Open Access Article
Open Access ArticleCatalyst-free chemoselective α-sulfenylation/β-thiolation for α,β-unsaturated carbonyl compounds†
Xi Huang,
Juan Li,
Xiang Li,
Jiayi Wang,
Yanqing Peng and
Gonghua Song *
*
Shanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology, Shanghai, 200237, PR China. E-mail: ghsong@ecust.edu.cn; Fax: +86-21-64252603
First published on 22nd August 2019
Abstract
A novel, efficient, catalyst-free and product-controllable strategy has been developed for the chemoselective α-sulfenylation/β-thiolation of α,β-unsaturated carbonyl compounds. An aromatic sulfur group could be chemoselectively introduced at α- or β-position of carbonyls with different sulfur reagents under slightly changed reaction conditions. A series of desired products were obtained in moderate to excellent yields. Mechanistic studies revealed that B2pin2 played the key role in activating the transformation towards the β-thiolation of α,β-unsaturated carbonyl compounds. This transition-metal-catalyst-free method provides a convenient and efficient tool for the highly chemoselective preparation of α-thiolation or β-sulfenylation products of α,β-unsaturated carbonyl compounds.
Introduction
Over decades, catalyst-free organic reactions have received much attention as they can not only prevent the need of expensive transition-metal complexes or toxic organocatalytic reagents, but also considerably reduce the risk of metal residues in pharmaceuticals.Organosulfur compounds widely exist in nature and are closely related to human life, due to their wide application in both pharmaceutical and material sciences. Among them, aryl sulfides are privileged motifs found in many drugs1 and materials.2 Over the past decades, several significant strategies have been successfully developed for the introduction of aromatic sulfur groups into α,β-unsaturated carbonyl compounds catalyzed by either metal complexes,3 or N-heterocyclic carbene catalysts.4 Although these methods provide useful tools for the construction of a C–S bond in an α,β-unsaturated carbonyl framework, they also exhibit some drawbacks such as high catalyst loading, complex and expensive ligands, harsh conditions, long reaction times and restricted substrate scope. Therefore, the development of a simple, efficient and catalyst-free method for the chemoselective construction of C–S bonds of α,β-unsaturated carbonyl compounds has remained an elusive goal.
Meanwhile, bis(pinacolato)diboron represent an important class of organoboron reagents and has been widely applied for various organic synthesis,5 especially the β-boration of α,β-unsaturated carbonyl compounds.6 However, other derivative reactions of α,β-unsaturated carbonyl compounds participated by bis(pinacolato)diboron are still rarely reported.
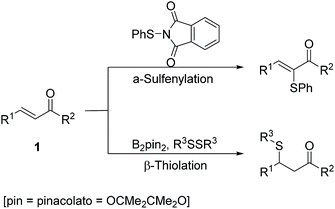
As our continuous efforts on the derivative reactions of α,β-unsaturated carbonyl compounds,7 herein, we firstly report a novel, efficient catalyst-free and chemoselective α-sulfenylation/β-thiolation of α,β-unsaturated carbonyl compounds. With two different sulfur reagents, various α-sulfenylation or β-thiolation products were obtained in moderate to excellent yields under slightly changed reaction conditions. This developed strategy provides a useful tool for the highly chemoselective preparation of α-thiolation or β-sulfenylation products of α,β-unsaturated carbonyl compounds (Scheme 1).
