Open Access Article
Open Access ArticleGrowth of carbon dioxide whiskers†
Avinash Kumar Both and
Chin Li Cheung
and
Chin Li Cheung *
*
University of Nebraska-Lincoln, Lincoln, Nebraska 68588, USA. E-mail: ccheung2@unl.edu
First published on 31st July 2019
Abstract
We report the growth of carbon dioxide (CO2) whiskers at low temperatures (−70 °C to −65 °C) and moderate pressure (4.4 to 1.0 bar). Their axial growth was assessed by optical video analysis. The identities of these whiskers were confirmed as CO2 solids by Raman spectroscopy. A vapor–solid growth mechanism was proposed based on the influence of the relative humidity on the growth.
1. Introduction
The study of phase stabilities of molecular solids at high pressures and extreme temperatures is important to understand the nature of chemical bonding, intermolecular interactions, and collective behavior of molecules in condensed phases. For simple molecular systems, it can yield new physical phenomena, ranging from solid–solid phase transitions to complex transformations where the molecular framework itself is profoundly perturbed and new extended materials are attained.1 This latter case usually occurs when the intermolecular and intramolecular distances converge so that the initial molecular identity is altered, and chemical bonds are reconstructed.2 Consequently, such new extended materials are usually revealed by their unique growth morphologies, phases, phase transitions and/or covalent bonding motifs. Examples of these systems include the formation of dexamethasone acetate hollow whiskers,3 metallic deuterium,4 natural gas clathrate hydrates,5 new phases of ice,6 and a new phase of solid iodine.7Carbon dioxide (CO2) is a chemically stable, linear molecule. It is one of the most important greenhouse gases owing to its ability to absorb and radiate energy in the infrared range and its high abundance in the atmosphere because of the fossil fuel burning activities.8 To reduce the amount of CO2 in the atmosphere, researchers have proposed sequestering CO2 in its solid form by storing it deep under the ground.9 Although CO2 is one of the most fundamental chemical species in nature, its solid-phase behavior under high pressures continues to confound the scientific community. Since the discovery of phase III of solid CO2 by powder X-ray diffraction in 1994,10 researchers have mapped out a rich CO2 phase diagram with many additional crystalline phases that range from molecular crystals at lower pressures11 and in confined nanoslits12 to extended covalent and ionic phases at high pressures.13 Experimental characterization of these crystal structures and their solid–solid phase boundaries has often been proven to be a daunting task because the considerable kinetic path-dependence and hysteresis occur in the phase transitions.14 In addition, the difficulty in acquiring high-quality diffraction data and maintaining sharp pressure gradients within samples that complicate spectroscopic measurements obscure new phase discoveries.15 As a result, the literature on high pressure solid CO2 contains numerous contradictory experimental interpretations16 and further verification studies are indispensable to advance the understanding of CO2 solids.
Herein, we report our observation of the growth of CO2 whiskers under low temperature (−70 °C to −65 °C) and moderate pressure (4.4 to 1.0 bar). The growth conditions were attained using a temperature-controlled microscopy pressure stage. The axial growth of these whiskers was evaluated by optical video analysis. A vapor–solid growth mechanism for these whiskers was proposed based on the observed growth process and the dependence of their growth on the relative humidity of the laboratory.
2. Experimental method
(100) Oriented 4′′-silicon wafers were purchased from University Wafer (Boston, MA). Solid dry ice and UHP grade CO2 (Matheson Tri-gas Inc., Montgomeryville, PA) were used as the CO2 source for the synthesis of CO2 whiskers.The growth of CO2 whiskers was performed inside a Linkam THMS600-PS temperature-controlled microscopy pressure stage (Linkam Scientific Instruments, Tadworth, United Kingdom) and was monitored using an Olympus SZ-STS optical microscope (Olympus Life Science, Center Valley, PA) (see Fig. S1†). Silicon wafers of approximately 1 × 1 cm2 size were used as substrates for growing the CO2 whisker materials. The relative humidity (R.H.) of the laboratory was maintained at ca. 42% or 65% using a humidifier (Levoit, Anaheim, CA). For each experiment, a silicon wafer was first placed on a quartz crucible located at the temperature-controlled platform inside the stage (see Fig. S1†). After the stage was sealed closed, the stage was first purged and then pressurized with UHP grade CO2 to 4.4 bar. The sample was cooled down to −70 °C at a cooling rate of 5 °C per minute to create a layer of solid CO2. To initiate the growth of the whisker-like structures, the pressure of the stage was decreased by venting the CO2 gas through a needle valve of the gas release valve assembly to the laboratory (see Fig. S1†). The pressure of the stage was observed to gradually drop from 4.4 bar to 1.3 bar in the first 30 seconds, but it continued to decrease at a slower rate of ca. 0.2 bar per minute for about 1.5 minutes until it reached atmospheric pressure (1.0 bar). During this period, the temperature of the substrate slightly increased from −70 °C to −65 °C. Eventually, the substrate was let warm up naturally to the room temperature. The evolution of the materials growth on the substrate was video recorded. To determine the dependence of whisker growth on R.H. of the laboratory, control experiments were performed either in a laboratory at ca. 25–30% R.H. or with the temperature-controlled microscopy pressure stage filled with Type 4A molecular sieves (8–12 mesh size beads, Sigma-Aldrich, Milwaukee, WI) to absorb moisture inside the stage.
