Open Access Article
Open Access ArticleDiscovery of diazahexa/hepta cyclic cage-like compounds with broad-spectrum antifungal activity against Candida and Cryptococcus species†
Anthony Weinstock‡
a,
Natarajan Arumugam‡ *b,
Abdulrahman I. Almansourb,
Raju Suresh Kumar
*b,
Abdulrahman I. Almansourb,
Raju Suresh Kumar b and
Shankar Thangamani
b and
Shankar Thangamani *c
*c
aArizona College of Osteopathic Medicine, Midwestern University, 19555 N. 59th Ave., Glendale, AZ 85308, USA
bDepartment of Chemistry, College of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia. E-mail: antarajan@ksu.edu.sa; Fax: +966 4675992; Tel: +966 4675907
cDepartment of Pathology and Population Medicine, College of Veterinary Medicine, Midwestern University, 19555 N. 59th Ave., Glendale, AZ 85308, USA. E-mail: sthang@midwestern.edu; Fax: +1 623 537 6399; Tel: +1 623 537 6378
First published on 20th September 2019
Abstract
Invasive fungal infections caused by Candida and Cryptococcus species lead to life threating infections in immunocompromised individuals. Furthermore, increasing incidence of fungal strains resistant to FDA-approved antifungal drugs along with the paucity of antifungal drugs warrants novel drugs to treat invasive fungal infections. In this study, we investigated the antifungal activity of a novel series of diazahexa/hepta cyclic cage-like compounds. Results indicate that compounds with unsubstituted and o-methyl substitution on aryl rings exhibit potent broad-spectrum antifungal activity against various fungal strains. In addition, these compounds showed significant inhibitory activity against Candida hyphae and biofilm formation. Collectively, results from this study indicate that these compounds are promising candidates to develop as novel antifungal drugs to treat drug-resistant fungal infections.
Introduction
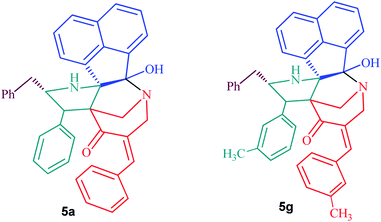
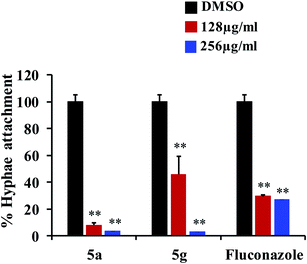
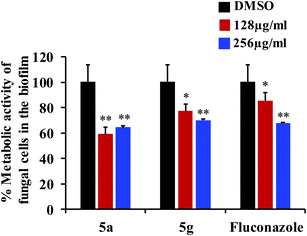
Fungal infections, especially invasive candidiasis, are among the most common blood stream infections in the hospital setting, particularly among cancer patients and patients in intensive care units.1–4 Cryptococcus spp. also demonstrates high prevalence in immunocompromised patients infected with human immunodeficiency virus (HIV).5 With increased dependence and utilization of broad-spectrum antifungal drugs, there has been a causal increase in resistance to antifungal drugs across Candida and Cryptococcus species.6–8 However, with the most recent antifungal drug approved in the 2000s, only a limited number of antifungal drugs are currently available to treat these fungal infections.9,10 Coupled with the fungal strains developing resistance to current drugs and paucity of FDA-approved antifungal agents, an unmet and urgent need to develop new antifungal drugs are necessary to treat the systemic invasive fungal infections.10–12In this study, we aim to identify novel compounds against the important human fungal pathogens, Candida and Cryptococcus species. Our results indicate that compounds 5a and 5g exhibit potent inhibitory activity against fungal strains and have the potential to develop as new antifungal drugs.
