. C. Sahoo, Synth. Commun., 2018, 48, 2683 CrossRef CAS; (b) M. M. Khan, Saigal, S. Khan, S. Shareef and S. C. Sahoo, RSC Adv., 2018, 8, 41892 RSC; (c) M. M. Khan, Saigal and S. Khan, J. Heterocycl. Chem., 2019, 56, 1020 CrossRef CAS.
- (a) M. M. Khan, Saigal, S. Khan, S. Shareef and M. Danish, ChemistrySelect, 2018, 3, 6830 CrossRef CAS; (b) M. M. Khan, Saigal, S. Khan, S. Shareef and S. Hussain, ChemistrySelect, 2018, 3, 2261 CrossRef CAS; (c) M. M. Khan, S. Khan, Saigal and S. C. Sahoo, ChemistrySelect, 2018, 3, 1371 CrossRef CAS; (d) M. M. Khan, S. Khan, S. Iqbal, Saigal and R. Yousuf, New J. Chem., 2016, 40, 7504 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1901104 and 1901105. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra04370e |
| This journal is © The Royal Society of Chemistry 2019 |