Open Access Article
Open Access ArticleSynthesis of a 1,2-cis-indoxyl galactoside as a chromogenic glycosidase substrate†
Sakuto Nagataa,
Hirotaka Tomidaa,
Haruka Iwai-Hiroseab,
Hide-Nori Tanakabc,
Hiromune Ando bc,
Akihiro Imamura
bc,
Akihiro Imamura *ab and
Hideharu Ishida*abc
*ab and
Hideharu Ishida*abc
aDepartment of Applied Bioorganic Chemistry, Gifu University, 1-1 Yanagido, Gifu 501-1193, Japan. E-mail: aimamura@gifu-u.ac.jp
bThe United Graduate School of Agricultural Science, Gifu University, 1-1 Yanagido, Gifu 501-1193, Japan
cCenter for Highly Advanced Integration of Nano and Life Sciences (G-CHAIN), Gifu University, 1-1 Yanagido, Gifu 501-1193, Japan
First published on 9th September 2019
Abstract
A synthetically challenging 1,2-cis-indoxyl galactoside, X-α-galactoside, was first prepared in this study using a cyclic ketone indoxyl acceptor and a glycosyl trichloroacetimidate donor to produce an enol glycoside and a 4,6-O-di-tert-butylsilylene-protected galactosyl donor to complete the synthesis. The target compound shows enzyme activity in the presence of α-galactosidase.
Introduction
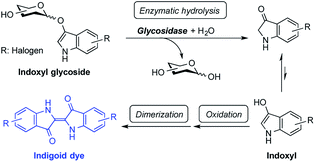
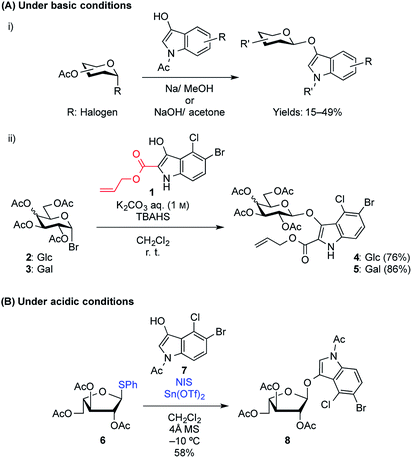
Indoxyl glycosides are chromogenic substrates that are useful in detecting glycosidase activity. These substrates have been widely used for this purpose in histochemical, biochemical, and bacteriological studies.1 In this process, the hydrolysis of an indoxyl substrate by glycosidase releases a sugar moiety and a free indoxyl, the liberated molecule of which then undergoes oxidation in the air followed by dimerization to generate an indigoid dye (Fig. 1). Indigoid dyes are insoluble in water, making the diffusion of the slightest amount of dye from enzymatic hydrolysis sites possible to determine the precise location of glycosidases.2 The color and physical properties (e.g., solubility and affinity for protein) of indigoid dyes depend on the nature of the halogen substituents located on the indole ring of the compound. For example, 5-bromo-4-chloro (greenish blue)-, 5-bromo-6-chloro (magenta)-, 5-chloro (red)-, and 5-iodo (purple)-substituted substrates are well known, and these different-colored compounds are used for tailored applications.1Many indoxyl glycosides are now commercially available, but halogen-substituted indoxyl glycosides are expensive because they are difficult to prepare. Their synthesis is difficult because of the low nucleophilicity of the indole hydroxy group, which suppresses the formation of the glycoside, in addition to side reactions that occur because of the indoxyl structure, leading to a decrease in the coupling yield. Furthermore, another synthetic challenge that exists for indoxyl glycosides is that they exist in the enol form and can be readily cleaved under normal glycosylation (acidic) conditions to release an aglycone in the keto form. Therefore, to date, reports on the synthesis of enol glycosides under acidic conditions have been extremely limited.3 SN2-like reaction between glycosyl halides and N-acetyl-indoxyl derivatives in the presence of NaOH in acetone or Na in MeOH is a commonly used synthetic approach used to produce indoxyl glycosides, which generally results in very low reaction yields of less than 50% (Fig. 2A-i).4 These basic conditions are required to generate the enolate form from the cyclic ketone version of the free indoxyl, which exists in keto–enol equilibrium in the reaction medium and almost none of the corresponding enol forms in nonpolar solvents.5 Unfortunately, these conditions result in low yields, in addition to the formation of various side products, including indigogenic compounds and acetyl migration and/or elimination products generated from the donor. In 2013, Thiem and coworkers