Results and discussion
Chemistry
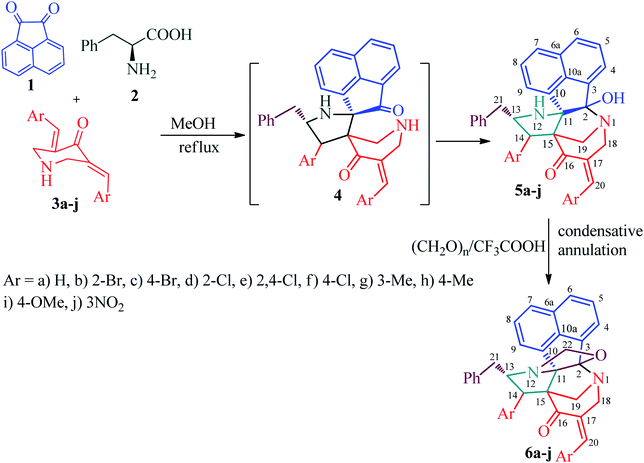
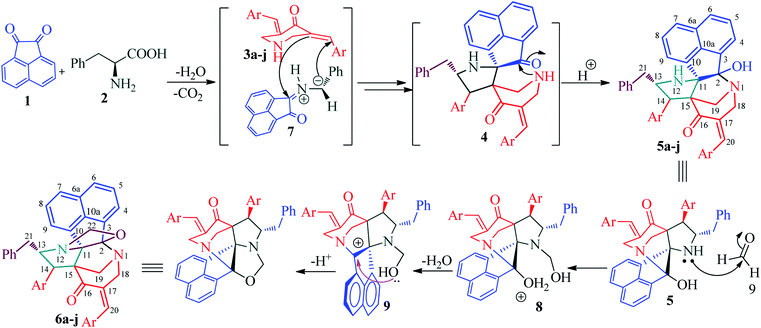
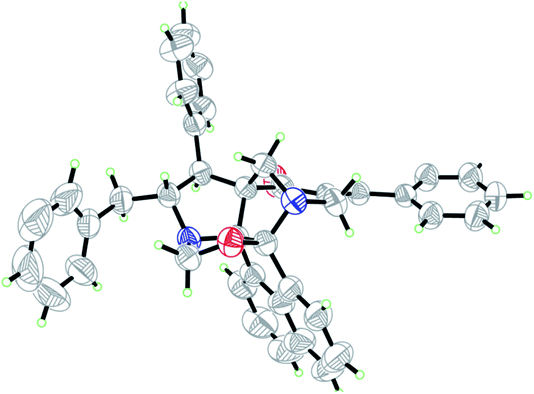
Recently, we synthesized a series of diazahexa-/hepta cyclic cage-like compounds (Fig. 1) via domino multicomponent protocol13 that involved (i) 1,3-dipolar cycloaddition and (ii) concomitant annulation steps (Scheme 1). 1,3-Dipolar cycloaddition reaction of the azomethine ylide generated in situ from an equimolar amount of acenapthenequinone (1) and L-phenylalanine (2), with a series of bisbenzylidenepiperidin-4-ones 3 in methanol under reflux condition afforded the cage-like systems in good to excellent yields. The crude cage-like product 5 obtained was purified through column chromatography and its structure was confirmed by spectroscopic studies. With a series of diaza hexacyclic cage-like compound 5 in hand, we proceeded further to explore their reaction with paraformaldehyde in the presence of trifluoroacetic acid, which led to the formation of structurally interesting diazahepta cyclic cage-like compounds 6 comprising [1,2-c]oxazolidine, pyrrolidine, and piperidine structural units. In a typical reaction, diazahexacyclic cage-like compounds 5 (1 mmol) were treated overnight with paraformaldehyde (1 mmol) in the presence of a catalytic amount trifluoroacetic acid (10 mol%) in CH2Cl2 at room temperature which afforded 6 in excellent yields (88–98%). The structure of the synthesized compound was confirmed by 1H and 13C NMR spectroscopic studies and their stereochemistry has been unambiguously ascertained by single X-ray crystal analysis (Fig. 2).14 The plausible mechanism for the formation of diazahexa/hepta cyclic cage-like compounds 5 and 6 is described in Scheme 2.Biology
| Entry | Compounds | MIC (μg mL−1) |
|---|---|---|
| 1 | 5a | 4 |
| 2 | 5b | 128 |
| 3 | 5c | 32 |
| 4 | 5d | 128 |
| 5 | 5e | 128 |
| 6 | 5f | 128 |
| 7 | 5g | 4 |
| 8 | 5h | 256 |
| 9 | 5i | 16 |
| 10 | 5j | 16 |
| 11 | 6a | 16 |
| 12 | 6c | 64 |
| 14 | 6d | 128 |
| 15 | 6g | 64 |
| 16 | 6i | 16 |
Next, we tested the antifungal activity of compounds 5a and 5g against clinical strains of Candida spp. and Cryptococcus neoformans in order to determine if the compounds possess broad-spectrum activity against a variety of fungal species. After 24 hours of incubation, both 5a and 5g demonstrated potent antifungal activity against C. albicans, Candida parapsilosis, and Candida tropicalis with the MICs ranging from 0.5 to 4 μg mL−1 (Table 2). Further, 5a and 5g also inhibited the growth of these fungal strains even after 48 hours of incubation with the MICs ranging from 2 to 8 μg mL−1 (Table 2). Compounds 5a and 5g also showed excellent antifungal activity against C. neoformans and C. glabrata with the MICs ranging from 0.5 to 2 μg mL−1 after 48 hours of incubation (Table 2).