Results and discussion
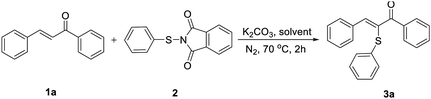
By employing the catalyst-free chemoselective α-sulfenylation of chalcone with N-(phenylthio)phthalimide as the model reaction, a range of reaction conditions was investigated as shown in Table 1. To our delight, the desired product was harvested in 96% yield when DMSO was used as the solvent (entry 2). Further studies confirmed the positive effects of nitrogen atmosphere and proper temperature (entries 6 and 7). When the reaction was conducted in the absence of base, no desired product was detected (entry 8). The investigation on the amount of N-(phenylthio)phthalimide (2) indicated that the product yield dropped sharply when either insufficient or excessive amount of 2 were used (entries 9 and 10). An almost stoichiometric amount of 3a was obtained by prolonging the reaction time to 3 h (entry 11). Thus, the optimized conditions were as follows: a mixture of chalcone (1a), 1.5 equiv. of N-(phenylthio)phthalimide (2), 0.1 equiv. of K2CO3 and 1.5 mL of DMSO was heated at 70 °C for 3 h under nitrogen.| Entry | Solvent | Equiv. of 2 | Yield of 3a [%] |
|---|---|---|---|
| a Reaction conditions: 0.5 mmol of chalcone (1a), N-(phenylthio)phthalimide (2), 0.1 equiv. of K2CO3, 1.5 mL of solvent in a reaction tube heated at 70 °C for 2 h under nitrogen atmosphere. GC yield (dodecane as internal standard).b The reaction was conducted under air atmosphere.c The reaction was heated at 50 °C.d The reaction was conducted in absence of K2CO3.e The reaction time was 3 h. | |||
| 1 | DMF | 1.5 | 72 |
| 2 | DMSO | 1.5 | 96 |
| 3 | THF | 1.5 | <1 |
| 4 | Toluene | 1.5 | <1 |
| 5 | 1,4-Dioxane | 1.5 | <1 |
| 6b | DMSO | 1.5 | 80 |
| 7c | DMSO | 1.5 | 48 |
| 8d | DMSO | 1.5 | <1 |
| 9 | DMSO | 1.25 | 85 |
| 10 | DMSO | 1.75 | 79 |
| 11e | DMSO | 1.5 | 99 |
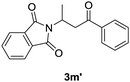
With the optimized conditions in hand, the catalyst-free α-sulfenylation of a range of α,β-unsaturated ketones was investigated (Table 2). To our delight, all substituted chalcones were compatible with this transformation, and gave the corresponding α-sulfenylation products in good to excellent yields (3a–3i). For chalcones bearing an electron-donating group, good yields were obtained by simply prolonging the reaction time (3b–3d). There was no significant correlation between the Z/E value and the electronic effect of the substituents. In addition, the strategy was also applicable for α,β-unsaturated ketones with a heterocycle group, and satisfied yields were obtained with strengthened conditions (3j–3k). For enones bearing an alkyl group, the reaction failed to give desired products (3l & 3m). It was interesting that when 1-phenylbut-2-en-1-one (1m) was adopted, the 1,4-addition by-product of 1m participated by N-(phenylthio)phthalimide, namely 2-(4-oxo-4-phenylbutan-2-yl)isoindoline-1,3-dione (3m′) was isolated in a yield of 51%, while no α-sulfenylation product (3m) was detected.