synthesized indoxylic acid allyl ester 1 to improve the reactivity and instability of the indoxyl acceptor by blocking the reactive 2-position.6 In this study, they found that phase transfer glycosylation using 1 suppressed the formation of indigogenic side products, resulting in a drastic increase in the yields by up to 86% (Fig. 2A-ii).7 Recently, Wei et al. found that solid–liquid phase transfer catalysis (S–L PTC) glycosylation between acetobromo sugars and an indoxyl acceptor could be used to synthesize indoxyl glycosides, resulting in moderate glycosylation yields of 46–65%.8 However, the formation of indoxyl glycosides via a standard glycosylation reaction under acidic conditions has scarcely been reported, although there are limited reports on the synthesis of X-α-L-arabinofuranosides using the Koenigs–Knorr method9 or thioglycoside activation.10 In the latter, the authors used thiophenyl L-arabinofuranosyl donor 6 and indoxyl 7 in the presence of NIS-Sn(OTf)2 in CH2Cl2 to give 8 in a 58% yield (Fig. 2B). To date, various indoxyl glycosides, including β-Glc,11 β-Gal,11,12 β-GlcNAc,11a β-GlcA,13 α-L-Araf,9,10 β-LacNAc,7b and β-xylobioside,14 have been chemically synthesized, but they are all 1,2-trans-oriented glycosides. To the best of our knowledge, there have been no reports on the synthesis of 1,2-cis-indoxyl glycosides, which is due not only to the difficulty in preparing these compounds, but also to the lack of reliable methods available to control the anomeric stereoselectivity in 1,2-cis-glycoside formation. However, a large number of 1,2-cis-glycosides (e.g., α-Gal, α-Glc, and α-Fuc) and the corresponding glycosidases can be found in nature, where they play pivotal roles in various biological processes.15 Thus, developing a synthetic route for 1,2-cis-indoxyl glycosides is, therefore, of great importance.
Results and discussion
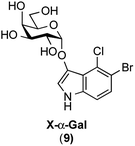
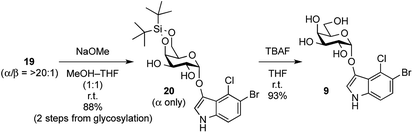
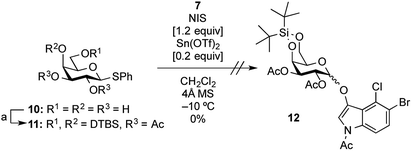
In this study, for the first time, the synthesis of X-α(1,2-cis-oriented)-galactoside (9, shown in Fig. 3, where X is the common abbreviation for 5-bromo-4-chloro-3-indoxyl) is reported. The first attempt to synthesize the target X-α-galactoside was made using thiophenyl 4,6-O-di-tert-butylsilylene- (DTBS-) protected galactosyl donor 11, readily prepared from known thiophenyl galactoside 10,16 and known indoxyl acceptor 7 (ref. 11b) to give 12. Based on our previous success in the synthesis of a large number of α-galactosides using DTBS-protected Gal donors, it was thought that this route would lead to a promising result.17 Furthermore, the abovementioned report on the synthesis of X-Araf using a thiophenyl arabinofuranosyl donor mediated by an NIS-Sn(OTf)2 system warranted further examination. However, the glycosylation reaction performed under the reported conditions did not proceed at all, and only the oxidative dimerization of 7 was observed, leading to a 91% recovery of the donor (Scheme 1). In this attempt, although 1.2 equiv. of the acceptor was initially used according to the preparation described in the literature,10 all of the acceptor was consumed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h, resulting in the precipitation of a blue dye. The use of 3.0 equiv. of the acceptor under the same conditions did not change the outcome of the reaction. These results suggest that the use of a thiophenyl pyranosyl donor was not feasible for the formation of X-glycosides, which might be due to the pyranoside donor being less reactive than its furanoside counterpart. In addition, in order to prevent the oxidative dimerization of the indoxyl acceptors, the use of thioglycosides, which usually have to be oxidatively activated, might be unsuitable in this study.