Confocal micro-Raman microscopy was performed on a DXR Raman microscope (Thermo Fisher Scientific, LLC, Waltham, MA) equipped with the Linkam temperature-controlled microscopy pressure stage for studying the vibrational signature of CO2 and water in the as-grown materials (see Fig. S2†). Whisker-like structures were grown using the same conditions as those experiments observed under the Olympus optical microscope. The whiskers and substrates were probed using a 532 nm laser with 5 mW power and a 0.25 μm pinhole aperture.
3. Results and discussion
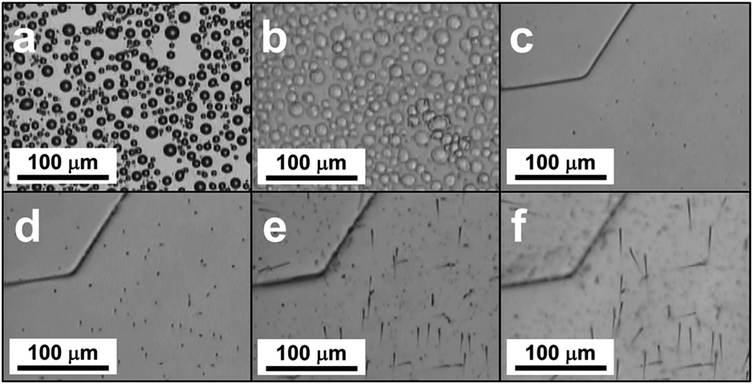
In our study, we examined the formation of CO2 whiskers on silicon wafers under moderate pressure (4.4 bar) of CO2 and at low temperature (−70 °C to −65 °C) inside a temperature-controlled microscopy pressure stage. As the CO2-pressurized microscopy stage was cooled from room temperature to −70 °C, we observed several sequential phenomena on the silicon substrate: (1) condensation of water vapor as hemispherical droplets, (2) freezing of the water droplets into ice crystals, and (3) condensation of gaseous CO2 and formation of solid CO2 (or dry ice) layer over the ice crystals (Fig. 1a–c). Upon the gradual depressurization of the stage from 4.4 bar to 1.0 bar of CO2 gas, nucleation of particles and growth of whiskers from these particles were observed (Fig. 1d–f and Video: CO2_whisker_growth.mp4). The chemical identity of the solid layer over the ice crystals was confirmed to be solid CO2 by confocal Raman spectroscopy. Typically, the Raman spectrum of CO2 gas molecules displays two peaks rather than a single peak representing the symmetric stretching of CO2 in the region of 1250 cm−1 to 1450 cm−1. Two peaks are observed because the energy level of the first excited state (ψ100) of the symmetric stretching mode (ν1) of CO2 is nearly the same as that of the energy level of the second excited state (ψ020) of the bending vibration (2ν2) and they have the same symmetry (Σg+). The mixing of these two states into two resulting symmetric states is known as the “Fermi resonance”.17,18 This leads to the re-representation of the symmetric CO2 vibration mode (ν1) via two experimentally observed Raman peaks at 1285 cm−1 and 1388 cm−1 in the Raman spectrum and they are commonly referred as the “Fermi diad”. This Fermi resonance phenomenon observed in CO2 is a classic example of strong anharmonic mode–mode coupling. It is not only evident in the gaseous state, but also is found to be pressure-dependent in the solid state.19 In our experiment, the Raman spectrum of the solid layer showed strong peaks at 1277 cm−1 and 1384 cm−1, which matched the locations of the vibration signatures of the Fermi diad in the spectra of our solid CO2 reference sample and literature data for phase I solid CO2 (ref. 20) (Fig. 2a). In addition, the intensity ratio of these two peaks (I(v1)/I(2v2) = 0.39) also agreed well with those of our solid CO2 reference. Since no vibration signatures indicating the hydroxyl (OH) stretching mode of water molecules were noticeable in the region of 3000 cm−1 to 3500 cm−1, this solid layer did not contain a detectable amount of water and thus was not CO2 gas clathrate hydrate. Note that upon the formation of the solid layer. As the stage was pressurized with CO2, gentle but continuous “bubbling” actions observed on this solid layer indicate the phase transition of the solid CO2 to gaseous CO2, probably due to the dynamic thermal gradient between the cooling stage and the casing of the stage (Fig. 1c and Video: CO2_whisker_growth.mp4).