Open Access Article
Open Access ArticleSynthesis and anti-phytopathogenic activity of 8-hydroxyquinoline derivatives†
Xiao-Dan
Yin
 a,
Yu
Sun
a,
Raymond Kobla
Lawoe
a,
Guan-Zhou
Yang
a,
Ying-Qian
Liu
*a,
Xiao-Fei
Shang
ab,
Hua
Liu
a,
Yu-Dong
Yang
a,
Jia-Kai
Zhu
a and
Xiao-Ling
Huang
a
a,
Yu
Sun
a,
Raymond Kobla
Lawoe
a,
Guan-Zhou
Yang
a,
Ying-Qian
Liu
*a,
Xiao-Fei
Shang
ab,
Hua
Liu
a,
Yu-Dong
Yang
a,
Jia-Kai
Zhu
a and
Xiao-Ling
Huang
a
aSchool of Pharmacy, Lanzhou University, Lanzhou 730000, People's Republic of China. E-mail: yqliu@lzu.edu.cn; Fax: +86-931-8915685; Tel: +86-931-8915686
bLanzhou Institute of Husbandry and Pharmaceutical Sciences, Chinese Academy of Agricultural Sciences, 335 Jiangouyan, Lanzhou 730050, P. R. China
First published on 24th September 2019
Abstract
Phytopathogenic fungi have become a serious threat to the quality of agricultural products, food security and human health globally, necessitating the need to discover new antifungal agents with de novo chemical scaffolds and high efficiency. A series of 8-hydroxyquinoline derivatives were designed and synthesized, and their antifungal activity was evaluated against five phytopathogenic fungi. In vitro assays revealed that most of the tested compounds remarkably impacted the five target fungi and their inhibitory activities were better than that of the positive control azoxystrobin. Compound 2, in particular, exhibited the highest potency among all the tested compounds, with an EC50 of 0.0021, 0.0016, 0.0124, 0.0059 and 0.0120 mM respectively against B. cinerea, S. sclerotiorum, F. graminearum, F. oxysporum and M. oryzae, followed by compound 5c. The morphological observations of optical microscopy and scanning electron microscopy revealed that compounds 2 and 5c caused mycelial abnormalities of S. sclerotiorum. Futhermore, the results of in vivo antifungal activity of compounds 2 and 5c against S. sclerotiorum showed that 5c possessed stronger protective and curative activity than that of 2, and the curative effects of 5c at 40 and 80 μg mL−1 (84.18% and 95.44%) were better than those of azoxystrobin (77.32% and 83.59%). Therefore, compounds 2 and 5c are expected to be novel lead structures for the development of new fungicides.
1. Introduction
Phytopathogenic fungi are often the main culprits in causing immense decrease in yield and quality of agricultural products, leading to serious losses in global agricultural and horticultural production, hence posing a great threat to global food security.1,2 More importantly, many phytopathogenic fungi can produce mycotoxins that are pernicious to human and animal health.1 Therefore, various antifungal agents have been discovered, developed and used for a long time to guarantee wholesome crops, increases in crop yields and economic benefits.3,4 However, excessive use and misuse of many traditional antifungal agents have led to heightened resistance in target phytopathogenic fungi, residual toxicity, and even environmental pollution in recent decades.5–7 The above concerns have called for discovery and development of novel antifungal compounds with lower application dose, higher efficiency and selectivity, unique mode of action and environmental compatibility.3,8N-Heterocycle plays a key role in drug design.9 Quinoline and its derivatives from natural products or synthetic biologically active sources are indispensable heterocyclic compounds endowed with a broad spectrum of pharmacological properties.10–13 Amidst quinoline core compounds, 8-hydroxyquinoline (HQ) has become a privileged scaffold for the design and synthesis of novel drug candidates due to its broad biological activities,14–17 such as cytotoxic,18–20 antifungal,20,21 antibacterial,22,23 antifilarial,24 and anti-HIV.25 The mode of action of HQ is related to many factors, according to reports, chelation with metal ions appears to be crucial because metal ions are cofactors for many physiologically active enzymes.17,26,27
Highly destructive phytopathogenic fungi S. sclerotiorum, B. cinerea, F. graminearum, F. oxysporum and M. oryzae have garnered considerable research attention owing to their typical pathogenic characteristics. In this investigation, we chose HQ as a primer molecule and introduced the nitro group into the HQ scaffold (Fig. 1). Motivated by compound 2 displaying superb antifungal activity than HQ and the positive control azoxystrobin against the five target phytopathogens tested, we further structurally derivatized compound 2. Interestingly, preliminary work showed that the 2-position modification of compound 2 resulted in a dramatic decrease in antifungal activity, whereas the 7-position modification of compound 2 with identical groups led to improved or comparable antifungal activity with HQ (ESI†). Thus, a series of HQ derivatives were synthesized to investigate their antifungal potential and structural activity relationships (SAR). Furthermore, optical microscope and scanning electron microscopy observations and effects of 8-hydroxyquinoline derivatives against S. sclerotiorum in vivo were performed to evaluate the antifungal properties of these compounds.
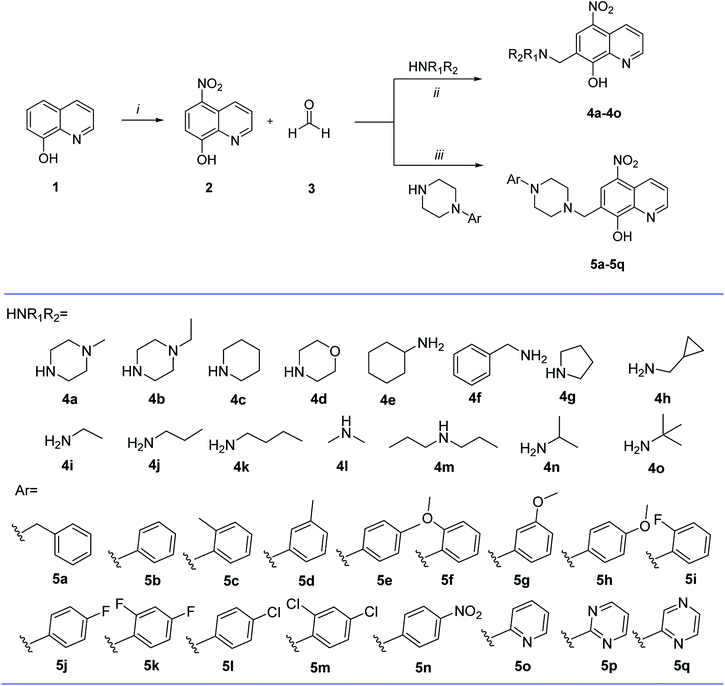 | ||
Fig. 1 Reagents and conditions: (i) NaNO2, concentrated HCl, 0 °C, 1 h; HNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3 H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 17 °C, 75 min; (ii) EtOH, reflux, 24 h; (iii) pyridine, 50 °C, 30 min. 2), 17 °C, 75 min; (ii) EtOH, reflux, 24 h; (iii) pyridine, 50 °C, 30 min. | ||
2. Results and discussion
2.1. Chemistry
The starting material 8-hydroxyquinoline (1) employed in the preparation of compound 2 was obtained from Sun Chemical Technology (Shanghai). Stirring the starting material 1 in concentrated HCl with NaNO2 aqueous solution at 0 °C, the precipitates formed were filtered, and added to a mixture of concentrated HNO3 and water (v/v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at 17 °C to be transformed into 2 (Fig. 1).28 Compounds 4a–4o and 5a–5q were conveniently assembled in a one-step synthesis according to classic Mannich reaction (Fig. 1). Compounds 4a–4o were prepared by refluxing 2 with formaldehyde and corresponding aliphatic amine in ethanol.29 Compounds 5a–5p were synthesized by refluxing compound 2 with formaldehyde and appropriate aromatic piperazine in pyridine.23 All the synthesized compounds were purified by recrystallization from absolute ethanol. Structures of all the synthesized compounds were supported by spectral data including 1H NMR, 13C NMR, and HRMS as reported in Experimental section.
2) at 17 °C to be transformed into 2 (Fig. 1).28 Compounds 4a–4o and 5a–5q were conveniently assembled in a one-step synthesis according to classic Mannich reaction (Fig. 1). Compounds 4a–4o were prepared by refluxing 2 with formaldehyde and corresponding aliphatic amine in ethanol.29 Compounds 5a–5p were synthesized by refluxing compound 2 with formaldehyde and appropriate aromatic piperazine in pyridine.23 All the synthesized compounds were purified by recrystallization from absolute ethanol. Structures of all the synthesized compounds were supported by spectral data including 1H NMR, 13C NMR, and HRMS as reported in Experimental section.