h, resulting in the precipitation of a blue dye. The use of 3.0 equiv. of the acceptor under the same conditions did not change the outcome of the reaction. These results suggest that the use of a thiophenyl pyranosyl donor was not feasible for the formation of X-glycosides, which might be due to the pyranoside donor being less reactive than its furanoside counterpart. In addition, in order to prevent the oxidative dimerization of the indoxyl acceptors, the use of thioglycosides, which usually have to be oxidatively activated, might be unsuitable in this study.
 | ||
| Scheme 1 Glycosylation of indoxyl 7 using DTBS-protected Gal thioglycoside donor 11. Reagents and conditions: (a) DTBS(OTf)2, Py, r.t.; Ac2O, r.t., 97% (2 steps in one-pot fashion). | ||
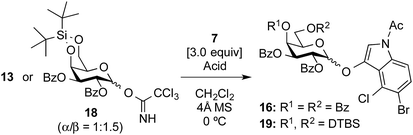
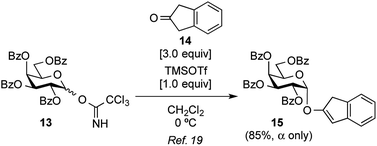
Attention was then turned to the acid activation of a trichloroacetimidate donor.18 Ding and coworkers reported the efficient direct α-glycosylation of a cyclic ketone using a glycosyl trichloroacetimidate donor, in which the coupling of 2,3,4,6-tetra-O-benzoyl-galactosyl trichloroacetimidate donor 13 (ref. 19) and cyclic ketone 14 in the presence of TMSOTf (1.0 equiv.) in CH2Cl2 at 0 °C provided the corresponding α-enol glycoside 15 as the sole product in an 85% yield (Scheme 2).20 The high α-stereo-selectivity observed was a result of the acid-catalyzed in situ anomerization of the kinetically formed β-isomer to the thermodynamic α-isomer. These glycosylation conditions were, thus, used in the present study (Table 1, entry 1). As a result, the reaction between 13 and 7 quantitatively proceeded to give the desired X-galactoside 16 contaminated with the dimerized dye product of 7, which was hard to remove by column chromatography. This result led to the first preparation of X-pyranosides under acidic conditions. However, the stereoselectivity in this reaction was very poor, with an α/β ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, favoring the unwanted β-isomer. The conversion of the β-isomer into the α-isomer was then attempted via in situ anomerization, in which the isolated β-isomer and 7 were stirred together under the above glycosylation conditions for 20
1.5, favoring the unwanted β-isomer. The conversion of the β-isomer into the α-isomer was then attempted via in situ anomerization, in which the isolated β-isomer and 7 were stirred together under the above glycosylation conditions for 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h. However, the formation of the α-isomer was not observed, with the reaction only resulting in the degradation of the β-isomer to the corresponding lactol and 7. Despite its poor stereoselectivity, the successful formation of an X-glycoside using a trichloroacetimidate donor prompted us to use DTBS-protected galactosyl donors again to improve the α-selectivity. Using 4,6-O-DTBS-protected galactosyl trichloroacetimidate donor 18, readily prepared from 10 via the corresponding thiophenyl glycoside 17, the glycosylation of 7 in the presence of TMSOTf (1.0 equiv.) was investigated (Table 1, entry 2). As anticipated, the reaction afforded the desired X-galactoside 19 in an almost quantitative yield with excellent α-selectivity (α/β ratio = >20
h. However, the formation of the α-isomer was not observed, with the reaction only resulting in the degradation of the β-isomer to the corresponding lactol and 7. Despite its poor stereoselectivity, the successful formation of an X-glycoside using a trichloroacetimidate donor prompted us to use DTBS-protected galactosyl donors again to improve the α-selectivity. Using 4,6-O-DTBS-protected galactosyl trichloroacetimidate donor 18, readily prepared from 10 via the corresponding thiophenyl glycoside 17, the glycosylation of 7 in the presence of TMSOTf (1.0 equiv.) was investigated (Table 1, entry 2). As anticipated, the reaction afforded the desired X-galactoside 19 in an almost quantitative yield with excellent α-selectivity (α/β ratio = >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The installation of the DTBS group to endow the product with low polarity made the removal of the dye from the reaction mixture easy, but separation of the stereoisomers was not possible at this stage. However, the structure of 19α as the major product was confirmed by 1H NMR spectrum of the crude