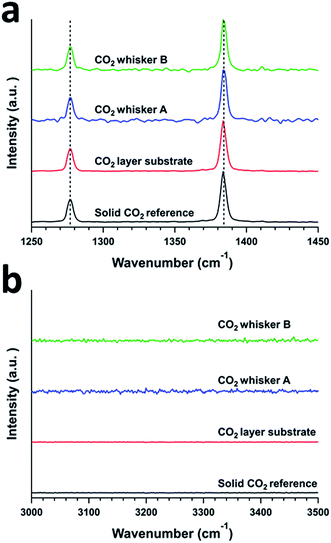 | ||
| Fig. 2 Confocal Raman spectra of a solid CO2 reference, a solid CO2 layer, and CO2 whiskers A and B. Ranges representing (a) Fermi diad and (b) OH-stretch peaks. | ||
After the microscopy stage was cooled to −70 °C, it was slowly depressurized to initiate the growth of the whisker structures, the pressure of the stage was observed to gradually drop from 4.4 bar to 1.3 bar in the first 30 seconds and it continued to decrease at a rate of ca. 0.2 bar per minute for about 1.5 minutes until it reached atmospheric pressure. At the same time, the temperature of the stage was let gradually rise to −65 °C. Nucleation of particles on the solid CO2 layer started to occur at the start of this process (Fig. 1d). Within 2 minutes, these particles initiated the growth many of small whiskers of lengths of 20 μm to 50 μm and diameters from 1 to 2 μm and they remained at the tip of these whiskers. The lengths of these whiskers grew to 50 μm to 100 μm within 3 minutes (Fig. S3†). Interestingly, the particles at the tips of whiskers were bigger when the experiment was carried out a laboratory at higher R.H. such as 65% vs. 42% (Fig. 3). In contrast, when we performed control experiments in a laboratory at low R.H. (such as 25% to 30%), using a microscopy stage filled with type 4A water-absorbing molecular sieves, no whisker-like structures were observed. Therefore, water was involved in the whisker growth process.
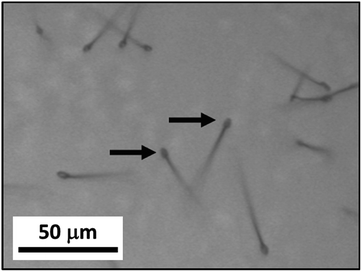 | ||
| Fig. 3 Optical image of CO2 whiskers grown when the laboratory R.H. is at 65%. Arrows are indicating the tips of the whiskers. | ||
Further Raman analysis of the as-grown whiskers revealed that they were composed of CO2 solids. Similar to the case of the CO2 solid layer grown on the substrates, the Raman spectra of these whiskers showed strong Fermi diad peaks representing vibrational bands at 1277 cm−1 and 1384 cm−1 (Fig. 2a). We ruled out that these whiskers were composed of typical CO2 clathrate hydrates or hydrated CO2. Though the growth of these whiskers was associated with the high R.H. of the laboratory, no OH vibrational signatures that indicate the presence of water in the whiskers were observed (Fig. 2b). In addition, while the locations of the Fermi diad for the whiskers were similar to those of CO2 clathrate hydrates or hydrated CO2 solids, the full width half maxima (FWHM) of these two peaks for the whiskers are ca. 4–5 cm−1, which were significantly smaller than the FWHM of similar peaks (ca. 8–10 cm−1) for CO2 clathrate hydrates or hydrated CO2 solids.21 Hence, we concluded that the whiskers were composed of mostly molecular CO2 solid.