2.2. Antifungal activity
The target compounds were evaluated for their antifungal activity against five economically important phytopathogenic fungi B. cinerea, S. sclerotiorum, F. graminearum, F. oxysporum and M. oryzae. The preliminary antifungal activity screening of the target compounds was determined at 25 and 50 μg mL−1 respectively, and the test results were shown in Table 1. Satisfactorily, compound 2 exhibited the most potent antifungal activity against the five test strains, and the inhibition rate reached 100% for each strain at 25 μg mL−1. Additionally, excluding 4e and 5a, most of the tested compounds presented the better antifungal activity against B. cinerea and S. sclerotiorum than that of the positive control azoxystrobin. Most of the compounds 5a–5q (5b, 5c, 5d, 5f, 5g, 5h, 5i, 5j, 5l) demonstrated moderate to remarkable antifungal activity against F. graminearum, F. oxysporum and M. oryzae, with inhibitory rates ranging from 76% to 100% (25 μg mL−1). However, compounds 4a–4o showed weak activity against the three fungi. To further explore the antifungal potential of the synthesized compounds, the most active compounds (whose inhibition rates >50% at 25 μg mL−1) in Table 1 were selected to determine their EC50 values against the five fungal strains.| Compd.a | Concb (μg mL−1) | Average inhibition rate ± SD (%) (n = 3) | ||||
|---|---|---|---|---|---|---|
| B. C. c | S. S. c | F. G. c | F. O. c | M. O. c | ||
| a Compd.: compound. b Conc: concentration. c B. C.: Botrytis cinerea. S. S.: Sclerotinia sclerotiorum. F. G.: Fusarium graminearum. F. O.: Fusarium oxysporum. f. sp. vasinfectum. M. O.: Magnaporthe oryzae. d ASB: azoxystrobin. | ||||||
| HQ | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 73.01 ± 0.33 | 100.00 ± 0.00 |
| 25 | 100.00 ± 0.00 | 100.00 ± 0.00 | 77.19 ± 0.31 | 36.29 ± 0.67 | 100.00 ± 0.00 | |
| 2 | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| 25 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| 4a | 50 | 97.92 ± 0.72 | 100.00 ± 0.00 | 0.00 ± 0.00 | 36.00 ± 0.74 | 31.56 ± 0.72 |
| 25 | 85.39 ± 0.65 | 100.00 ± 0.00 | 0.00 ± 0.00 | 24.89 ± 0.72 | 11.11 ± 0.72 | |
| 4b | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 85.00 ± 0.01 | 93.78 ± 0.72 | 100.00 ± 0.00 |
| 25 | 98.33 ± 0.72 | 86.04 ± 0.97 | 78.75 ± 0.01 | 79.56 ± 0.72 | 74.44 ± 0.95 | |
| 4c | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 0.00 ± 0.00 | 25.78 ± 0.72 | 35.56 ± 0.72 |
| 25 | 93.00 ± 0.41 | 97.92 ± 0.72 | 0.00 ± 0.00 | 16.44 ± 0.72 | 17.56 ± 0.95 | |
| 4d | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 12.92 ± 0.01 | 28.44 ± 0.44 | 27.11 ± 0.34 |
| 25 | 85.08 ± 0.49 | 89.80 ± 0.49 | 0.00 ± 0.00 | 15.33 ± 0.63 | 12.44 ± 0.72 | |
| 4e | 50 | 26.31 ± 0.19 | 0.00 ± 0.00 | 47.50 ± 0.02 | 31.25 ± 0.02 | 27.11 ± 0.07 |
| 25 | 29.71 ± 0.16 | 0.00 ± 0.00 | 19.17 ± 0.02 | 14.89 ± 0.95 | 11.56 ± 0.72 | |
| 4f | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 0.00 ± 0.00 | 33.73 ± 0.25 | 40.44 ± 0.72 |
| 25 | 97.24 ± 0.01 | 97.92 ± 0.72 | 0.00 ± 0.00 | 16.67 ± 0.62 | 33.78 ± 0.72 | |
| 4g | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 0.00 ± 0.00 | 20.00 ± 0.36 | 18.67 ± 0.17 |
| 25 | 100.00 ± 0.00 | 100.00 ± 0.00 | 0.00 ± 0.00 | 13.76 ± 0.74 | 0.00 ± 0.00 | |
| 4h | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 0.00 ± 0.00 | 18.22 ± 0.60 | 29.78 ± 0.15 |
| 25 | 100.00 ± 0.00 | 100.00 ± 0.00 | 0.00 ± 0.00 | 4.89 ± 0.72 | 11.11 ± 0.72 | |
| 4i | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 10.42 ± 0.01 | 43.11 ± 0.91 | 32.44 ± 0.60 |
| 25 | 100.00 ± 0.00 | 100.00 ± 0.00 | 0.00 ± 0.00 | 19.99 ± 0.01 | 20.89 ± 0.72 | |
| 4j | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 18.33 ± 0.01 | 44.89 ± 0.72 | 28.89 ± 0.91 |
| 25 | 99.58 ± 0.72 | 100.00 ± 0.00 | 0.00 ± 0.00 | 26.00 ± 0.62 | 16.22 ± 0.36 | |
| 4k | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 00.00 ± 0.00 | 32.89 ± 0.72 | 27.56 ± 0.60 |
| 25 | 97.88 ± 0.31 | 100.00 ± 0.00 | 0.00 ± 0.00 | 23.02 ± 0.80 | 17.78 ± 0.72 | |
| 4l | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 93.33 ± 0.01 | 78.67 ± 0.05 | 34.67 ± 0.25 |
| 25 | 100.00 ± 0.00 | 100.00 ± 0.00 | 0.00 ± 0.00 | 43.56 ± 0.72 | 20.53 ± 0.66 | |
| 4m | 50 | 99.58 ± 0.72 | 100.00 ± 0.00 | 21.67 ± 0.03 | 75.56 ± 0.91 | 47.56 ± 0.60 |
| 25 | 77.14 ± 0.74 | 97.92 ± 0.72 | 12.50 ± 0.03 | 52.67 ± 0.62 | 41.96 ± 0.63 | |
| 4n | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 28.75 ± 0.03 | 36.00 ± 0.25 | 36.89 ± 0.72 |
| 25 | 98.33 ± 0.72 | 100.00 ± 0.00 | 3.33 ± 0.03 | 19.56 ± 0.72 | 25.78 ± 0.72 | |
| 4o | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 0.00 ± 0.00 | 32.89 ± 0.72 | 33.78 ± 0.91 |
| 25 | 98.33 ± 0.72 | 100.00 ± 0.00 | 0.00 ± 0.00 | 18.22 ± 0.72 | 27.08 ± 0.75 | |
| 5a | 50 | 52.33 ± 0.86 | 0.00 ± 0.00 | 9.58 ± 0.03 | 20.89 ± 0.44 | 48.89 ± 0.73 |
| 25 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 38.22 ± 0.72 | |
| 5b | 50 | 99.11 ± 0.09 | 99.14 ± 0.07 | 97.92 ± 0.01 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| 25 | 97.92 ± 0.18 | 97.25 ± 0.77 | 93.79 ± 0.01 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| 5c | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 95.42 ± 0.01 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| 25 | 100.00 ± 0.00 | 100.00 ± 0.00 | 92.79 ± 0.01 | 100.00 ± 0.00 | 98.17 ± 0.48 | |
| 5d | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 94.17 ± 0.03 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| 25 | 100.00 ± 0.00 | 100.00 ± 0.00 | 85.21 ± 0.01 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| 5e | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 16.67 ± 0.02 | 44.89 ± 0.60 | 66.22 ± 0.61 |
| 25 | 100.00 ± 0.00 | 100.00 ± 0.00 | 7.08 ± 0.01 | 34.09 ± 0.64 | 59.11 ± 0.72 | |
| 5f | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 99.17 ± 0.01 | 100.00 ± 0.00 | 84.00 ± 0.03 |
| 25 | 100.00 ± 0.00 | 100.00 ± 0.00 | 88.24 ± 0.01 | 80.34 ± 0.82 | 80.69 ± 0.73 | |
| 5g | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 98.75 ± 0.01 | 99.56 ± 0.72 | 100.00 ± 0.00 |
| 25 | 100.00 ± 0.00 | 100.00 ± 0.00 | 91.27 ± 0.00 | 75.96 ± 0.69 | 98.88 ± 0.60 | |
| 5h | 50 | 97.85 ± 0.08 | 100.00 ± 0.00 | 98.33 ± 0.01 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| 25 | 90.68 ± 0.73 | 91.06 ± 0.39 | 88.86 ± 0.02 | 95.94 ± 0.16 | 100.00 ± 0.00 | |
| 5i | 50 | 97.92 ± 0.72 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| 25 | 97.91 ± 0.39 | 100.00 ± 0.00 | 89.50 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| 5j | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 99.17 ± 0.01 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| 25 | 100.00 ± 0.00 | 97.92 ± 0.72 | 80.87 ± 0.02 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| 5k | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 97.85 ± 0.05 | 60.00 ± 0.17 | 44.89 ± 0.44 |
| 25 | 98.33 ± 0.72 | 100.00 ± 0.00 | 57.08 ± 0.01 | 32.89 ± 0.72 | 31.16 ± 0.69 | |
| 5l | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 93.75 ± 0.01 | 100.00 ± 0.70 | 99.13 ± 0.41 |
| 25 | 95.78 ± 0.12 | 100.00 ± 0.00 | 91.21 ± 0.00 | 97.67 ± 0.93 | 94.92 ± 0.65 | |
| 5m | 50 | 83.11 ± 0.70 | 93.03 ± 0.80 | 34.58 ± 0.03 | 86.67 ± 0.17 | 83.11 ± 0.72 |
| 25 | 86.46 ± 0.53 | 91.63 ± 0.53 | 23.33 ± 0.02 | 79.16 ± 0.79 | 76.67 ± 0.63 | |
| 5n | 50 | 88.66 ± 0.39 | 100.00 ± 0.00 | 48.33 ± 0.02 | 73.75 ± 0.50 | 63.56 ± 0.91 |
| 25 | 81.50 ± 0.36 | 97.36 ± 0.92 | 24.17 ± 0.05 | 0.00 ± 0.00 | 55.11 ± 0.72 | |
| 5o | 50 | 87.00 ± 0.00 | 90.55 ± 0.00 | 39.17 ± 0.01 | 37.78 ± 0.72 | 32.89 ± 0.52 |
| 25 | 85.82 ± 0.81 | 77.30 ± 0.23 | 4.17 ± 0.02 | 28.75 ± 0.72 | 23.33 ± 0.62 | |
| 5p | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 73.75 ± 0.01 | 54.67 ± 0.25 | 31.56 ± 0.71 |
| 25 | 100.00 ± 0.00 | 100.00 ± 0.00 | 44.17 ± 0.02 | 41.62 ± 0.83 | 23.33 ± 0.62 | |
| 5q | 50 | 100.00 ± 0.00 | 100.00 ± 0.00 | 11.25 ± 0.03 | 47.56 ± 0.72 | 34.22 ± 0.60 |
| 25 | 98.33 ± 0.72 | 98.13 ± 0.63 | 0.00 ± 0.00 | 43.56 ± 0.72 | 20.44 ± 0.72 | |
| ASBd | 50 | 47.88 ± 0.43 | 45.57 ± 0.29 | 61.85 ± 0.06 | 36.42 ± 0.43 | 33.31 ± 0.25 |
| 25 | 27.00 ± 0.23 | 30.43 ± 0.98 | 57.32 ± 0.28 | 31.87 ± 0.61 | 29.49 ± 0.70 | |
Results of antifungal evaluation (Table 2) indicated that 31 out of the 33 tested compounds showed moderate to strong inhibitory activity against B. cinerea with EC50 values of 0.0021–0.0827 mM, which were higher than the positive control azoxystrobin (EC50 = 0.3551 mM), and 19 of compounds with EC50 values of 0.0021–0.0330 mM demonstrated superior activity than HQ (EC50 = 0.0331 mM). The antifungal activity of 31 out of the 33 tested compounds against S. sclerotiorum was better than azoxystrobin (EC50 = 0.1629 mM), with EC50 values between 0.0016 and 0.0636 mM. Compound 2 showed the greatest activity, with EC50 value of 0.0016 mM, followed by 5c (EC50 = 0.0030 mM) respectively. 10 of the tested compounds showed higher activity against F. graminearum, with EC50 values of 0.0124–0.0211 mM, than HQ (EC50 = 0.0931 mM) and azoxystrobin (EC50 = 0.0229 mM) respectively. Furthermore, 10 out of 33 of the tested compounds exhibited stronger activity against F. oxysporum than HQ (EC50 = 0.1840 mM) and azoxystrobin (EC50 = 0.1265 mM) respectively, with EC50 values of 0.0059–0.0365 mM. Similarly, antifungal activity of 10 of the synthesized compounds against M. oryzae was better than HQ (EC50 = 0.0964 mM) and azoxystrobin (EC50 > 10.0000 mM) respectively, with EC50 values of 0.0120–0.0159 mM. The above results revealed that compound 2 was the most effective compound, followed by compound 5c.