| Strain | Description | MIC Fluconazole (μg mL−1) | MIC 5a (μg mL−1) | MIC 5g (μg mL−1) | |||
|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | ||
| a Not detected (n.d.). | |||||||
| C. albicans NR-29434 | Bloodstream isolate from a patient with candidemia from Winnipeg, Manitoba, Canada in 2000 | 0.125 | 32 | 4 | 8 | 2 | 4 |
| C. albicans NR-29449 | Vaginal isolate from a patient with vaginitis from Ann Arbor, Michigan, USA between 1990 and 1992 | 0.5 | 32 | 4 | 8 | 4 | 8 |
| C. albicans NR-29435 | Bloodstream isolate from a patient with candidemia from Iowa City, Iowa, USA in 2000 | 0.5 | 0.5 | 2 | 4 | 2 | 4 |
| C. albicans NR-29448 | Clinical isolate from a person with candidemia from Arizona, USA | 1 | 32 | 2 | 8 | 2 | 4 |
| C. albicans NR-29437 | Bloodstream isolate from a patient with candidemia from Brussels, Belgium in 2000 | 0.25 | 32 | 4 | 8 | 2 | 4 |
| C. albicans NR-29446 | Bloodstream isolate from a patient with candidemia from Utah, USA | 16 | 32 | 1 | 4 | 0.5 | 4 |
| C. albicans NR5-29453 | Clinical isolate from a patient with thrush and HIV from Pretoria, South Africa | 0.0625 | 0.0625 | 4 | 4 | 2 | 4 |
| C. albicans NR-29438 | Bloodstream isolate from a patient with candidemia from Tel-Hashomer, Israel, in 2000 | 0.0625 | 0.25 | 4 | 8 | 2 | 4 |
| C. albicans NR-29367 | Clinical isolate from China | 0.0625 | 0.0625 | 4 | 4 | 2 | 4 |
| C. albicans NR-29439 | Bloodstream isolate from a patient with candidemia from Omaha, Nebraska, USA, in 2000 | 0.5 | 32 | 2 | 8 | 2 | 4 |
| C. albicans NR-29440 | Bloodstream isolate from a patient with candidemia from Lille, France, in 2000 | 0.25 | 0.5 | 4 | 8 | 2 | 4 |
| C. albicans NR-29441 | Bloodstream isolate from a patient with candidemia from Iowa City, Iowa, USA, in 2000 | 0.25 | 16 | 2 | 8 | 1 | 4 |
| C. albicans NR-29442 | Bloodstream isolate from a patient with candidemia from Ottawa, Ontario, Canada, in 2000 | 0.25 | 32 | 4 | 8 | 2 | 4 |
| C. albicans NR-29444 | Oral isolate from a patient with vaginitis collected in Ann Arbor, Michigan, USA between 1990 and 1992. | 1 | 16 | 2 | 4 | 2 | 4 |
| C. parapsilosis ATCC 22019 | Clinical isolate from a patient with celiac disease from Puerto Rico | 1 | 4 | 0.5 | 2 | 0.5 | 2 |
| C. tropicalis ATCC 13803 | FDA provided isolate | 8 | 32 | 4 | 8 | 2 | 4 |
| C. glabrata ATCC 90030 | Bloodstream isolate from a patient from Iowa | n.d. | 4 | n.d. | 0.5 | n.d. | 0.5 |
| C. albicans ATCC 10231 | Clinical isolate from a patient with bronchomycosis | 2 | 4 | 2 | 4 | 1 | 4 |
| C. neoformans NR-41291 | Cerebrospinal fluid isolate from a patient from China in July 2011 | n.d. | 32 | n.d. | 2 | n.d. | 2 |
| C. neoformans NR-41292 | Cerebrospinal fluid isolate from a patient from China in February 2012 | n.d. | 32 | n.d. | 1 | n.d. | 1 |
| C. neoformans NR-41296 | Cerebrospinal fluid isolate from a patient from China in February 2012 | n.d. | 4 | n.d. | 1 | n.d. | 1 |
| C. neoformans NR-41295 | Cerebrospinal fluid isolate from a patient from China in February 2012 | n.d. | 32 | n.d. | 1 | n.d. | 1 |