| Entry | R1 | R2 | Time [h] | Yield [%] | Z/Eb | |
|---|---|---|---|---|---|---|
| a Reaction conditions: 0.5 mmol of α,β-unsaturated carbonyl ketones (1), 1.5 equiv. of N-(phenylthio)phthalimide (2), 0.1 equiv. of K2CO3 and 1.5 mL of DMSO in a reaction tube heated at 70 °C for 3 h (general procedure B). Y = isolated yield; t = reaction time.b The Z/E value was measured by 1H NMR.c 2.5 equiv. of N-(phenylthio)phthalimide (2) was used.d Massive 1,4-addition by-product participated by N-(phenylthio)phthalimide was detected in GC-MS, and isolated yield of by-product was indicated in parentheses. | ||||||
| 1 | Ph | Ph | 3 | 91 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
3a |
| 2 | 4-CH3C6H4 | Ph | 4.5 | 93 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
3b |
| 3 | 4-OCH3C6H4 | Ph | 4.5 | 92 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
3c |
| 4 | Ph | 4-OCH3C6H4 | 6 | 83 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
3d |
| 5 | 4-BrC6H4 | Ph | 3 | 91 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
3e |
| 6 | 4-ClC6H4 | Ph | 3 | 85 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
3f |
| 7 | Ph | 4-ClC6H4 | 3 | 92 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
3g |
| 8 | Ph | 4-FC6H4 | 3 | 89 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
3h |
| 9 | 4-CF3C6H4 | Ph | 3 | 88 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
3i |
| 10 | 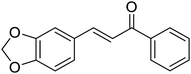 |
4.5 | 78 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
3j | |
| 11 | 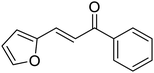 |
6 | 90c | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
3k | |
| 12 | Ph | CH3 | 3 | <1 | — | 3l |
| 13 | CH3 | Ph | 3 | <1 (51)d | — | 3m (3m′) |
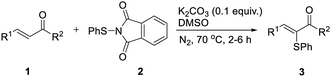
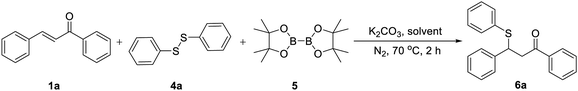
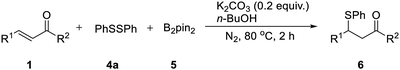
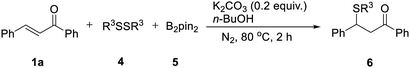
We then tried to explore a method to introduce a phenyl sulfide group at the β-position of a carbonyl compound without commonly used sulfur reagent thiophenol which is stinky and toxic. By employing the catalyst-free chemoselective β-thiolation of chalcone with 1,2-diphenyldisulfide and bis(pinacolato)diboron as the model reaction, a range of reaction conditions was investigated as shown in Table 3. The β-thiolation of α,β-unsaturated carbonyl compounds was preferred to proceed in the protonic solvents (entries 1–7). When the reaction was conducted in n-BuOH, the desired β-thiolation product was harvested