| Compd.a | EC50 | ||||
|---|---|---|---|---|---|
| B. C. b | S. S. b | F. G. b | F. O. b | M. O. b | |
| a Compd.: compound. b B. C.: Botrytis cinerea. S. S.: Sclerotinia sclerotiorum. F. G.: Fusarium graminearum. F. O.: Fusarium oxysporum. f. sp. vasinfectum. M. O.: Magnaporthe oryzae. c ASB: azoxystrobin. | |||||
| HQ | 0.0331 | 0.0181 | 0.0931 | 0.1840 | 0.0964 |
| 2 | 0.0021 | 0.0016 | 0.0124 | 0.0059 | 0.0120 |
| 4a | 0.0827 | 0.0424 | — | — | — |
| 4b | 0.0165 | 0.0355 | — | — | — |
| 4c | 0.0537 | 0.0490 | — | — | — |
| 4d | 0.0536 | 0.0636 | — | — | — |
| 4f | 0.0317 | 0.0468 | — | — | — |
| 4g | 0.0444 | 0.0443 | — | — | — |
| 4h | 0.0298 | 0.0434 | — | — | — |
| 4i | 0.0496 | 0.0483 | — | — | — |
| 4j | 0.0356 | 0.0215 | — | — | — |
| 4k | 0.0277 | 0.0432 | — | — | — |
| 4l | 0.0222 | 0.0234 | — | — | — |
| 4m | 0.0790 | 0.0593 | — | — | — |
| 4n | 0.0285 | 0.0211 | — | — | — |
| 4o | 0.0330 | 0.0361 | — | — | — |
| 5b | 0.0386 | 0.0362 | 0.0190 | 0.0226 | 0.0156 |
| 5c | 0.0124 | 0.0030 | 0.0140 | 0.0146 | 0.0140 |
| 5d | 0.0150 | 0.0205 | 0.0167 | 0.0208 | 0.0150 |
| 5e | 0.0349 | 0.0343 | — | — | — |
| 5f | 0.0328 | 0.0320 | 0.0192 | 0.0348 | 0.0313 |
| 5g | 0.0172 | 0.0195 | 0.0228 | 0.0365 | 0.0154 |
| 5h | 0.0623 | 0.0463 | 0.0193 | 0.0233 | 0.0142 |
| 5i | 0.0307 | 0.0184 | 0.0183 | 0.0200 | 0.0156 |
| 5j | 0.0359 | 0.0458 | 0.0211 | 0.0206 | 0.0159 |
| 5k | 0.0348 | 0.0306 | — | — | — |
| 5l | 0.0233 | 0.0145 | 0.0211 | 0.0233 | 0.0144 |
| 5m | 0.0232 | 0.0417 | — | — | — |
| 5n | 0.0125 | 0.0190 | — | — | — |
| 5o | 0.0325 | 0.0419 | — | — | — |
| 5p | 0.0192 | 0.0328 | — | — | — |
| 5q | 0.0241 | 0.0324 | — | — | — |
| ASBc | 0.3551 | 0.1629 | 0.0229 | 0.1265 | >10.0000 |
2.3. Structure–activity relationships
Different substituent groups were introduced into the compound 2 scaffold to synthesize different analogs of 2 and their structure activity relationships (SAR) were investigated. The values indicated in Table 2, it was inferred that the presence of tertiary or secondary amine on the synthesized compound was essential for the antifungal activity of the tested compounds 4a–4o against B. cinerea and S. sclerotiorum. When NHR1R2 was directly substituted with aliphatic ring, the corresponding target compound displayed a very poor antifungal activity as exemplified by 4e. In contrast, when the NHR1R2 was replaced by substituted phenylpiperazines, a spike in antifungal activity was observed for the corresponding phenylpiperazine derivatives (5b–5q). It was therefore extrapolated that, the presence of substituted phenylpiperazine derivatives played a pivotal role in their impressive antifungal activity. However, benzylpiperazine 8-hydroxyquinoline derivative 5a showed weak activity against B. cinerea and S. sclerotiorum. Through analysis of the EC50 data in Table 2, revealed that unlike compounds 4a–4o, most of the tested compounds 5a–5q bearing aromatic piperazine substituent groups showed remarkable inhibitory activity against F. graminearum, F. oxysporum and M. oryzae respectively. From these observations, it was inferred that when the aromatic ring of aromatic piperazine on the target compound was benzene ring, the target compound demonstrated great antifungal activity. However, the presence of other aromatic rings, such as pyridine, pyrimidine and pyrazine on the target compounds 5o, 5p and 5q respectively, led to no antifungal activity. On the other hand, it was telling that the number of substituent groups (mono- versus di-) on the phenyl ring also impacted antifungal activity of the target compounds against the various fungi tested. Premised on the above observation, direct pairwise comparisons of antifungal activity of the tested compounds (5jversus5k, 5lversus5m) against the three fungi were analyzed in terms of their EC50 values. It was discovered that the mono-substituted compounds 5j and 5l exhibited better antifungal activity than their 2,4-dihalogenated counterparts 5k and 5m respectively. This observation showed that the number of substituents on the phenyl ring played a key role in inhibitory activity of the tested compounds. In order to determine the optimum position for mono-substitution on the phenyl ring, comparisons of compounds 5cversus5d; 5fversus5g; 5iversus5j were examined, and it was found that substitution at the ortho position (compounds 5c, 5i and 5f) conferred greater antifungal activity than substitution at the meta or para position (5d, 5j and 5g). Interestingly, the tested compounds bearing electron-donating groups such as methyl and methoxy on the aromatic piperazine (5c, 5d, 5g and 5h), exhibited remarkable antifungal activity against the three fungi. In addition, the introduction of halogen atoms such as F and Cl on phenylpiperazine augmented antifungal activity compounds 5i, 5j and 5l (EC50 values were <0.0250 mM) against the three fungi.2.4. Effects of compounds 2 and 5c on hyphal morphology of S. sclerotiorum
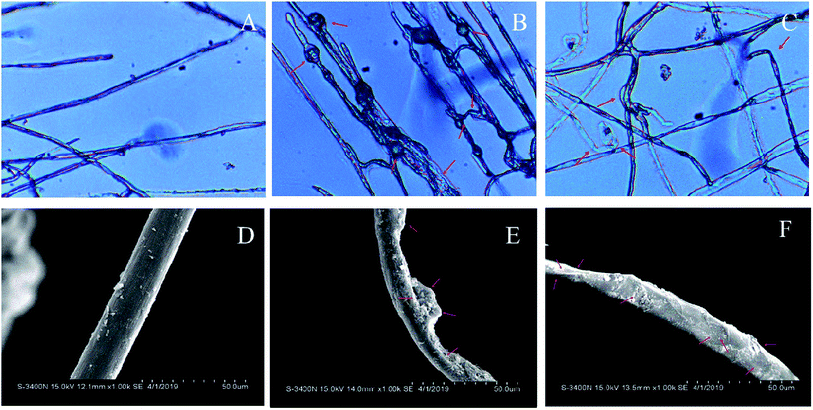
The hyphae morphology of S. sclerotiorum treated with EC50 of the two most effective compounds 2 and 5c were observed by optical microscope (Fig. 2A, B and C) and scanning electron microscopy (Fig. 2D, E and F), respectively. Satisfactorily, the two experiments yielded consistent results. The mycelia of the control group displayed a normal morphology with smooth, linear, regular, homogeneous and a constant diameter (Fig. 2A and D). However, mycelial morphology of the two tested compounds treated showed significant alteration. Hyphae of S. sclerotiorum treated with the EC50 of compound 2 were abnormal, with distinct bulges (Fig. 2B), correspondingly, scanning electron microscope observation results showed that hyphae become clear swelling or shrinking (Fig. 2E); the hyphae treated with the EC50 of compound 5c appeared obviously contort and wrinkle in the observation of optical microscope (Fig. 2C), further than that, scanning electron microscope observation results showed that hyphae appeared shriveled and collapsed (Fig. 2F). From the above observations, it was inferred that the two compounds destroyed the cell membrane and wall of S. sclerotiorum, culminating in the death of hyphae.2.5. Effects of compounds 2 and 5c against S. sclerotiorum in vivo
The results of pot experiments showed that compounds 2 and 5c exhibited moderate to excellent curative and protective effects in vivo (Table 3). Three conclusions were deduced from the data in Table 3: firstly, the curative and protective effects of the two compounds exhibited concentration-dependent properties; secondly, compound 5c possessed stronger protective and curative activity than that of 2; thirdly, the curative effects of compounds 5c and 2 at 80 μg mL−1 (95.44%, 87.91%) were better than the control azoxystrobin (83.59%) and the protective effects of compounds 5c and 2 at 80 μg mL−1 (91.09%, 90.93%) were close to that of azoxystrobin (91.32%). Underivatized compound 2 exhibited better activity in vitro, but the compound 5c possessed superior activity in vivo, it was therefore extrapolated that the introduction of 1-(2-methylphenyl)piperazine significantly improved the absorbability of compound 2 scaffold in plants. From pictures in Fig. 3, it was deduced that compounds 2 and 5c demonstrated no obvious phytotoxicity on oilseed rape leaves at a high concentration (80 μg mL−1), which were benign to the oilseed rape.| Compd.a | Concentration (μg mL−1) | Curative effect | Protective effect | ||
|---|---|---|---|---|---|
| Lesion length (mm ± SD) | Control efficacy (%) | Lesion length (mm ± SD) | Control efficacy (%) | ||
| a Compd.: compound. b ASB: azoxystrobin. | |||||
| 2 | 80 | 6.17 ± 0.38 | 87.91 | 5.87 ± 0.72 | 90.93 |
| 40 | 8.86 ± 0.74 | 60.05 | 7.08 ± 0.92 | 78.29 | |
| 20 | 12.80 ± 0.95 | 19.15 | 9.27 ± 0.55 | 55.39 | |
| 5c | 80 | 5.44 ± 0.40 | 95.44 | 5.85 ± 0.69 | 91.09 |
| 40 | 6.53 ± 0.65 | 84.18 | 7.06 ± 0.92 | 78.42 | |
| 20 | 12.71 ± 0.96 | 20.14 | 8.66 ± 0.98 | 61.68 | |
| ASBb | 80 | 6.58 ± 0.98 | 83.59 | 5.83 ± 0.52 | 91.32 |
| 40 | 7.19 ± 0.59 | 77.32 | 5.86 ± 0.52 | 91.02 | |
| 20 | 9.54 ± 0.94 | 52.94 | 6.78 ± 0.54 | 81.35 | |
| Control | — | 18.04 ± 0.76 | — | 20.53 ± 0.75 | — |
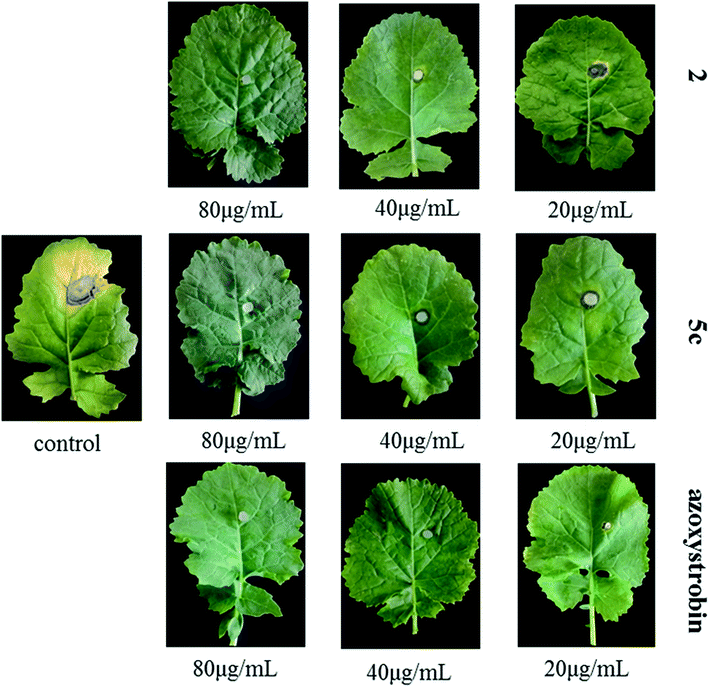 | ||