mixture, where the J1,2 value of 3.5 Hz for H-1 of the Gal residue (δ 5.94, doublet) and the presence of H-2 of the indole ring (δ 7.16, singlet) indicated the α-configuration of X-galactoside. In entry 3 of Table 1, it can be seen that reducing the amount of TMSOTf to 0.1 equiv. led to a sluggish reaction as well as a decrease in the coupling yield to 84%. Next, the acid catalyst was changed to triflic acid (Table 1, entry 4), but the outcome of the glycosylation was almost the same as that using TMSOTf. Subsequently, tin(II) triflate, which was used in the abovementioned thioglycoside activation, gave a result similar to that of TMSOTf (Table 1, entry 5). The results of both experiments, shown in entries 4 and 5 in Table 1, suggest that the nature of the acid species is not important in this reaction. Consequently, the use of a trichloroacetimidate donor was found to be of great importance in the synthesis of indoxyl glycosides.
1). The installation of the DTBS group to endow the product with low polarity made the removal of the dye from the reaction mixture easy, but separation of the stereoisomers was not possible at this stage. However, the structure of 19α as the major product was confirmed by 1H NMR spectrum of the crude mixture, where the J1,2 value of 3.5 Hz for H-1 of the Gal residue (δ 5.94, doublet) and the presence of H-2 of the indole ring (δ 7.16, singlet) indicated the α-configuration of X-galactoside. In entry 3 of Table 1, it can be seen that reducing the amount of TMSOTf to 0.1 equiv. led to a sluggish reaction as well as a decrease in the coupling yield to 84%. Next, the acid catalyst was changed to triflic acid (Table 1, entry 4), but the outcome of the glycosylation was almost the same as that using TMSOTf. Subsequently, tin(II) triflate, which was used in the abovementioned thioglycoside activation, gave a result similar to that of TMSOTf (Table 1, entry 5). The results of both experiments, shown in entries 4 and 5 in Table 1, suggest that the nature of the acid species is not important in this reaction. Consequently, the use of a trichloroacetimidate donor was found to be of great importance in the synthesis of indoxyl glycosides.
 | ||
| Scheme 2 The successful conditions for the stereoselective glycosylation of cyclic ketone derivative 14 using Gal trichloroacetimidate donor 13 reported by Liu et al. | ||
| Entry | Donora | Acid (equiv.) | Time [h] | Prod. | % Yieldb | α/β ratioc |
|---|---|---|---|---|---|---|
| a One equivalent of the donor was used.b Isolated yield.c Determined based on 1H NMR spectra of the isomer mixtures.d Contaminated with the dimerization product of 7 (dye).e The trichloroacetimidate donor 18 was prepared from 10 over four steps via the corresponding thiophenyl glycoside 17. | ||||||
| 1 | 13 | TMSOTf (1.0) | 0.5 | 16 | >100d | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
| 2 | 18e | TMSOTf (1.0) | 0.5 | 19 | 96 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 18 | TMSOTf (0.1 + 0.1 + 0.1) | 2 | 19 | 84 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 18 | TfOH (1.0) | 0.5 | 19 | 91 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 18 | Sn(OTf)2 (1.0) | 0.5 | 19 | 90 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
In order to complete the synthesis of X-α-galactoside, its protecting groups, including acyl groups and the DTBS group, were removed (Scheme 3). Before acyl group deprotection, it was thought that the 1,2-cis-enol glycosides would be alkali-sensitive because the 1,2-cis-configuration could lead to β-elimination at the anomeric carbon, producing the enolate form of the indoxyl and the 1,2-ene derivative of the sugar. Fortunately, these fears proved to be unfounded and the acyl groups in 19 (a mixture of α- and β-isomers) were cleaved under Zemplén conditions, affording 20 as a single isomer in good yield without any degradation of the enol glycoside. Subsequently, clean removal of the DTBS group by TBAF in THF furnished the target X-α-Gal 9 in excellent yield. During the deprotection process, it should be noted that the protected X-glycoside derivatives 19 and 20 were not very stable during even short-term storage in the freezer and underwent degradation to the corresponding lactol and indigoid dye. Therefore, after the glycosylation of the indoxyl, global deprotection should be performed as soon as possible.