in 74% yield (entry 6). Further investigation confirmed the positive effect of nitrogen atmosphere (entry 8). A higher yield of 88% was obtained when the amount of K2CO3 was increasing to 0.2 equiv. (entry 9). Moreover, when the amount of 1,2-diphenyldisulfide (4a) was decreased from 1.5 equiv. to 1.0 equiv. (entry 10), the yield remained at high level. Interestingly, a yield of 76% was obtained when 0.5 equiv. of 4a were adopted, which indicated that both thiophenyl groups produced by the homolytic scission of 4a participated in this transformation (entry 11). When the reaction was conducted at 80 °C, the β-thiolation product was obtained in 97% yield (entry 12). Thus, the optimized conditions were as follows: a mixture of chalcone (1a), 1.0 equiv. of 1,2-diphenyldisulfide (4a) and 1.5 equiv. of bis(pinacolato)diboron (5), 0.2 equiv. of K2CO3 and 1.5 mL of n-BuOH was heated at 80 °C for 2 h under nitrogen.
| Entry | Solvent | Equiv. of 4a | Yield of 6a [%] |
|---|---|---|---|
| a Reaction conditions: 0.5 mmol of chalcone (1a), 1,2-diphenyldisulfide (4a), 1.5 equiv. of bis(pinacolato)diboron (5), 0.1 equiv. of K2CO3, 1.5 mL of solvent in a reaction tube heated at 70 °C for 2 h under nitrogen atmosphere. LC yield (biphenyl as internal standard).b The reaction was conducted under air atmosphere.c 0.2 equiv. of K2CO3 was used.d The reaction was heated at 80 °C. | |||
| 1 | DMF | 1.5 | 3 |
| 2 | DMSO | 1.5 | <1 |
| 3 | Toluene | 1.5 | <1 |
| 4 | 1,4-Dioxane | 1.5 | <1 |
| 5 | EtOH | 1.5 | 15 |
| 6 | n-BuOH | 1.5 | 74 |
| 7 | t-BuOH | 1.5 | 14 |
| 8b | n-BuOH | 1.5 | 62 |
| 9c | n-BuOH | 1.5 | 88 |
| 10c | n-BuOH | 1.0 | 89 |
| 11c | n-BuOH | 0.5 | 76 |
| 12c,d | n-BuOH | 1.0 | 97 |
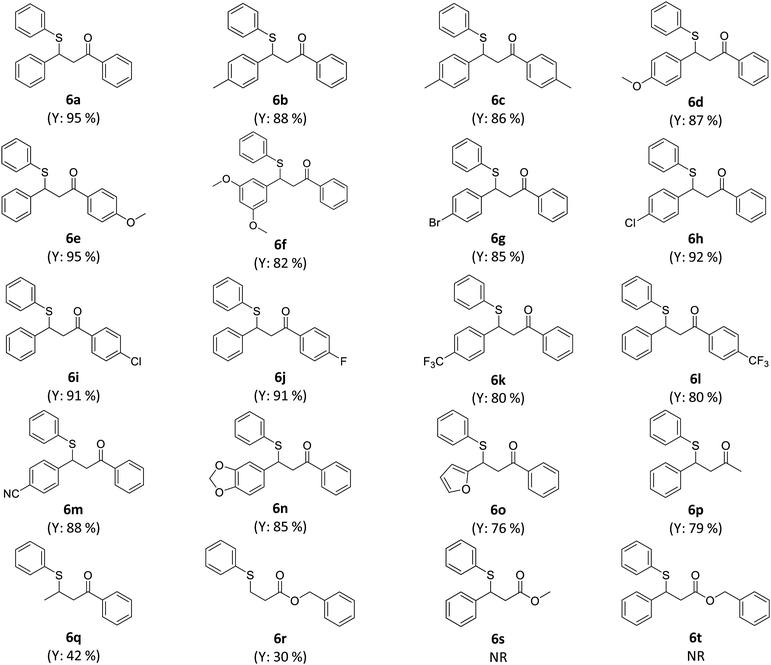
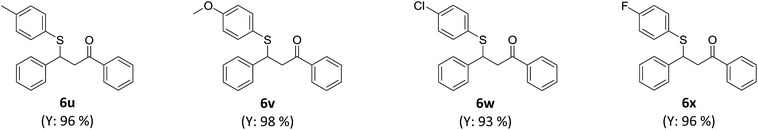
The catalyst-free β-thiolation of various α,β-unsaturated carbonyl compounds (Table 4) with different 1,2-diaryldisulfides (Table 5) was then investigated. To our delight, all substituted chalcones were compatible with this transformation and the β-thiolation products were obtained in good to excellent yields (Table 4, 6a–6m). In addition, the strategy was also applicable for α,β-unsaturated enones with a heterocycle (6n & 6o) or an alkyl group (6p–6q). Unfortunately, when α,β-unsaturated esters were applied to this transformation, only benzyl acrylate could gave the desired product in a relatively low yield (6r). The β-thiolation of cinnamate esters could hardly proceed (6s & 6t). When 1,2-diaryldisulfides bearing electron-donating or electron withdrawing groups was adopted, no obvious substituent effect was observed and the corresponding β-thiolation products of chalcone were always obtained in excellent yield (Table 5, 6u–6x).