| Fig. 3 In vivo protective efficacy of compounds 2, 5c and azoxystrobin against S. sclerotiorum on rape leaves. | ||
2.6. Antifungal and antibacterial spectrum of compound 2
Compound 2 possessed the highest antifungal activity in vitro among all of 8-hydroxyquinoline derivatives, making it a promising lead compound for the development of novel antifungal agents. Hence, the antifungal spectrum of this compound was investigated and the results revealed that compound 2 showed impressive antifungal activity against many deleterious fungal pathogens, including Rhizoctonia solani, Mycosphaerlla melonis, Phyllosticta zeae, Colletotrichum gossypii, Phytophthora capsici and Pythium aphanidermatum. When it came to M. melonis, C. gossypii, P. capsici and P. aphanidermatum, compound 2 revealed excellent antifungal activity with EC50 of 0.0081, 0.0068, 0.0019 and 0.0043 mmol, respectively (Table 4). Additionally, the inhibitory activity of compound 2 against eight agricultural pathogenic bacteria (Table 5) was explored. The data in Table 5 indicated that compound 2 exhibited inhibitory effects against the plant pathogenic bacteria tested, and the activity against Acidovorax avenae subsp. citrulli, Pseudomanas solanacearum, Xanthomonas oryzae pv. oryzae and Xanthomonas oryzae pv. oryzicola was higher than that of the control streptomycin sulfate. The above results showed that compound 2 has a broad spectrum of antifungal and antibacterial activities.| Fungi | EC50 (mM) | 95% Cl |
|---|---|---|
| R. solani | 0.0149 | 0.0120–0.0185 |
| M. melonis | 0.0081 | 0.0059–0.0111 |
| P. zeae | 0.0127 | 0.0077–0.0208 |
| C. gossypii | 0.0068 | 0.0058–0.0081 |
| P. capsici | 0.0019 | 0.0016–0.0023 |
| P. aphanidermatum | 0.0043 | 0.0034–0.0055 |
| Bacterium | MIC (mM) | |
|---|---|---|
| Compound 2 | Streptomycin sulfate | |
| Acidovorax avenae subsp. citrulli | 0.0263 | 0.0412 |
| Agrobacterium tumefaciens | 0.1578 | 0.1372 |
| Erwinia carotovora | 0.1578 | 0.1372 |
| Pseudomonas syringae pv. actinidiae | 0.1578 | 0.0069 |
| Pseudomonas syringae pv. lachrymans | 0.1578 | 0.0206 |
| Pseudomanas solanacearum | 0.3155 | — |
| Xanthomonas oryzae pv. oryzae | 0.2104 | — |
| Xanthomonas oryzae pv. oryzicola | 0.2104 | — |
3. Conclusion
A series of 8-hydroxyquinoline derivatives were designed, synthesized and their antifungal activity was evaluated against five phytopathogenic fungi. Most of the tested compounds exhibited stronger antifungal activity against the five fungi than the primer molecule HQ. Especially, compound 2 demonstrated the best antifungal activity in vitro against B. cinerea, S. sclerotiorum, F. graminearum, F. oxysporum and M. oryzae with EC50 values of 0.0021, 0.0016, 0.0124, 0.0059 and 0.0120 mM respectively, followed by 5c. Moreover, compound 5c exhibited better protective and curative activity than that of compound 2in vivo, and the curative effects of compounds 5c and 2 at 80 μg mL−1 (95.44%, 87.91%) respectively were better than the positive control azoxystrobin (83.59%), compounds 2 and 5c effectively controlled the disease development in S. sclerotiorum infected oilseed rape in vivo, indicating great potential of these two compounds to control fungal diseases. The obvious teratogenic effect of compounds 2 and 5c on hyphal morphology of S. sclerotiorum will provide valuable insights into understanding the antifungal mechanism of 8-hydroxyquinoline derivatives. Additionally, compound 2 also displayed remarkable activity against eight agricultural pathogenic bacteria.4. Experimental section
4.1. General methods
All reactions were performed using commercially available regents without further purification. Thin-layer chromatography (TLC) was employed to monitor all reactions and column chromatography was performed with silica gel (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China). Melting points (mp) were determined using glass capillary tubes on a WRS-2U melting point apparatus (Shanghai Precision Instrument Co., Ltd., Shanghai, China) and were uncorrected. Mass spectrometry was performed using ESI mode on a Bruker Daltonics APEXII49e spectrometer (Bruker Daltonics Inc., Billerica, MA, US). Nuclear magnetic resonance (1H NMR and 13C NMR) spectra were recorded at 400 and 100 MHZ on a Bruker AM-400 (Bruker Company, Billerica, MA, and US.) spectrometer with TMS as an internal standard.The commercial fungicide azoxystrobin (analytical grade, 98% purity) provided by Jiangsu Balling Agrochemical Co., Ltd. Jiangying, China was used as a positive control in vitro experiment. And the commercial bactericide streptomycin sulfate (analytical grade, 98% purity) (J&K) was used as a positive control in minimal inhibitory concentration (MIC) test of compound 2 against the eight phytopathogenic bacterial.
Botrytis cinerea, Sclerotinia sclerotiorum, Fusarium graminearum, Fusarium oxysporum f. sp. vasinfectum, Magnaporthe oryzae, Rhizoctonia solani, Mycosphaerlla melonis, Phyllosticta zeae, Colletotrichum gossypii, Phytophthora capsici and Pythium aphanidermatum were provided by the Institute of Plant Protection, Gansu Academy of Agricultural Science, and Lanzhou, China. Acidovorax avenae subsp. citrulli, Agrobacterium tumefaciens, Erwinia carotovora, Pseudomonas syringae pv. actinidiae, Pseudomonas syringae pv. lachrymans, Pseudomanas solanacearum, Xanthomonas oryzae pv. oryzae and Xanthomonas oryzae pv. oryzicola, which were obtained from Shenyang Research Institute of Chemical Industry, and Shenyang, China.
4.2. Synthesis and characterization of compounds
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at 17 °C. The nitrosoquinoline was rapidly converted to the insoluble nitro compound. The mixture was diluted with water after 75 min stirring, cooled to 0 °C and made alkaline with sodium acetate. The product was washed with water and recrystallized from ethanol. Yellow solid; yield 65%; mp 226–228 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 7.17 (d, J = 8.8 Hz, 1H, ArH), 7.86 (dd, J = 8.8, 4.0 Hz, 1H, ArH), 8.52 (d, J = 8.8 Hz, 1H, ArH), 8.99 (d, J = 4.0 Hz, 1H, ArH), 9.18 (d, J = 8.8 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 110.45, 122.97, 125.67, 129.51, 132.84, 135.51, 137.72, 149.62, 161.09. HRMS calcd for C9H6N2O3, [M + H]+, 190.0378; found 190.2645.
2) at 17 °C. The nitrosoquinoline was rapidly converted to the insoluble nitro compound. The mixture was diluted with water after 75 min stirring, cooled to 0 °C and made alkaline with sodium acetate. The product was washed with water and recrystallized from ethanol. Yellow solid; yield 65%; mp 226–228 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 7.17 (d, J = 8.8 Hz, 1H, ArH), 7.86 (dd, J = 8.8, 4.0 Hz, 1H, ArH), 8.52 (d, J = 8.8 Hz, 1H, ArH), 8.99 (d, J = 4.0 Hz, 1H, ArH), 9.18 (d, J = 8.8 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 110.45, 122.97, 125.67, 129.51, 132.84, 135.51, 137.72, 149.62, 161.09. HRMS calcd for C9H6N2O3, [M + H]+, 190.0378; found 190.2645.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOH–H2O to yield the final product.
1 EtOH–H2O to yield the final product.
4.2.2.1. 7-((4-Methylpiperazin-1-yl)methyl)-5-nitroquinolin-8-ol (4a). Yellow solid; yield 66%; mp 206–208 °C; 1H NMR (400 MHz, chloroform-d, δ ppm): 2.36 (s, 3H, CH3), 2.62–2.77 (m, 8H, piperazine), 3.98 (s, 2H, CH2), 7.66 (dd, J = 8.9, 4.1 Hz, 1H, ArH), 8.42 (s, 1H, ArH), 8.96 (d, J = 4.0 Hz, 1H, ArH), 9.28 (d, J = 8.8 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 42.34, 48.45, 48.45, 50.52, 53.47, 53.47, 114.57, 117.46, 123.96, 126.39, 132.88, 135.11, 136.69, 147.99, 158.85. HRMS calcd for C15H18N4O3, [M + H]+, 303.1412; found 303.2014.
4.2.2.2. 7-((4-Ethylpiperazin-1-yl)methyl)-5-nitroquinolin-8-ol (4b). Yellow solid; yield 90%; mp 187–189 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 1.06 (t, J = 7.1 Hz, 3H, CH3), 2.74–2.84 (m, 8H, 4CH2piperazine), 3.08 (s, 2H, CH2), 3.91 (s, 2H, CH2), 7.61 (dd, J = 8.9, 4.1 Hz, 1H, ArH), 8.52 (s, 1H, ArH), 8.69 (d, J = 4.0 Hz, 1H, ArH), 9.32 (d, J = 8.8 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 9.42, 49.64, 49.64, 49.64, 50.98, 53.84, 53.84, 115.29, 118.22, 123.04, 126.02, 131.71, 134.20, 137.06, 148.71, 159.17. HRMS calcd for C16H20N4O3, [M + H]+, 317.1569; found 317.2228.