The growth mechanism for the whiskers was proposed to be similar to the vapor–liquid–solid mechanism (VLS)22 or the vapor–solid mechanism (VS)23 for the growth of semiconductor nanowires and microwires with catalyst seeds. Our observed rapid whisker growth process with distinguished tip features mirrored well with those typically observed for the semiconductor whiskers grown by the VLS or VS processes. The depressurization of the microscopy stage to the laboratory at high relative humidity was found critical to induce the growth process. This suggests that water vapor probably leaked into the stage even though the stage was pressurized above atmospheric pressure. Based on the observed phenomena under the experimental conditions, after the formation of the solid CO2 layer on the substrate at −70 °C and 4.4 bar of CO2, the formation mechanisms of CO2 whiskers was postulated to follow the sequential steps shown in Fig. 4. First, as the stage was depressurized from 4.4 bar, water vapor leaked into the microscopy stage through the vent valve assembly and condensed as small nuclei saturated with CO2 onto the cold surface of the solid CO2 layer at −70 °C. Second, these condensed nuclei grew into particles supersaturated with CO2 by continuously absorbing gaseous CO2 and water vapor available inside the stage. The feasibility for the formation of these complex particles could be inferred from the CO2–H2O pressure–temperature (P–T) phase diagram compiled by Genov.24 In the P–T region of 1 to 4 bar and −70 to −65 °C, a phase boundary existed between solid CO2 and the coexistence of gaseous CO2, water ice, and CO2 hydrate (Fig. S3†), suggesting that the proposed particles of complex compositions could occur under these conditions. Since the stage was continuously depressurized, the solubility of CO2 in these “seeds” decreased. As the solid CO2 layer was maintained at about −70 °C, these “seeds” preferentially “precipitated” excess CO2 on the cold CO2 layer in the form of whiskers in the third step. Lastly, as more CO2 dissolved through the tops of these “seeds” to balance the rapid change of their CO2 concentration, the CO2 whiskers continued to grow in length with the “seeds” remained at their tops. Note that the whisker growth could be halted by closing the gas release valve during the growth process. This further confirmed that the change in chemical potential of CO2 in the “seeds” was necessary for the whisker growth.
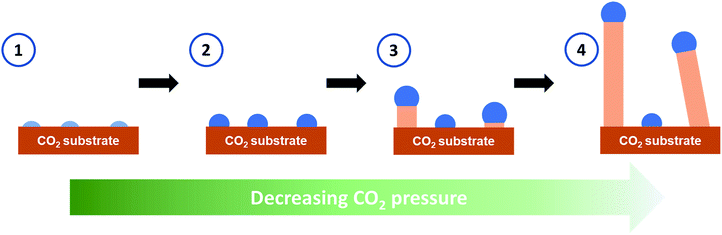 | ||
| Fig. 4 Schematics for the growth mechanism of the CO2 whiskers. Light blue: nuclei containing water saturated with CO2. Blue: particles supersaturated with CO2. Beige: solid CO2. | ||
As aforementioned, water was demonstrated to participate in the CO2 whiskers growth process, and we postulated its critical role in the nucleation of the CO2 whiskers. However, similar as for the whiskers, the Raman analysis performed at the whiskers' tips also did not show the OH vibration signatures of water (data not shown). This was possibly due to the weak detection sensitivity to water for the Raman technique25 and relatively small amount of water in these tips. While the OH stretching vibrations of water are well-known to exhibit strong absorption when examined by the infrared spectroscopy, they have a comparatively much weaker signal in the Raman spectrum of water.26,27 As the vibrations of the highly polar OH bond does not greatly change its polarizability, the derivative of its polarizability is nearly zero and, hence, the intensity of the OH Raman band is weak. Given that only a miniscule amount of water could be present at the whiskers' tips, it was not unexpected that we might have reached our Raman instrument's detection limit of water at these tips and detect no water signatures. Furthermore, we attempted to perform Raman spectroscopy of the initial nuclei of the tips which might contain higher water content in our postulated mechanism. Nonetheless, as evident in our video data, the whisker growth rate was too fast for us to capture informative Raman spectra using our current instrument.