| C. neoformans NR-41294 | Cerebrospinal fluid isolate from a patient from China in June 2011 | n.d. | 4 | n.d. | 0.5 | n.d. | 0.5 |
| C. neoformans NR-41297 | Cerebrospinal fluid isolate from a patient from China in February 2012 | n.d. | 8 | n.d. | 1 | n.d. | 1 |
| C. neoformans NR-41298 | Cerebrospinal fluid isolate from a patient from China in February 2012 | n.d. | 16 | n.d. | 2 | n.d. | 2 |
| C. neoformans NR-41299 | Cerebrospinal fluid isolate from a patient from China in August 2009 | n.d. | 8 | n.d. | 1 | n.d. | 1 |
Fluconazole was used as a metric of comparison, due to its status as FDA-approved antifungal drug with widespread clinical use. Fluconazole inhibited the Candida strains with a wide range of MICs from 0.0625 to 16 μg mL−1 after 24 hours of incubation (Table 2). However, after 48 hours, the MICs of fluconazole for all strains were increased several fold with most strains inhibited at 32 μg mL−1 (Table 2). Surprisingly, unlike fluconazole, both compounds 5a and 5g showed potent inhibitory activity against most of the Candida strains, even after 48 hours of incubation without considerable increase in the MIC values. In addition, 5a and 5g also showed excellent antifungal activity against C. glabrata and C. neoformans after 48 hours of incubation compared to fluconazole (Table 2). Collectively, compounds 5a and 5g exhibit potent broad-spectrum antifungal activity compared to fluconazole against all the fungal strains tested in this study.
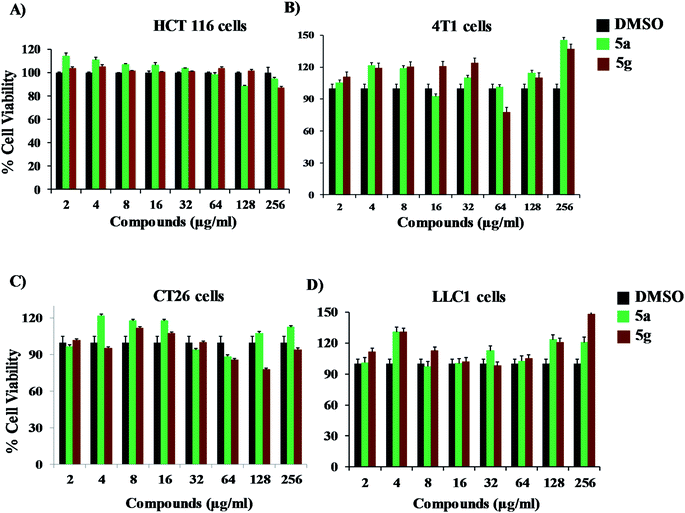
Results from this study indicate that compounds 5a and 5g possess potent antifungal and antivirulence activities without causing mammalian host toxicity. In addition, 5a and 5g showed excellent activity compared to fluconazole. Further studies to determine the pharmacokinetic and physicochemical profile of these compounds is essential to move these compounds to the next stage of the drug development pipeline. The structure of 5a and 5g contain the nitrogen (N1) atom in the diazahexa cage-like compounds, a characteristic shared by the benzylamine and allylamine antifungals. These antifungals act as inhibitors of squalene epoxidase, a key enzyme in the synthesis of sterols by fungi. The nitrogen (N12) atom in the pyrrolidine ring shares similar bonding to that of echinocandins, which act as beta-1, 3-D-glucan synthase inhibitors. The carbonyl unit of piperidone at position sixteen shares similarities to that of the flavonoids, which have become a new area of study for their antifungal activity. Therefore, we speculate that these novel compounds may target pathways specific to fungi including fungal 3b-glucan synthase and ergosterol biosynthesis.25 However, future studies will be needed to understand the antifungal mechanism of these compounds. In addition, further structure modifications to enhance the activities of 5a and 5g should be also a promising avenue. Taken together, compounds 5a and 5g have strong potential to develop as novel antifungal drugs.