4.2.2.3. 5-Nitro-7-(piperidin-1-ylmethyl)quinolin-8-ol (4c). Yellow solid; yield 90%; mp 206–207 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 1.53–1.72 (m, 2H, CH2piperidine), 1.75 (t, J = 5.8 Hz, 4H, 2CH2piperidine), 3.13 (t, J = 4.8 Hz, 4H, 2CH2piperidine), 4.17 (s, 2H, CH2), 7.55 (dd, J = 8.7, 4.1 Hz, 1H, ArH), 8.58 (s, 1H, ArH), 8.60 (d, J = 3.0 Hz, 1H, ArH), 9.31 (d, J = 8.8, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 21.55, 22.96, 22.96, 52.48, 52.48, 53.69, 112.13, 124.11, 126.50, 132.48, 133.33, 135.06, 136.74, 148.08, 162.83. HRMS calcd for C15H17N3O3, [M + H]+, 288.1303; found 288.2205.
4.2.2.4. 7-(Morpholinomethyl)-5-nitroquinolin-8-ol (4d). Yellow solid; yield 89%; mp 219–220 °C; 1H NMR (400 MHz, chloroform-d, δ ppm): 2.71 (t, J = 3.4 Hz, 4H, 2CH2morpholine), 3.83 (t, J = 4.7 Hz, 4H, 2CH2morpholine), 3.97 (s, 2H, CH2), 7.69 (dd, J = 8.9, 4.2 Hz, 1H, ArH), 8.52 (s, 1H, ArH), 8.96 (d, J = 4.0 Hz, 1H, ArH), 9.28 (d, J = 8.9 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 51.58, 51.58, 53.99, 63.74, 63.74, 117.71, 124.27, 126.56, 132.30, 133.46, 135.27, 136.67, 147.94, 163.05. HRMS calcd for C14H15N3O4, [M + H]+, 290.1096; found 290.1586.
4.2.2.5. 7-((Cyclohexylamino)methyl)-5-nitroquinolin-8-ol (4e). Yellow solid; yield 85%; mp 206–207 °C; 1H NMR (400 MHz, chloroform-d, δ ppm): 2.53–1.31 (m, 10H, 5CH2cyclohexane), 3.13 (s, 1H, CH), 4.05 (s, 2H, CH2), 7.55 (dd, J = 8.7, 4.1 Hz, 1H, ArH), 7.92 (s, 1H, ArH), 8.62 (d, J = 3.0 Hz, 1H, ArH), 9.28 (d, J = 8.9 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 24.39, 24.39, 25.17, 29.12, 29.12, 42.08, 56.95, 117.46, 126.32, 126.32, 131.94, 132.83, 134.76, 136.60, 148.50, 161.60. HRMS calcd for C16H19N3O3, [M + H]+, 302.1460; found 302.2065.
4.2.2.6. 7-((Benzylamino)methyl)-5-nitroquinolin-8-ol (4f). Yellow solid; yield 50%; mp 204–207 °C; 1H NMR (400 MHz, chloroform-d, δ ppm): 4.07 (s, 4H, 2CH2), 4.80 (s, NH), 7.46–7.33 (m, 5H, ArH), 8.57 (dd, J = 8.9, 4.1 Hz, 1H, ArH), 8.88 (s, 1H, ArH), 9.24 (d, J = 8.8 Hz, 1H, ArH), 9.32 (d, J = 8.8 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 53.82, 59.41, 114.24, 117.12, 119.99, 124.29, 126.30, 129.09, 129.45, 130.49, 131.50, 132.57, 134.50, 136.88, 145.63, 148.35, 158.69. HRMS calcd for C17H15N3O3, [M + H]+, 310.1147; found 310.1745.
4.2.2.7. 7-(Pyrrolidin-1-ylmethyl)-5-nitroquinolin-8-ol (4g). Yellow-green solid; yield 80%; mp 218–218 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 1.73 (t, J = 5.9 Hz, 4H, 2CH2pyrrole), 3.11 (t, J = 5.5 Hz, 4H, 2CH2pyrrole), 4.17 (s, 2H, CH2), 7.56 (dd, J = 8.7, 4.1 Hz, 1H, ArH), 8.59 (s, 1H, ArH), 8.61 (d, J = 4.0 Hz, 1H, ArH), 9.33 (d, J = 8.7 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 22.96, 22.96, 51.59, 53.73, 53.73, 113.65, 124.07, 126.47, 132.43, 132.43, 135.17, 136.65, 148.08, 162.40. HRMS calcd for C14H15N3O3, [M + H]+, 274.1147; found 274.1811.
4.2.2.8. 7-(((Cyclopropylmethyl)amino)methyl)-5-nitroquinolin-8-ol (4h). Yellow solid; yield 44%; mp 219–219 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 0.35 (d, J = 5.6 Hz, 2H, CH2cyclopropnane), 0.57 (d, J = 7.6 Hz, 2H, CH2cyclopropnane), 1.13 (s, 1H, CHcyclopropnane), 2.87 (d, J = 7.4 Hz, 2H, CH2), 4.13 (s, 2H, CH2), 7.54 (dd, J = 8.7, 4.1 Hz, 1H, ArH), 8.55 (s, 1H, ArH), 8.62 (d, J = 3.0 Hz, 1H, ArH), 9.34 (d, J = 8.7 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 4.42, 4.42, 7.54, 44.80, 51.83, 114.46, 116.21, 123.99, 126.16, 132.14, 134.51, 137.28, 148.21, 158.64.
HRMS calcd for C14H15N3O3, [M + H]+, 274.1147; found 274.1638.
4.2.2.9. 7-((Ethylamino)methyl)-5-nitroquinolin-8-ol (4i). Yellow solid; yield 90%; mp 210–214 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 1.25 (d, J = 9.1 Hz, 3H, CH3), 1.74 (s, 2H, CH2), 4.31 (s, 2H, CH2), 7.78 (dd, J = 8.7, 4.1 Hz, 1H, ArH), 8.62 (s, 1H, ArH), 8.95 (d, J = 4.0 Hz, 1H, ArH), 9.21 (d, J = 9.6 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 11.41, 42.52, 44.36, 114.33, 123.68, 126.31, 131.89, 132.70, 134.68, 136.72, 148.49, 161.81. HRMS calcd for C12H13N3O3, [M + H]+, 248.0990; found 248.1824.
4.2.2.10. 7-((Propylamino)methyl)-5-nitroquinolin-8-ol (4j). Yellow solid; yield 90%; mp 204–205 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 0.91 (d, J = 7.7 Hz, 3H, CH3), 1.83–1.57 (m, 2H, CH2), 2.89 (d, J = 8.2 Hz, 2H, CH2), 4.08 (s, 2H, CH2), 7.54 (dd, J = 8.7, 4.1 Hz, 1H, ArH), 8.55 (s, 1H, ArH), 8.61 (d, J = 4.0 Hz, 1H, ArH), 9.34 (d, J = 8.8 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 11.38, 19.45, 44.76, 48.85, 114.26, 123.68, 126.34, 131.96, 132.77, 134.76, 136.66, 148.47, 161.75. HRMS calcd for C13H15N3O3, [M + H]+, 262.1147; found 262.1171.
4.2.2.11. 7-((Butylamino)methyl)-5-nitroquinolin-8-ol (4k). Yellow solid; yield 90%; mp 194–196 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 0.89 (t, J = 7.4 Hz, 3H, CH3), 1.30–1.37 (m, 2H, CH2), 1.64 (s, 2H, CH2), 2.94 (t, J = 7.8 Hz, 2H, CH2), 4.08 (s, 2H, CH2), 7.55 (dd, J = 8.7, 4.1 Hz, 1H, ArH), 8.55 (s, 1H, ArH), 8.61 (d, J = 4.0 Hz, 1H, ArH), 9.35 (d, J = 8.7 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 13.96, 19.72, 27.91, 44.79, 47.05, 114.29, 123.69, 126.34, 131.96, 132.74, 134.77, 136.65, 148.46, 161.75. HRMS calcd for C14H17N3O3, [M + H]+, 276.1303; found 276.1975.
4.2.2.12. 7-((Dimethylamino)methyl)-5-nitroquinolin-8-ol (4l). Yellow solid; yield 75%; mp 210–212 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 2.70 (s, 6H, 2CH3), 4.15 (s, 2H, CH2), 7.57 (d, J = 4.1 Hz, 1H, ArH), 8.58 (s, 1H, ArH), 8.64 (d, J = 3.4 Hz, 1H, ArH), 9.38 (d, J = 8.7, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 42.67, 42.67, 54.60, 112.72, 124.26, 126.52, 132.24, 133.04, 135.31, 136.62, 147.91, 162.91. HRMS calcd for C12H13N3O3, [M + H]+, 248.0990; found 248.1636.
4.2.2.13. 7-((Dipropylamino)methyl)-5-nitroquinolin-8-ol (4m). Yellow solid; yield 14%; mp 156–158 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 0.88 (t, J = 3.2 Hz, 6H, 2CH3), 1.72 (t, J = 12.0 Hz, 4H, 2CH2), 3.07–2.91 (m, 4H, 2CH2), 4.25 (s, 2H, CH2), 7.58 (dd, J = 9.0, 4.1 Hz, 1H, ArH), 8.58 (s, 1H, ArH), 8.65 (d, J = 4.0 Hz, 1H, ArH), 9.29 (d, J = 8.9 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 11.47, 11.47, 17.35, 17.35, 54.16, 54.16, 54.78, 114.45, 124.94, 126.44, 132.47, 134.05, 136.59, 141.92, 146.53, 150.06. HRMS calcd for C16H21N3O3, [M + H]+, 304.1616; found 204.2135.
4.2.2.14. 7-((Isopropylamino)methyl)-5-nitroquinolin-8-ol (4n). Yellow solid; yield 90%; mp 207–211 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 1.67–0.88 (m, 6H, 2CH3), 3.60 (s, 1H, CH), 4.08 (s, 2H, CH2), 4.57 (s, 1H, NH), 7.56 (s, 1H, ArH), 8.62 (d, J = 17.7 Hz, 2H, ArH), 9.37 (d, J = 9.3 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 19.12, 19.12, 42.30, 50.52, 117.18, 123.62, 126.30, 131.85, 132.90, 134.73, 136.62, 148.54, 161.49. HRMS calcd for C13H15N3O3, [M + H]+, 262.1147; found 262.1806.