Our study illustrated the complex behavior of the CO2–H2O system and the kinetic-and-composition dependence for the growth of solid CO2 whiskers. In most CO2 nucleation models proposed by various theoretical studies,12,28 CO2 whisker structures have not been reported. We postulated that it was because these CO2 whiskers were kinetic products during the phase transitions. We expect that our findings would be of special interests to scientists and engineers working on CO2 processing research such as CO2 sequestration. Particularly, understanding the impact of supersaturation of CO2 in the Earth's and interplanetary environments29 has lately attracted the attention among the Earth science, space science, and astrophysics communities. For example, many theoretical models have been reported in recent years on understanding the effect of supersaturation of CO2 in lakes30,31 and in the formation of caves.32 In addition, recent studies have reported the formation of solid CO2 snow33,34 and CO2 clathrate hydrate35 on the surface of Mars. Therefore, our results could help to stimulate renewed understanding of the supersaturation of CO2 and the phase behavior of water–CO2 system in environmental and space research.
4. Conclusions
The growth of CO2 whiskers was demonstrated under moderate pressure (4.4 bar to 1.0 bar) and low temperature (−70 °C to −65 °C). The Fermi diad vibration signatures of CO2 observed in the confocal Raman spectra of these whisker structures and the absence of OH-stretching vibration signatures indicated that these whiskers were composed of CO2 with non-detectable water content. Our findings had led to the postulation of a vapor–solid growth mechanism based on the supersaturation of CO2 initialed by the condensation of water on the substrates for the formation of whisker-like structures. Additionally, these examples could potentially serve as benchmarks for theoretical models of solid CO2. Through the re-evaluation of the roles of CO2 supersaturation, this work could pave a way towards a better understanding of the stochastic nucleation and growth of other one-dimensional molecular solids under phase transition conditions. To resolve more detailed involvement of water in the whisker growth mechanism, other local probe characterization techniques such as in situ TEM36 and confocal infrared spectroscopy,37 which have higher detection sensitivities for water than Raman spectroscopy, are recommended for future studies.Conflicts of interest
The authors declare no conflicts of interests.Acknowledgements
The authors thank the National Science Foundation (Grant #: CHE 1665324) for financial support. We are grateful to Dr Alexander Sinitskii for assistance with the Raman data collection.Notes and references
- S. A. Bonev, F. Gygi, T. Ogitsu and G. Galli, Phys. Rev. Lett., 2003, 91, 065501 CrossRef CAS PubMed.
- R. Bini, Acc. Chem. Res., 2004, 37, 95–101 CrossRef CAS PubMed.
- F. Mallet, S. Petit, S. Lafont, P. Billot, D. Lemarchand and G. Coquerel, Cryst. Growth Des., 2004, 4, 965–969 CrossRef CAS.
- P. M. Celliers, M. Millot, S. Brygoo, R. S. McWilliams, D. E. Fratanduono, J. R. Rygg, A. F. Goncharov, P. Loubeyre, J. H. Eggert, J. L. Peterson, N. B. Meezan, S. Le Pape, G. W. Collins, R. Jeanloz and R. J. Hemley, Science, 2018, 361, 677–682 CrossRef CAS PubMed.
- X. Cao, Y. Huang, W. Li, Z. Zheng, X. Jiang, Y. Su, J. Zhao and C. Liu, Phys. Chem. Chem. Phys., 2015, 18, 3272–3279 RSC.
- C. Lobban, J. L. Finney and W. F. Kuhs, Nature, 1998, 391, 268–270 CrossRef CAS.
- Q. Zeng, Z. He, X. San, Y. Ma, F. Tian, T. Cui, B. Liu, G. Zou and H.-k. Mao, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 4999–5001 CrossRef CAS PubMed.
- J. Hansen, D. Johnson, A. Lacis, S. Lebedeff, P. Lee, D. Rind and G. Russell, Science, 1981, 213, 957–966 CrossRef CAS PubMed.
- S. Holloway, Annu. Rev. Energy, 2001, 26, 145–166 CrossRef.
- K. Aoki, H. Yamawaki, M. Sakashita, Y. Gotoh and K. Takemura, Science, 1994, 263, 356–358 CrossRef CAS PubMed.
- J. Li, O. Sode, G. A. Voth and S. Hirata, Nat. Commun., 2013, 4, 2647 CrossRef PubMed.