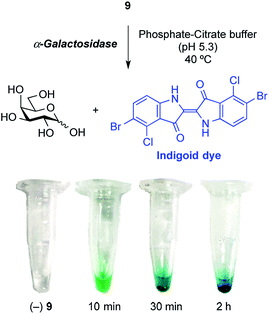
Finally, enzymatic hydrolysis of the synthesized X-α-Gal 9 using an α-galactosidase (from green coffee bean) was carried out (Fig. 4). The glycosidase activity in a phosphate-citrate buffer (pH 5.3) at 40 °C was monitored in Eppendorf tubes for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min, 30
min, 30 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min, and 2
min, and 2 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h. A greenish-blue color evolved over 10
h. A greenish-blue color evolved over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min, after which the precipitation of dye particles was observed after 30
min, after which the precipitation of dye particles was observed after 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min and 2
min and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h. In the tube, the substrate was hydrolyzed by glycosidase, and the liberated indoxyl was gradually oxidized and then dimerized to form a water-insoluble indigoid dye, providing a clear indication of enzyme activity. This result demonstrated that the synthesized X-α-Gal was applicable to the detection of α-galactosidase activity.
h. In the tube, the substrate was hydrolyzed by glycosidase, and the liberated indoxyl was gradually oxidized and then dimerized to form a water-insoluble indigoid dye, providing a clear indication of enzyme activity. This result demonstrated that the synthesized X-α-Gal was applicable to the detection of α-galactosidase activity.
Conclusions
This was the first successful preparation of a synthetically challenging 1,2-cis-oriented indoxyl galactoside, the reaction steps of which proceeded in a facile and high-yielding manner. The formation of the α-galactosidic linkage was achieved using a 4,6-O-DTBS-protected galactosyl donor with excellent stereoselectivity. Importantly, the use of a trichloroacetimidoyl leaving group was found to be effective in the formation of an enol glycoside. Upon exposure to α-galactosidase, the solution of the synthesized X-α-Gal in a buffer underwent a color change as the substrate was hydrolyzed by the enzyme, oxidized by the air, and then dimerized, showing that the target X-α-Gal exhibits enzyme activity. The successful synthesis of a 1,2-cis-indoxyl galactoside in this study can be expanded upon to allow access to a variety of indoxyl glycosides. We are currently underway with the efficient synthesis of other 1,2-cis-indoxyl glycosides including X-α-D-glucoside and X-α-L-fucoside. The results will be reported in the near future.Experimental
Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc). The reaction mixture was poured on crushed ice in a beaker and stirred quickly by glass rod. The precipitate formed was filtered off and washed with generous quantities of 1% solution of sodium acetate followed by water. The resulting residue was exposed to high vacuum, giving 7 (856 mg, 98%) as yellow solid. Prepared 7 should be stored in a temperature of less than −60 °C until use. 1H NMR (500 MHz, CDCl3): δ 8.42 (d, 1H, 3Jortho = 8.5 Hz, Ar), 7.82 (d, 1H, Ar), 4.36 (s, 2H, CH2), 2.33 (s, 3H, Ac); 13C NMR (125 MHz, CDCl3): δ 190.7, 168.1, 154.1, 140.9, 131.8, 122.8, 119.0, 117.8, 56.4, 24.3. HRMS (ESI-TOF): m/z calcd for C8H5BrClNO: 267.9135 [M + Na]+; found: 267.9135.