4.2.2.15. 7-((tert-Butylamino)methyl)-5-nitroquinolin-8-ol (4o). Yellow solid; yield 90%; mp 215–216 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 1.44 (s, 9H, 3CH3), 4.01 (s, 2H, CH2), 7.44 (dd, J = 8.7, 4.1 Hz, 1H, ArH), 8.46 (s, 1H, ArH), 8.56 (d, J = 4.2 Hz, 1H, ArH), 9.23 (d, J = 9.2 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 25.62, 25.62, 25.6, 55.31, 57.38, 114.22, 117.10, 123.62, 126.33, 131.90, 134.63, 136.69, 148.69, 158.67. HRMS calcd for C14H17N3O3, [M + H]+, 276.1303; found 276.1962.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOH–H2O to yield the final product.
1 EtOH–H2O to yield the final product.
4.2.3.1. 7-((4-Benzylpiperazin-1-yl)methyl)-5-nitroquinolin-8-ol (5a). Yellow solid; yield 50%; mp 204–207 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 2.62 (t, J = 4.8 Hz, 4H, 2CH2piperazine), 2.99 (t, J = 4.4 Hz, 4H, 2CH2piperazine), 3.57 (s, 2H, CH2), 4.07 (s, 2H, CH2), 7.53–7.09 (m, 5H, ArH), 7.65 (dd, J = 8.7, 4.2 Hz, 1H, ArH), 8.57 (s, 1H, ArH), 8.73 (d, J = 4.1 Hz, 1H, ArH), 9.25 (d, J = 8.3 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 49.44, 49.69, 53.68, 59.41, 115.33, 118.26, 123.26, 126.11, 129.21, 129.21, 129.60, 131.25, 131.25, 132.01, 133.29, 134.38, 136.96, 148.54, 158.80. HRMS calcd for C21H22N4O3, [M + H]+, 379.1725; found 379.2229.
4.2.3.2. 7-((4-Phenylpiperazin-1-yl)methyl)-5-nitroquinolin-8-ol (5b). Yellow solid; yield 21%; mp 197–199 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 2.98 (t, J = 4.8 Hz, 4H, 2CH2piperazine), 3.29 (t, J = 4.8 Hz, 4H, 2CH2piperazine), 4.06 (s, 2H, CH2), 6.81 (t, J = 7.2 Hz, 1H, ArH), 6.95 (d, J = 8.2 Hz, 2H, ArH), 7.23 (t, J = 7.8 Hz, 2H, ArH), 7.73 (dd, J = 8.7, 4.1 Hz, 1H, ArH), 8.64 (s, 1H, ArH), 8.84 (d, J = 4.7 Hz, 1H, ArH), 9.26 (d, J = 8.7 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 7.52, 7.52, 44.47, 44.47, 51.90, 114.35, 114.35, 114.98, 117.90, 123.62, 126.32, 131.87, 131.87, 132.81, 134.76, 136.61, 148.46, 158.61, 158.96, 161.58. HRMS calcd for C20H20N4O3, [M + H]+, 365.1569; found 365.2229.
4.2.3.3. 7-((4-(o-Tolyl)piperazin-1-yl)methyl)-5-nitroquinolin-8-ol (5c). Yellow solid; yield 44%; mp 188–189 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 2.25 (s, 3H, CH3), 3.15–2.99 (m, 8H, 4CH2piperazine), 4.14 (s, 2H, CH), 7.03–6.96 (m, 2H, ArH), 7.19–7.12 (m, 2H, ArH), 7.66 (dd, J = 8.8, 4.2 Hz, 1H, ArH), 8.62 (s, 1H, ArH), 8.76 (d, J = 4.1 Hz, 1H, ArH), 9.24 (d, J = 8.8 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 17.88, 48.76, 48.76, 52.14, 52.14, 53.61, 111.99, 114.83, 117.73, 119.42, 124.26, 126.55, 127.16, 131.45, 132.54, 133.51, 135.23, 136.72, 148.00, 150.16, 163.00. HRMS calcd for C21H22N4O3, [M + H]+, 379.1725; found 379.1820.
4.2.3.4. 7-((4-(m-Tolyl)piperazin-1-yl)methyl)-5-nitroquinolin-8-ol (5d). Yellow solid; yield 81%; mp 187–188 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 2.25 (s, 3H, CH3), 3.03 (t, J = 5.0 Hz, 4H, 2CH2piperazine), 3.30 (t, J = 5.4 Hz, 4H, 2CH2piperazine), 4.07 (s, 2H, CH2), 6.63 (d, J = 7.4 Hz, 1H, ArH), 6.77 (s, 1H, ArH), 7.10 (t, J = 7.8 Hz, 1H, ArH), 7.39 (d, J = 13.4 Hz, 1H, ArH), 7.68 (dd, J = 8.8, 4.1 Hz, 1H, ArH), 8.60 (s, 1H, ArH), 8.77 (d, J = 4.1 Hz, 1H, ArH), 9.23 (d, J = 8.7 Hz, 1H). 13C NMR (100 MHz, DMSO-d6, δ ppm): 21.80, 46.01, 46.01, 51.26, 51.26, 53.53, 111.96, 113.60, 117.01, 121.22, 124.28, 126.55, 129.41, 132.30, 133.53, 135.28, 136.69, 138.75, 147.94, 149.95, 163.07. HRMS calcd for C21H22N4O3, [M + H]+, 379.1725; found 379.2224.
4.2.3.5. 7-((4-(p-Tolyl)piperazin-1-yl)methyl)-5-nitroquinolin-8-ol (5e). Yellow solid; yield 51%; mp 206–206 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 2.20 (s, 3H, CH3), 2.94 (t, J = 5.3 Hz, 4H, 2CH2piperazine), 3.21 (t, J = 5.5 Hz, 4H, 2CH2piperazine), 4.03 (s, 2H, CH2), 6.85 (d, J = 8.3 Hz, 2H, ArH), 7.03 (d, J = 8.3 Hz, 2H, ArH), 7.73 (dd, J = 8.9, 4.2 Hz, 1H, ArH), 8.64 (s, 1H, ArH), 8.83 (s, 1H, ArH), 9.28 (d, J = 8.9 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 20.48, 46.42, 46.42, 51.28, 51.28, 53.52, 111.96, 116.65, 116.65, 117.94, 124.28, 126.56, 129.40, 130.00, 130.00, 132.29, 133.53, 135.29, 136.70, 147.83, 163.08. HRMS calcd for C21H22N4O3, [M + H]+, 379.1725; found 379.1804.
4.2.3.6. 7-((4-(2-Methoxyphenyl)piperazin-1-yl)methyl)-5-nitroquinolin-8-ol (5f). Yellow solid; yield 79%; mp 196–201 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 3.09–3.15 (m, 8H, 4CH2piperazine), 3.78 (s, 3H, OCH3), 4.13 (s, 2H, CH2), 7.15–6.76 (m, 4H, ArH), 7.68 (dd, J = 8.8, 4.1 Hz, 1H, ArH), 8.62 (s, 1H, ArH), 8.76 (d, J = 5.8 Hz, 1H, ArH), 9.26 (d, J = 10.4 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 45.91, 45.91, 51.19, 51.19, 53.52, 55.42, 102.73, 105.58, 108.84, 111.95, 117.76, 124.29, 126.56, 130.34, 132.31, 133.53, 135.30, 136.69, 147.94, 151.26, 160.73. HRMS calcd for C21H22N4O4, [M + H]+, 395.1675; found 395.2277.
4.2.3.7. 7-((4-(3-Methoxyphenyl)piperazin-1-yl)methyl)-5-nitroquinolin-8-ol (5g). Yellow solid; yield 79%; mp 177–178 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 3.01 (t, J = 5.2 Hz, 4H, 2CH2piperazine), 3.32 (t, J = 5.1 Hz, 4H, 2CH2piperazine), 3.71 (s, 3H, OCH3), 4.05 (s, 2H, CH2), 6.40 (d, J = 8.1 Hz, 1H, ArH), 6.47 (s, 1H, ArH), 6.53 (d, J = 8.4 Hz, 1H, ArH), 7.12 (t, J = 8.2 Hz, 1H, ArH), 7.68 (dd, J = 8.8, 4.2 Hz, 1H, ArH), 8.60 (s, 1H, ArH), 8.78 (d, J = 3.9 Hz, 1H, ArH), 9.23 (d, J = 8.8 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 47.27, 47.27, 52.23, 52.23, 55.37, 56.20, 102.29, 105.01, 108.67, 111.95, 116.70, 124.68, 125.08, 130.17, 132.78, 135.30, 147.66, 152.12, 158.63, 159.99, 160.68. HRMS calcd for C21H22N4O4, [M + H]+, 395.1675; found 395.2348.
4.2.3.8. 7-((4-(4-Methoxyphenyl)piperazin-1-yl)methyl)-5-nitroquinolin-8-ol (5h). Yellow solid; yield 64%; mp 172–173 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 3.00 (t, J = 4.0 Hz, 4H, 2CH2piperazine), 3.18 (t, J = 4.6 Hz, 4H, 2CH2piperazine), 3.68 (s, 3H, OCH3), 4.07 (s, 2H, CH2), 6.99–6.78 (m, 4H, ArH), 7.72 (dd, J = 8.8, 4.2 Hz, 1H, ArH), 8.63 (s, 1H, ArH), 8.81 (d, J = 4.2 Hz, 1H, ArH), 9.26 (d, J = 8.8 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 47.32, 47.32, 51.46, 51.46, 53.53, 55.66, 111.96, 114.85, 114.85, 118.49, 118.49, 124.29, 126.57, 132.31, 133.52, 135.32, 136.68, 144.15, 147.94, 154.16, 163.06. HRMS calcd for C21H22N4O4, [M + H]+, 395.1675; found 395.2326.
4.2.3.9. 7-((4-(2-Fluorophenyl)piperazin-1-yl)methyl)-5-nitroquinolin-8-ol (5i). Yellow solid; yield 68%; mp 160.0–161 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 2.96 (s, 4H, 2CH2piperazine), 3.16 (t, J = 5.4 Hz, 4H, 2CH2piperazine), 4.02 (s, 2H, CH2), 7.39 (dd, J = 7.5, 5.1 Hz, 4H, ArH), 8.58 (s, 1H, ArH), 8.63 (s, 1H, ArH), 8.87 (s, 1H, ArH), 9.26 (d, J = 9.0 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 47.55, 47.55, 51.57, 51.57, 53.65, 111.89, 114.65, 117.55, 120.11, 125.45, 126.56, 127.39, 132.34, 133.52, 135.29, 136.69, 143.29, 145.80, 147.94, 163.05. HRMS calcd for C20H19FN4O3, [M + H]+, 383.1475; found 383.2014.