| a Reaction conditions: 0.5 mmol of α,β-unsaturated carbonyl compounds (1), 1.0 equiv. of 1,2-diphenyldisulfide (4a), 1.5 equiv. of bis(pinacolato)diboron (5), 0.2 equiv. of K2CO3, 1.5 mL of n-BuOH in a reaction tube heated at 80 °C for 2 h under nitrogen atmosphere (general procedure D). Y = isolated yield. NR = no reaction. |
|---|
 |
| a Reaction conditions: 0.5 mmol of chalcone (1a), 1.0 equiv. of 1,2-diaryldisulfide (4), 1.5 equiv. of bis(pinacolato)diboron (5), 0.2 equiv. of K2CO3, 1.5 mL of n-BuOH in a reaction tube heated at 80 °C for 2 h under nitrogen atmosphere (general procedure E). Y = isolated yield. |
|---|
 |
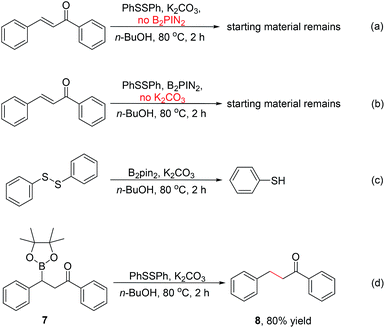
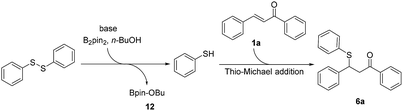
To identify the key role of bis(pinacolato)diboron in this β-thiolation of α,β-unsaturated carbonyl compounds, several control experiments were conducted. No reaction occurred in the absence of either B2pin2 or K2CO3 ((a) & (b), Scheme 2). The results indicated that both B2pin2 and base are critical factors for the success of the transformation. When chalcone was removed from the reaction system, almost all of 1,2-diphenyldisulfide was converted into thiophenol ((c), Scheme 2). The result manifested that 1,2-diphenyldisulfide could react with bis(pinacolato)diboron in n-BuOH to generate thiophenol in situ. Finally, the β-thiolation of 1,3-diphenyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)propan-1-one (7) was used to replace chalcone and was treated under the standard reaction condition except the presence of B2pin2 ((d) Scheme 2). Interestingly, the 1,4-reduction product of chalcone was obtained in 80% yield. This meant that compound 7 underwent a protodeboronation process instead of a cross-coupling process. The control experiment results excluded the possible reaction mechanism in which β-boration/cross-coupling tandem process was dominant.
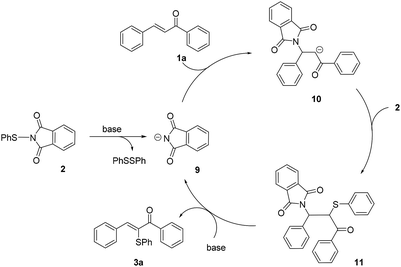
Based on the experimental results and the literature report,4f plausible reaction mechanisms for the chemoselective α-sulfenylation/β-thiolation of α,β-unsaturated carbonyl compounds was proposed respectively. For the α-sulfenylation of α,β-unsaturated ketones, a plausible reaction mechanism was shown in Scheme 3, where substrate chalcone was used as the example. The reaction is initiated by the formation of a phthalimide anion 9 from N-(phenylthio)phthalimide under alkaline conditions. Subsequent 1,4-addition of 9 to the electrophilic β-carbon of chalcone affords an enolate 10. Sequent attack by the electrophilic sulfur reagent 2 gives the intermediate 11. Further deprotonation and liberation of nucleophile induced by a base completes the catalytic cycle and gives desired α-sulfenylation product 3a. The proposed mechanism is in accord with the experimental evidence that massive 1,2-diphenyldisulfide in the α-sulfenylation reaction mixtures was detected by GC-MS, and the 1,4-addition by-product 3m′ was obtained in 51% yield. For the β-thiolation of α,β-unsaturated carbonyl ketones/esters, a plausible reaction mechanism was shown in Scheme 4. The reaction is initiated by the in situ generation of thiophenol from 1,2-diphenyldisulfide. As a Lewis acid, B2pin2 reacts with 1,2-diphenyldisulfide in n-BuOH to generate thiophenol in situ along with compound 12 which was detected by GC-MS8. Subsequent thio-Michael addition of chalcone with thiophenol gives the desired β-thiolation product 6a. Massive thiophenol in the β-thiolation reaction mixtures was detected by LC-MS, which is in accordance with proposed mechanism diagram.