Materials and methods
Chemistry
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane (7
hexane (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) as eluent.
3 v/v) as eluent.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane (1
hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v) as the eluent and recrystallized from EtOAc.
4 v/v) as the eluent and recrystallized from EtOAc.Biology
Conflicts of interest
The authors declare that there are no conflicts of interest.Acknowledgements
The authors acknowledge the Deanship of Scientific Research at King Saud University for the funding this work through the Research Grant RGP-026.References
- T. P. McCarty and P. G. Pappas, Invasive Candidiasis, Infect. Dis. Clin. N. Am., 2016, 30(1), 103–124 CrossRef PubMed.
- M. Bassetti, D. R. Giacobbe, A. Vena, C. Trucchi, F. Ansaldi and M. Antonelli, et al., Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: results of the EUCANDICU project, Crit. Care, 2019, 23(1), 219 CrossRef PubMed.
- E. Ghrenassia, D. Mokart, J. Mayaux, A. Demoule, I. Rezine and L. Kerhuel, et al., Candidemia in critically ill immunocompromised patients: report of a retrospective multicenter cohort study, Ann. Intensive Care, 2019, 9(1), 62 CrossRef PubMed.
- Z. Xiao, Q. Wang, F. Zhu and Y. An, Epidemiology, species distribution, antifungal susceptibility and mortality risk factors of candidemia among critically ill patients: a retrospective study from 2011 to 2017 in a teaching hospital in China, Antimicrob. Resist. Infect. Contr., 2019, 8, 89 CrossRef PubMed.
- J. McKenney, S. Bauman, B. Neary, R. Detels, A. French and J. Margolick, et al., Prevalence, correlates, and outcomes of cryptococcal antigen positivity among patients with AIDS, United States, 1986-2012, Clin. Infect. Dis., 2015, 60(6), 959–965 CrossRef PubMed.
- S. G. Whaley, E. L. Berkow, J. M. Rybak, A. T. Nishimoto, K. S. Barker and P. D. Rogers, Azole Antifungal Resistance in, Front. Microbiol., 2016, 7, 2173 Search PubMed.
- F. Bongomin, R. O. Oladele, S. Gago, C. B. Moore and M. D. Richardson, A systematic review of fluconazole resistance in clinical isolates of Cryptococcus species, Mycoses, 2018, 61(5), 290–297 CrossRef CAS PubMed.
- J. R. Perfect, W. E. Dismukes, F. Dromer, D. L. Goldman, J. R. Graybill and R. J. Hamill, et al., Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america, Clin. Infect. Dis., 2010, 50(3), 291–322 CrossRef PubMed.
- D. W. Denning, Echinocandins: a new class of antifungal, J. Antimicrob. Chemother., 2002, 49(6), 889–891 CrossRef CAS PubMed.
- T. Roemer and D. J. Krysan, Antifungal drug development: challenges, unmet clinical needs, and new approaches, Cold Spring Harbor Perspect. Med., 2014, 4(5), 1–14 Search PubMed.
- B. de Pauw, Is there a need for new antifungal agents?, Clin. Microbiol. Infect., 2000, 6(2), 23–28 CrossRef PubMed.
- D. J. Krysan, The unmet clinical need of novel antifungal drugs, Virulence, 2017, 8(2), 135–137 CrossRef PubMed.