4.2.3.10. 7-((4-(4-Fluorophenyl)piperazin-1-yl)methyl)-5-nitroquinolin-8-ol (5j). Yellow solid; yield 55%; mp 168–171 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 3.02 (t, J = 3.2 Hz, 4H, 2CH2piperazine), 3.27 (t, 4H, 2CH2piperazine), 4.06 (s, 2H, CH2), 6.97 (t, J = 4.5 Hz, 2H, ArH), 7.06 (t, J = 8.9 Hz, 2H, ArH), 7.70 (dd, J = 8.8, 4.1 Hz, 1H, ArH), 8.61 (s, 1H, ArH), 8.79 (d, J = 2.5 Hz, 1H), 9.22 (d, J = 8.8 Hz, 1H). 13C NMR (100 MHz, DMSO-d6, δ ppm): 48.89, 50.97, 50.97, 53.71, 53.74, 115.09, 118.0, 118.00, 118.00, 120.92, 123.38, 126.16, 133.19, 133.19, 134.54, 136.92, 148.45, 158.88, 159.23, 161.53. HRMS calcd for C20H19FN4O3, [M + H]+, 383.1475; found 383.2049.
4.2.3.11. 7-((4-(2,4-Difluorophenyl)piperazin-1-yl)methyl)-5-nitroquinolin-8-ol (5k). Yellow solid; yield 62%; mp 197–203 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 2.96–2.54 (m, 8H, 4CH2piperazine), 3.92 (s, 2H, CH2), 7.39 (d, J = 6.8 Hz, 1H, ArH), 7.61 (dd, J = 8.7, 4.1 Hz, 1H, ArH), 7.79 (s, 1H, ArH), 8.52 (s, 1H, ArH), 8.69 (s, 1H, ArH), 9.31 (d, J = 8.8 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 48.84, 48.84, 50.98, 50.98, 53.67, 111.95, 114.85, 117.76, 117.76, 120.67, 123.51, 126.23, 126.23, 132.31, 133.05, 134.70, 136.86, 148.36, 159.14, 161.74. HRMS calcd for C20H18F2N4O3, [M + H]+, 401.1381; found 401.2038.
4.2.3.12. 7-((4-(4-Chlorophenyl)piperazin-1-yl)methyl)-5-nitroquinolin-8-ol (5l). Yellow solid; yield 61%; mp 192–197 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 2.99 (t, J = 5.0 Hz, 4H, 2CH2piperazine), 3.32 (t, J = 4.4 Hz, 4H, 2CH2piperazine), 4.04 (s, 2H, CH2), 6.96 (d, J = 8.6 Hz, 2H, ArH), 7.24 (d, J = 8.9 Hz, 2H, ArH), 7.71 (dd, J = 8.8, 4.1 Hz, 1H, ArH), 8.60 (s, 1H, ArH), 8.80 (d, J = 4.1 Hz, 1H, ArH), 9.21 (d, J = 8.9 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 45.81, 45.81, 51.08, 51.08, 53.55, 111.93, 114.86, 117.94, 117.94, 124.04, 126.57, 129.28, 129.28, 132.27, 133.53, 135.34, 136.68, 147.91, 148.78, 163.10. HRMS calcd for C20H19ClN4O3, [M + H]+, 399.1146; found 399.1839.
4.2.3.13. 7-((4-(2,4-Dichlorophenyl)piperazin-1-yl)methyl)-5-nitroquinolin-8-ol (5m). Yellow solid; yield 83%, mp 165–165 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 2.84 (t, J = 4.0 Hz, 4H, 2CH2piperazine), 3.31 (t, J = 4.2 Hz, 4H, 2CH2piperazine), 3.95 (s, 2H, CH2), 6.94 (d, J = 4.5 Hz, 1H, ArH), 7.15 (d, J = 4.8 Hz, 1H, ArH), 7.39 (s, 1H, ArH), 7.77 (dd, J = 8.8, 4.1 Hz, 1H, ArH), 8.60 (s, 1H, ArH), 8.86 (d, J = 2.5 Hz, 1H, ArH), 9.25 (d, J = 9.0 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 45.34, 45.34, 50.87, 50.87, 53.59, 111.90, 116.31, 117.45, 121.28, 124.33, 126.58, 127.40, 131.11, 132.12, 133.55, 135.36, 136.67, 147.90, 149.64, 158.66. HRMS calcd for C20H18Cl2N4O3, [M + H]+, 434.0726; found 434.3588.
4.2.3.14. 5-Nitro-7-((4-(4-nitrophenyl)piperazin-1-yl)methyl)quinolin-8-ol (5n). Yellow solid; yield 73%; mp 176–176 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 2.81 (t, J = 5.1 Hz, 4H, 2CH2piperazine), 3.57 (t, J = 5.0 Hz, 4H, 2CH2piperazine), 3.95 (s, 2H, CH2), 7.05 (d, J = 9.5 Hz, 2H, ArH), 7.39 (dd, J = 7.7, 5.7 Hz, 1H, ArH), 7.95–7.67 (m, 2H, ArH), 8.64 (s, 1H, ArH), 8.92 (d, J = 10.5 Hz, 1H, ArH), 9.22 (d, J = 9.2 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 44.18, 44.18, 50.77, 50.77, 53.65, 112.96, 113.94, 113.94, 124.31, 126.10, 126.10, 128.39, 132.26, 133.52, 135.30, 136.71, 138.44, 147.91, 154.14, 163.13. HRMS calcd for C20H19N5O5, [M + H]+, 410.1420; found 410.2100.
4.2.3.15. 5-Nitro-7-((4-(pyridin-2-yl)piperazin-1-yl)methyl)quinolin-8-ol (5o). Yellow solid; yield 88%; mp 190–191 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 2.97 (t, J = 5.1 Hz, 4H, 2CH2piperazine), 3.68 (t, J = 4.8 Hz, 4H, 2CH2piperazine), 4.05 (s, 2H, CH2), 6.68 (dd, J = 7.2, 4.8 Hz, 1H, ArH), 6.87 (d, J = 8.6 Hz, 1H, ArH), 7.39 (t, 7.36, 1H, ArH), 7.70 (dd, J = 8.8, 4.2 Hz, 1H, ArH), 7.79 (t, J = 6.7 Hz, 1H, ArH), 8.61 (s, 1H, ArH), 8.79 (d, J = 4.0 Hz, 1H, ArH), 9.22 (d, J = 8.7 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 42.64, 42.64, 50.87, 50.87, 53.64, 109.30, 111.9, 114.53, 117.81, 127.14, 132.33, 133.48, 135.27, 136.70, 139.67, 143.84, 146.01, 147.94, 163.05. HRMS calcd for C19H19N5O3, [M + H]+, 366.1521; found 366.2084.
4.2.3.16. 5-Nitro-7-((4-(pyrimidin-2-yl)piperazin-1-yl)methyl)quinolin-8-ol (5p). Yellow solid; yield 94%; mp 191–192 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 2.95 (t, J = 5.2 Hz, 4H, 2CH2piperazine), 3.92 (t, J = 5.2 Hz, 4H, 2CH2piperazine), 4.02 (s, 2H, CH2), 6.68 (t, J = 4.7 Hz, 1H, ArH), 7.39 (t, J = 4.7 Hz, 1H, ArH), 7.69 (dd, J = 8.6, 4.0 Hz, 1H, ArH), 8.39 (d, J = 4.7 Hz, 1H, ArH), 8.58 (s, 1H, ArH), 8.79 (d, J = 4.1 Hz, 1H, ArH), 9.19 (d, J = 8.8 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 40.55, 40.55, 51.01, 51.01, 53.68, 111.93, 124.24, 127.28, 132.38, 133.44, 135.21, 136.70, 143.51, 145.45, 147.97, 158.60, 158.60, 161.09. HRMS calcd for C18H18N6O3, [M + H]+, 367.1474; found 367.2326.
4.2.3.17. 5-Nitro-7-((4-(pyrazin-2-yl)piperazin-1-yl)methyl)quinolin-8-ol (5q). Yellow solid; yield 53%; mp 206–207 °C; 1H NMR (400 MHz, DMSO-d6, δ ppm): 2.89 (t, J = 5.2 Hz, 4H, 2CH2piperazine), 3.72 (t, J = 5.2 Hz, 4H, 2CH2piperazine), 3.99 (s, 2H, CH2), 7.39 (dd, J = 7.6, 5.7 Hz, 1H, ArH), 7.87 (d, J = 2.6 Hz, 1H, ArH), 8.16–8.05 (m, 1H, ArH), 8.35 (d, J = 2.3 Hz, 1H, ArH), 8.62 (s, 1H, ArH), 8.84 (d, J = 2.5 Hz, 1H, ArH), 9.21 (d, J = 8.8 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6, δ ppm): 41.74, 41.74, 50.77, 50.77, 53.69, 111.48, 114.33, 117.21, 127.65, 132.11, 133.48, 135.24, 141.99, 142.64, 146.65, 147.92, 154.21, 158.75. HRMS calcd for C18H18N6O3, [M + H]+, 367.1474; found 367.2580.
4.3. Antifungal activity
The in vitro antifungal activity against B. cinerea, S. sclerotiorum, F. graminearum, F. oxysporum, M. oryzae, R. solani, M. melonis, P. zeae, C. gossypii, P. capsici and P. aphanidermatum were assayed by mycelium linear growth rate method as previously reported.1 The strains were removed from their storage tubes and grown on potato dextrose agar (PDA) mediums for one week at 25 °C to allow the mycelia growth to be used for antifungal assays. The tested compounds were dissolved in DMSO and water containing Tween-80, and then added to the PDA to obtain mediums with different drug concentrations. The final concentration of DMSO was 0.5% because it had been shown to have no significant effect on the growth of the tested fungi. Azoxystrobin with different concentrations in the PDA mediums containing 0.5% DMSO (v/v) and 0.5% DMSO in the PDA medium were used as positive control and blank control respectively. A 5 mm diameter disc of fungus cut from subcultured Petri dishes was placed at the center of Petri dishes which contained PDA mediums with different drug concentrations. The diameter of mycelia was measured when the fungi in the blank control completely covered the Petri dish. The inhibition percentages were calculated using the formula:30I (%) = ([(C − d) − (T − d)])/((C − d)) × 100, where d is diameter of the cut fungus (5 mm), I is the inhibition (%), and C and T are the average colony diameters of the mycelium of the blank control and treatment respectively.After the preliminary antifungal activity screening, compounds with better activity were selected to further determine their medium effective concentrations (EC50) according to the same methods described above. A series of PDA mediums containing 50, 25, 10, 5, 2.5 μg mL−1 respectively of the tested compounds were prepared. Azoxystrobin was used as a positive control and 0.5% DMSO as a blank control respectively. Each test was performed in triplicate.