- J. Bai, J. S. Francisco and X. C. Zeng, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 10263 CrossRef CAS PubMed.
- C.-S. Yoo, Phys. Chem. Chem. Phys., 2013, 15, 7949–7966 RSC.
- W. Sontising, Y. N. Heit, J. L. McKinley and G. J. O. Beran, Chem. Sci., 2017, 8, 7374–7382 RSC.
- M. Santoro and F. A. Gorelli, Chem. Soc. Rev., 2006, 35, 918–931 RSC.
- R. C. Hanson, J. Phys. Chem., 1985, 89, 4499–4501 CrossRef CAS.
- P. F. Bernath, Spectra of Atoms and Molecules, Oxford University Press, 1995 Search PubMed.
- F. A. Cotton, Chemical applications of group theory, Wiley, 1990 Search PubMed.
- O. Sode, M. Keçeli, K. Yagi and S. Hirata, J. Chem. Phys., 2013, 138, 074501 CrossRef PubMed.
- R. C. Hanson and K. Bachman, Chem. Phys. Lett., 1980, 73, 338–342 CrossRef CAS.
- M. Berkesi, K. Hidas, T. Guzmics, J. Dubessy, R. J. Bodnar, C. Szabó, B. Vajna and T. Tsunogae, J. Raman Spectrosc., 2009, 40, 1461–1463 CrossRef CAS.
- R. S. Wagner and W. C. Ellis, Appl. Phys. Lett., 1964, 4, 89–90 CrossRef CAS.
- A. I. Persson, M. W. Larsson, S. Stenström, B. J. Ohlsson, L. Samuelson and L. R. Wallenberg, Nat. Mater., 2004, 3, 677–681 CrossRef CAS PubMed.
- G. Y. Genov, PhD thesis, Georg-August-Universität Göttingen, 2005.
- M. Reichenbächer and J. Popp, Challenges in Molecular Structure Determination, Springer Berlin Heidelberg, 2012 Search PubMed.
- S. M. Ali, F. Bonnier, H. Lambkin, K. Flynn, V. McDonagh, C. Healy, T. C. Lee, F. M. Lyng and H. J. Byrne, Anal. Methods, 2013, 5, 2281–2291 RSC.
- L. E. Rodriguez-Saona, M. M. Giusti and M. Shotts, in Advances in Food Authenticity Testing, ed. G. Downey, Woodhead Publishing, 1st edn, 2016, ch. 4, pp. 71–116 Search PubMed.
- A. Määttänen, H. Vehkamäki, A. Lauri, S. Merikallio, J. Kauhanen, H. Savijärvi and M. Kulmala, J. Geophys. Res., 2005, 110, E02002 CrossRef.
- B. M. Jakosky, Planet. Space Sci., 2019, 175, 52–59 CrossRef CAS.
- R. Marcé, B. Obrador, J.-A. Morguí, J. Lluís Riera, P. López and J. Armengol, Nat. Geosci., 2015, 8, 107 CrossRef.
- J. K. Lazzarino, R. W. Bachmann, M. V. Hoyer and D. E. Canfield, Hydrobiologia, 2009, 627, 169–180 CrossRef CAS.
- J. S. Herman, in Encyclopedia of Caves, ed. W. B. White, D. C. Culver and T. Pipan, Academic Press, 3rd edn, 2019, ch. 133, pp. 1136–1143 Search PubMed.
- H. E. Chinnery, A. Hagermann, E. Kaufmann and S. R. Lewis, J. Geophys. Res.: Planets, 2019, 124, 337–348 Search PubMed.
- D. L. Glandorf, A. Colaprete, M. A. Tolbert and O. B. Toon, Icarus, 2002, 160, 66–72 CrossRef CAS.
- A. Dobrovolskis and A. P. Ingersoll, Icarus, 1975, 26, 353–357 CrossRef CAS.
- S. M. Ghodsi, S. Anand, R. Shahbazian-Yassar, T. Shokuhfar and C. M. Megaridis, ACS Nano, 2019, 13, 4677–4685 CrossRef CAS PubMed.
- P. F. Bernath, Phys. Chem. Chem. Phys., 2002, 4, 1501–1509 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental setups, additional images of CO2 whiskers, CO2–H2O phase diagram, and whiskers growth video. See DOI: 10.1039/c9ra04583j |
| This journal is © The Royal Society of Chemistry 2019 |