- N. Arumagam, A. I. Almansour, R. Suresh Kumar, S. Perumal, H. A. Ghabbour and H.-K. Fun, A 1,3-dipolar cycloaddition-annulation protocol for the expedient regio-, stereo- and product-selective construction of novel hybrid heterocycles comprising seven rings and seven contiguous stereocentres, Tetrahedron Lett., 2013, 2515–2519 CrossRef.
- Crystallographic data (excluding structure factors) for heptacyclic compound 6a in this letter have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 917844.
- S. Thangamani, M. Maland, H. Mohammad, P. E. Pascuzzi, L. Avramova and C. M. Koehler, et al., Repurposing Approach Identifies Auranofin with Broad Spectrum Antifungal Activity That Targets Mia40-Erv1 Pathway, Front. Cell. Infect. Microbiol., 2017, 7, 4 Search PubMed.
- C. A. Kumamoto and M. D. Vinces, Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence, Cell. Microbiol., 2005, 7(11), 1546–1554 CrossRef CAS PubMed.
- R. A. Calderone and W. A. Fonzi, Virulence factors of Candida albicans, Trends Microbiol., 2001, 9(7), 327–335 CrossRef CAS PubMed.
- R. Pukkila-Worley, A. Y. Peleg, E. Tampakakis and E. Mylonakis, Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model, Eukaryotic Cell, 2009, 8(11), 1750–1758 CrossRef CAS PubMed.
- J. Guinan, S. Wang, T. R. Hazbun, H. Yadav and S. Thangamani, Antibiotic-induced decreases in the levels of microbial-derived short-chain fatty acids correlate with increased gastrointestinal colonization of Candida albicans, Sci. Rep., 2019, 9(1), 8872 CrossRef PubMed.
- J. Guinan, P. Villa and S. Thangamani, Secondary bile acids inhibit Candida albicans growth and morphogenesis, Pathog. Dis., 2018, 76(3), 1–8 Search PubMed.
- L. Mathé and P. Van Dijck, Recent insights into Candida albicans biofilm resistance mechanisms, Curr. Genet., 2013, 59(4), 251–264 CrossRef PubMed.
- G. Ramage, S. Bachmann, T. F. Patterson, B. L. Wickes and J. L. Lopez-Ribot, Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms, J. Antimicrob. Chemother., 2002, 49(6), 973–980 CrossRef CAS PubMed.
- C. A. Muzny and J. R. Schwebke, Biofilms: An Underappreciated Mechanism of Treatment Failure and Recurrence in Vaginal Infections, Clin. Infect. Dis., 2015, 61(4), 601–606 CrossRef CAS PubMed.
- J. V. Desai, A. P. Mitchell and D. R. Andes, Fungal biofilms, drug resistance, and recurrent infection, Cold Spring Harbor Perspect. Med., 2014, 4(10), 1–18 CAS.
- M. A. Ghannoum and L. B. Rice, Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance, Clin. Microbiol. Rev., 1999, 12(4), 501–517 CrossRef CAS PubMed.
- S. Thangamani, H. E. Eldesouky, H. Mohammad, P. E. Pascuzzi, L. Avramova and T. R. Hazbun, et al., Ebselen exerts antifungal activity by regulating glutathione (GSH) and reactive oxygen species (ROS) production in fungal cells, Biochim. Biophys. Acta, Gen. Subj., 2017, 1861(1), 3002–3010 CrossRef CAS PubMed.
- P. Villa, N. Arumugam, A. I. Almansour, R. Suresh Kumar, S. M. Mahalingam and K. Maruoka, et al., Benzimidazole tethered pyrrolo[3,4-b]quinoline with broad-spectrum activity against fungal pathogens, Bioorg. Med. Chem. Lett., 2019, 29(5), 729–733 CrossRef CAS PubMed.
- C. G. Pierce, P. Uppuluri, A. R. Tristan, F. L. Wormley, E. Mowat and G. Ramage, et al., A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing, Nat. Protoc., 2008, 3(9), 1494–1500 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra05777c |
| ‡ Co-first authors. |
| This journal is © The Royal Society of Chemistry 2019 |