4.4. Effects of compounds 2 and 5c on hyphal morphology of S. sclerotiorum
A mycelial disk (5 mm diameter) was taken from the periphery of the colony grown on PDA mediums containing EC50 (0.0016 mM) of compound 2 and EC50 (0.0030 mM) of compound 5c respectively. The samples were inoculated to microscope slides on the first day, and observed the mycelial morphology by optical microscope (Motic AE31E) on the third day, respectively. Scanning electron microscopy observations on the hyphae of S. sclerotiorum were conducted according to the method of previous studies.32 A mycelial disk (5 mm diameter) was taken from the periphery of the colony grown on PDA mediums containing EC50 (0.0016 mM) of compound 2 and EC50 (0.0030 mM) of compound 5c respectively. Samples were fixed in 2.5% glutaraldehyde for 24 h at room temperature, and were washed for 15 min with 0.1 mol L−1 phosphate buffer for three times, followed another 1 h fixation in 1% OsO4 solution. The specimens were dehydrated in a grated ethanol series (20%, 50%, 80% and 100% respectively, 5 min for each alcohol dilution). After drying at critical point and gold coating, SEM observations were carried out with a scanning electron microscope (Hitachi, S-3400N, Japan) at an accelerating voltage of 15.0 kV.4.5. Effects of compounds 2 and 5c against S. sclerotiorum in vivo
The control efficacy (protective and curative activity) of compounds 2 and 5c against S. sclerotiorum in leaves of oilseed rape was assessed with pot experiments according to the method described by Yan et al.33 Firstly, 30 day-old oilseed rape leaves were washed with distilled water. For curative effect assay, the mycelial plugs were inoculated to the leaves on the first day, on the second day, the compounds 2 and 5c solutions as well as the positive control azoxystrobin with different concentrations (20, 40 and 80 μg mL−1) respectively (containing 0.1% Tween 80 as surfactant) were sprayed on the leaves. Plants sprayed with water (plus 0.1% Tween 80) were used as a negative control. Then the plants were placed in a greenhouse at 25 °C with 100% relative humidity. After 3 days, the lesion diameter was measured and the curative efficacy of compounds 2 and 5c was calculated according to the following formula: (diameter of lesion in negative control − diameter of lesion in the treatment)/diameter of lesion in negative control. There were three replicates for each treatment, and the experiment was repeated at least twice. For protection assay, the mycelial plugs were inoculated to the leaves for one day after the leaves were sprayed with test sample solutions. The rest of the steps were the same as the above.4.6. Minimal inhibitory concentration (MIC) test of compound 2 against plant pathogenic bacteria
MIC values were determined by the broth microdilution method in 96-well microtiter plates.31 Dilutions of compound 2, ranging from 1 to 1000 μg mL−1 were incubated with corresponding bacterial suspensions adjusted to 5 × 105 CFU mL−1 in Mueller–Hinton Broth (MHB). Streptomycin sulfate was used as a positive control, and the vehicle was used as a negative control. The microtiter plates were incubated at 37 °C, after 24 h of incubation, readings were performed by visual reading and optical-density (OD 595 nm) determination in a BioTek microplate reader (Highland Park, Winooski, USA). The MIC value was defined as the lowest compound concentration that prevented bacterial growth after a 24 h incubation. MIC values were determined by three independent replicates.Conflicts of interest
The authors state no conflict of interest.Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (21672092, 21877056) and the National Key Research and Development Program of China (2017YFD0201404); Support was also supplied by the Key Program for International S&T Cooperation Projects of China Gansu Province (18YF1WA115).References
- R. Yang, Z. F. Gao, J. Y. Zhao, W. B. Li, L. Zhou and M. Fang, J. Agric. Food Chem., 2015, 63, 1906–1914 CrossRef CAS.
- Y. B. Bai, A. L. Zhang, J. J. Tang and J. M. Gao, J. Agric. Food Chem., 2013, 61, 2789–2795 CrossRef CAS.
- X. F. Cao, F. Li, M. Hu, W. C. Lu, G. A. Yu and S. H. Liu, J. Agric. Food Chem., 2008, 56, 11367–11375 CrossRef CAS.
- H. J. Kim, H. J. Suh, C. H. Lee, J. H. Kim, S. C. Kang, S. Park and J. H. Kim, J. Agric. Food Chem., 2010, 58, 9483–9487 CrossRef CAS.
- Z. Hou, L. F. Zhu, X. C. Yu, M. Q. Sun, F. Miao and L. Zhou, Design, J. Agric. Food Chem., 2016, 64, 2847–2854 CrossRef CAS.
- G. Gellerman, N. Pariente, Z. Paz, A. Shnaiderman and O. Yarden, J. Agric. Food Chem., 2009, 57, 8303–8307 CrossRef CAS.
- P. Laborda, Y. Y. Zhao, J. Ling, R. X. Hou and F. Q. Liu, J. Agric. Food Chem., 2018, 66, 630–636 CrossRef CAS.
- S. S. Zhang, D. D. Li, Z. H. Song, C. L. Zhang and X. S. Song, J. Agric. Food Chem., 2017, 65, 9013–9021 CrossRef CAS.
- L. B. Freitas, T. F. Borgati, R. P. Freitas, A. T. Ruiz, G. M. Marchetti, J. D. Carvalho, E. F. Cunha, T. C. Ramalho and R. B. Alves, Eur. J. Med. Chem., 2014, 84, 595–604 CrossRef CAS.
- K. D. Thomas, A. V. Adhikari and N. S. Shetty, Eur. J. Med. Chem., 2010, 45, 3803–3810 CrossRef CAS.
- K. H. Lam, R. Gambari, K. H. Lee, Y. X. Chen, S. H. Kok, R. S. Wong, F. Y. Lau, C. H. Cheng, W. Y. Wong, Z. X. Bian, A. S. Chan, J. C. Tang and C. H. Chui, Bioorg. Med. Chem. Lett., 2014, 24, 367–370 CrossRef CAS.
- K. V. Sashidhara, A. Kumar, G. Bhatia, M. M. Khan, A. K. Khanna and J. K. Saxena, Eur. J. Med. Chem., 2009, 44, 1813–1818 CrossRef CAS.
- L. B. Freitas, T. F. Borgati, R. P. Freitas, A. L. Ruiz, G. M. Marchetti, J. E. Carvalho, E. F. Cunha, T. C. Ramalho and R. B. Alves, Eur. J. Med. Chem., 2014, 84, 595–604 CrossRef CAS.
- E. Serrao, B. Debnath, H. Otake, Y. Kuang, F. Christ, Z. Debyser and N. Neamati, J. Med. Chem., 2013, 56, 2311–2322 CrossRef CAS PubMed.
- S. Madona, C. Beclin, Y. Laras, V. Moret, A. Macowycz, D. Lamoral, J. Dubois, M. b. Requin, G. Lenglet, S. Depauw, T. Cresteil, G. Aubert, V. Monnier, R. Kiss, M. D. Cordonnier and J. L. Kraus, Eur. J. Med. Chem., 2010, 45, 623–638 CrossRef PubMed.
- J. Kos, L. Zadrazilova, E. Nevin, S. Michal, T. Gonec, P. Kollar, M. Oravec, A. Coffey, J. O. Mahony, T. Liptaj, K. Kralova and J. Jampilek, Bioorg. Med. Chem., 2015, 23, 4188–4196 CrossRef CAS.
- V. Oliveri and G. Vecchio, Eur. J. Med. Chem., 2016, 120, 252–274 CrossRef CAS.
- V. Moret, Y. Laras, T. Cresteil, G. Aubert, D. Q. Ping, C. Di, M. B. Requin and C. Beclin, Eur. J. Med. Chem., 2009, 44, 558–567 CrossRef CAS.
- A. E. Rashad, W. A. Ei-sayed, A. M. Mohamed and M. Mamdouh, Arch. Pharm. Chem. Life Sci., 2010, 8, 440–448 CrossRef.
- R. K. Arafa, G. H. Hegazy, G. A. Piazza and A. H. Abadi, Eur. J. Med. Chem., 2013, 63, 826–832 CrossRef CAS.
- R. Musiol, J. Jampilek, V. Buchta, L. Silva, H. Niedbala, B. Podeszwa, A. Palka, B. Oleksyn and J. Polanski, Bioorg. Med. Chem., 2006, 14, 3592–3598 CrossRef CAS.
- R. Musiol, J. Jampilek, J. E. Nycz, M. Pesko, J. Carroll, K. Kralova, M. Vejsova, J. O. Mahony, A. Coffey, A. Mrozek and J. Polanski, Molecules, 2010, 15, 288–304 CrossRef CAS.
- P. A. Enquist, A. Gylfe, U. Hagglund, P. Lindstrom, H. N. Scherman, C. Sundin and M. Elofsson, Bioorg. Med. Chem. Lett., 2012, 22, 3550–3553 CrossRef CAS.
- S. S. Chhajed, P. Manisha, V. A. Bastikar, H. Animeshchandra, V. N. Ingle, C. D. Upasani and S. S. Wazalwar, Bioorg. Med. Chem. Lett., 2010, 20, 3640–3644 CrossRef CAS.
- J. Polanski, H. Niedbala, R. Musiol, B. Podeszwa, D. Tabak, A. Palka, A. Mencel, J. Finster, J. F. Mouscadet and M. L. Bret, Lett. Drug Des. Discovery, 2006, 3, 175–178 CrossRef CAS.
- S. Madonna, P. Maher and J. L. Kraus, Bioorg. Med. Chem. Lett., 2010, 20, 6966–6998 CrossRef CAS PubMed.
- N. C. Warshakoon, S. Wu, A. Boyer, R. Kawamoto, J. Sheville, S. Renock, K. Xu, M. Pokross, S. T. Zhou, C. Winter, R. Walter, M. Mekel and A. G. Evdokimov, Bioorg. Med. Chem. Lett., 2006, 16, 5517–5522 CrossRef CAS.
- U. K. Mazumder, M. Gupta, S. Bhattacharya, S. S. Karki, S. Rathinasany and S. Thangavel, J. Enzyme Inhib. Med. Chem., 2004, 19, 185–192 CrossRef CAS PubMed.
- P. Wangtrakuldee, M. S. Byrd, C. G. Campos, M. W. Henderson, Z. Zhang, M. Clare, A. Masoudi, P. J. Myler, J. R. Horn, P. A. Cotter and T. J. Hagen, ACS Med. Chem. Lett., 2013, 4, 699–703 CrossRef CAS.
- M. Agarwal, S. Walia, S. Dhingra and B. P. Khambay, Pest Manage. Sci., 2001, 57, 289–300 CrossRef CAS.
- A. A. Armijio, N. Glibota, M. Frias, M. P. Frias, J. Altarejos, A. Galvez, S. Salido and E. O. Morente, J. Agric. Food Chem., 2018, 66, 2151–2158 CrossRef.
- X. Liu, L. P. Wang, Y. C. Li, T. Yu and X. D. Zheng, J. Appl. Microbiol., 2009, 107, 1450–1456 CrossRef CAS.
- H. Yan, Z. Xiong, N. Xie, S. Z. Liu, L. L. Zhang, F. Xu, W. H. Guo and J. T. Feng, Ind. Crops Prod., 2018, 121, 352–359 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra05712a |
| This journal is © The Royal Society of Chemistry 2019 |