Conclusions
In conclusion, we firstly reported a highly efficient strategy for the chemoselective α-sulfenylation/β-thiolation of α,β-unsaturated carbonyl compounds. With two different sulfur reagent, various α-sulfenylation or β-thiolation products were obtained in moderate to excellent yields under slightly different reaction conditions. This method provides an efficient and convenient tool for the selective introduction of an aromatic sulfur group at the α- or β-position of carbonyl of an α,β-unsaturated carbonyl compound under catalyst-free condition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial supports for this study from National Natural Science Foundation of China (Grant No. 21572060) and National Key Research and Development Plan (Grant No. 2017YFD0200504) are gratefully acknowledged.Notes and references
- (a) G. Liu, J. R. Huth, E. T. Olejniczak, R. Mendoza, P. DeVries, S. Leitza, E. B. Reilly, G. F. Okasinski, S. W. Fesik and T. W. von Geldern, J. Med. Chem., 2001, 44, 1202 CrossRef CAS PubMed; (b) G. D. Martino, M. C. Edler, G. La Regina, A. Coluccia, M. C. Barbera, D. Barrow, R. I. Nicholson, G. Chiosis, A. Brancale, E. Hamel, M. Artico and R. Silvestri, J. Med. Chem., 2006, 49, 947 CrossRef PubMed; (c) A. Gangjee, Y. Zeng, T. Talreja, J. J. McGuire, R. L. Kisliuk and S. F. Queener, J. Med. Chem., 2007, 55, 3046 CrossRef PubMed; (d) S. Aiello, G. Wells, E. L. Stone, H. Kadri, R. Bazzi, D. R. Bell, M. F. G. Stevens, C. S. Matthews, T. D. Bradshaw and A. D. Westwell, J. Med. Chem., 2008, 51, 5135 CrossRef CAS PubMed; (e) E. A. Ilardi, E. Vitaku and J. T. Njardarson, J. Med. Chem., 2014, 57, 2832 CrossRef CAS PubMed.
- (a) K. Lee and J. Lee, Chem. Mater., 2006, 18, 4519 CrossRef CAS; (b) M. Nakano and K. Takimiya, Chem. Mater., 2017, 29, 256 CrossRef CAS.
- For reviews, see: (a) T. Kondo and T. Mitsudo, Chem. Rev., 2000, 100, 3205 CrossRef CAS PubMed; (b) I. P. Beletskaya and V. P. Ananikov, Chem. Rev., 2011, 111, 1596 CrossRef CAS PubMed; (c) W. Liu and X. Zhao, Synthesis, 2013, 45, 2051 CrossRef CAS; (d) P. Chauhan, S. Mahajan and D. Enders, Chem. Rev., 2014, 114, 8807 CrossRef CAS PubMed.
- (a) T. Uno, T. Inokuma and Y. Takemoto, Chem. Commun., 2012, 48, 1901 RSC; (b) S. Singh and L. D. S. Yadav, Tetrahedron Lett., 2012, 53, 5136 CrossRef CAS; (c) L. He, H. Guo, Y. Z. Li, G. F. Du and B. Dai, Chem. Commun., 2014, 50, 3719 RSC; (d) Y. Z. Li, Y. Wang, G. F. Du, H. Y. Zhang, H. L. Yang and L. He, Asian J. Org. Chem., 2015, 4, 327 CrossRef CAS; (e) J. A. Chen, S. X. Meng, L. M. Wang, H. M. Tang and Y. Huang, Chem. Sci., 2015, 6, 4184 RSC; (f) Y. T. Dong, Q. Jin, L. Zhou and J. Chen, Org. Lett., 2016, 18, 5708 CrossRef CAS PubMed.
- D. G. Hall, Boronic Acids, John Wiley & Sons, Weiheim, Germany, 2012 Search PubMed.
- E. Hartmann, D. J. Vyas and M. Oestreich, Chem. Commun., 2011, 47, 7917 RSC.
- (a) W. Chen, L. Sun, X. Huang, J. Y. Wang, Y. Q. Peng and G. H. Song, Adv. Synth. Catal., 2015, 357, 1474 CrossRef CAS; (b) X. Huang, J. J. Hu, M. Y. Wu, J. Y. Wang, Y. Q. Peng and G. H. Song, Green Chem., 2018, 20, 255 RSC.
- D. A. Spiegel, K. B. Wiberg, L. N. Schacherer, M. R. Medeiros and J. L. Wood, J. Am. Chem. Soc., 2005, 127, 12513 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra05708k |
| This journal is © The Royal Society of Chemistry 2019 |