1 n-hexane/EtOAc). The reaction mixture was poured on crushed ice in a beaker and stirred quickly by glass rod. The precipitate formed was filtered off and washed with generous quantities of 1% solution of sodium acetate followed by water. The resulting residue was exposed to high vacuum, giving 7 (856 mg, 98%) as yellow solid. Prepared 7 should be stored in a temperature of less than −60 °C until use. 1H NMR (500 MHz, CDCl3): δ 8.42 (d, 1H, 3Jortho = 8.5 Hz, Ar), 7.82 (d, 1H, Ar), 4.36 (s, 2H, CH2), 2.33 (s, 3H, Ac); 13C NMR (125 MHz, CDCl3): δ 190.7, 168.1, 154.1, 140.9, 131.8, 122.8, 119.0, 117.8, 56.4, 24.3. HRMS (ESI-TOF): m/z calcd for C8H5BrClNO: 267.9135 [M + Na]+; found: 267.9135.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc). Subsequently, acetic anhydride (3.45 mL, 36.7 mmol) was added to the reaction mixture at 0 °C. After stirring for 1 h at rt, the completion of the reaction was confirmed by TLC (5
1 n-hexane/EtOAc). Subsequently, acetic anhydride (3.45 mL, 36.7 mmol) was added to the reaction mixture at 0 °C. After stirring for 1 h at rt, the completion of the reaction was confirmed by TLC (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc). The reaction was quenched by the addition of dry methanol at 0 °C. Solvents were removed by co-evaporation with toluene, and then the residue was diluted with CHCl3, washed with 2 M HCl, H2O, satd aq NaHCO3, and brine. The organic layer was subsequently dried over Na2SO4, filtered, and concentrated. The resulting residue was purified by silica gel column chromatography (5
1 n-hexane/EtOAc). The reaction was quenched by the addition of dry methanol at 0 °C. Solvents were removed by co-evaporation with toluene, and then the residue was diluted with CHCl3, washed with 2 M HCl, H2O, satd aq NaHCO3, and brine. The organic layer was subsequently dried over Na2SO4, filtered, and concentrated. The resulting residue was purified by silica gel column chromatography (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) to give 11 (1.76 g, 97%) as white foamy material. [α]D +33.8 (c 1.0 in CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.50–7.25 (m, 5H, Ph), 5.47 (t, 1H, J1,2 = J2,3 = 10.0 Hz, H-2), 4.80 (dd, 1H, J3,4 = 3.0 Hz, H-3), 4.73 (d, 1H, H-1), 4.69 (d, 1H, H-4), 4.25 (dd, 1H, J5,6a = 1.5 Hz, Jgem = 13.0 Hz, H-6a), 4.22 (dd, 1H, J5,6b = 2.0 Hz, H-6a), 3.49 (s, 1H, H-5), 2.10 (s, 3H, Ac), 2.08 (s, 3H, Ac), 1.11 (s, 9H, tBu), 1.01 (s, 9H, tBu); 13C NMR (125 MHz, CDCl3): δ 170.6, 169.5, 134.0, 132.0, 128.9, 127.7, 87.2, 77.3, 74.9, 74.8, 70.1, 67.4, 67.0, 27.5, 27.5, 23.2, 20.9, 20.8, 20.6. HRMS (ESI-TOF): m/z calcd for C24H36O7SSi: 519.1843 [M + Na]+; found: 519.1844.
1 n-hexane/EtOAc) to give 11 (1.76 g, 97%) as white foamy material. [α]D +33.8 (c 1.0 in CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.50–7.25 (m, 5H, Ph), 5.47 (t, 1H, J1,2 = J2,3 = 10.0 Hz, H-2), 4.80 (dd, 1H, J3,4 = 3.0 Hz, H-3), 4.73 (d, 1H, H-1), 4.69 (d, 1H, H-4), 4.25 (dd, 1H, J5,6a = 1.5 Hz, Jgem = 13.0 Hz, H-6a), 4.22 (dd, 1H, J5,6b = 2.0 Hz, H-6a), 3.49 (s, 1H, H-5), 2.10 (s, 3H, Ac), 2.08 (s, 3H, Ac), 1.11 (s, 9H, tBu), 1.01 (s, 9H, tBu); 13C NMR (125 MHz, CDCl3): δ 170.6, 169.5, 134.0, 132.0, 128.9, 127.7, 87.2, 77.3, 74.9, 74.8, 70.1, 67.4, 67.0, 27.5, 27.5, 23.2, 20.9, 20.8, 20.6. HRMS (ESI-TOF): m/z calcd for C24H36O7SSi: 519.1843 [M + Na]+; found: 519.1844.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc). The reaction was quenched by the addition of satd aq NaHCO3 at 0 °C. The precipitate was filtered through Celite pad and the pad was washed with EtOAc. The combined filtrate and washings were washed with satd aq NaHCO3, 2 M HCl, and brine. The organic layer was subsequently dried over Na2SO4, filtered, and concentrated. The resulting residue was purified by silica gel column chromatography (7
1 n-hexane/EtOAc). The reaction was quenched by the addition of satd aq NaHCO3 at 0 °C. The precipitate was filtered through Celite pad and the pad was washed with EtOAc. The combined filtrate and washings were washed with satd aq NaHCO3, 2 M HCl, and brine. The organic layer was subsequently dried over Na2SO4, filtered, and concentrated. The resulting residue was purified by silica gel column chromatography (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) to give 16 (a mixture of the stereoisomers, α/β = 1/1.5), 7, and the corresponding dye (426 mg as the mixture). 16β (contaminated with some compounds): 1H NMR (500 MHz, CDCl3): δ 8.23–7.27 (m, 22H, Ar), 7.25 (s, 1H,
1 n-hexane/EtOAc) to give 16 (a mixture of the stereoisomers, α/β = 1/1.5), 7, and the corresponding dye (426 mg as the mixture). 16β (contaminated with some compounds): 1H NMR (500 MHz, CDCl3): δ 8.23–7.27 (m, 22H, Ar), 7.25 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–N), 6.20 (dd, 1H, J1,2 = 7.5 Hz, J2,3 = 10.0 Hz, H-2), 6.08 (d, 1H, J3,4 = 3.5 Hz, H-4), 5.72 (dd, 1H, H-3), 5.36 (d, 1H, H-1), 4.76 (dd, 1H, J5,6a = 7.0 Hz, Jgem = 12.0 Hz, H-6a), 4.58 (dd, 1H, J5,6b = 4.5 Hz, H-6b), 4.54 (m, 1H, H-5), 2.24 (s, 3H, Ac); 13C NMR (125 MHz, CDCl3): δ 168.3, 166.2, 165.6, 165.6, 165.2, 139.7, 133.8, 133.6, 133.5, 133.4, 133.4, 130.5, 130.1, 129.8, 129.8, 129.8, 129.7, 129.3, 129.1, 128.8, 128.6, 128.6, 128.5, 128.4, 125.3, 122.5, 118.4, 116.1, 112.8, 101.2, 72.4, 71.4, 69.1, 68.2, 63.0, 23.4. HRMS (ESI-TOF): m/z calcd for C44H33BrClNO11: 888.0818 [M + Na]+; found: 888.0814.
CH–N), 6.20 (dd, 1H, J1,2 = 7.5 Hz, J2,3 = 10.0 Hz, H-2), 6.08 (d, 1H, J3,4 = 3.5 Hz, H-4), 5.72 (dd, 1H, H-3), 5.36 (d, 1H, H-1), 4.76 (dd, 1H, J5,6a = 7.0 Hz, Jgem = 12.0 Hz, H-6a), 4.58 (dd, 1H, J5,6b = 4.5 Hz, H-6b), 4.54 (m, 1H, H-5), 2.24 (s, 3H, Ac); 13C NMR (125 MHz, CDCl3): δ 168.3, 166.2, 165.6, 165.6, 165.2, 139.7, 133.8, 133.6, 133.5, 133.4, 133.4, 130.5, 130.1, 129.8, 129.8, 129.8, 129.7, 129.3, 129.1, 128.8, 128.6, 128.6, 128.5, 128.4, 125.3, 122.5, 118.4, 116.1, 112.8, 101.2, 72.4, 71.4, 69.1, 68.2, 63.0, 23.4. HRMS (ESI-TOF): m/z calcd for C44H33BrClNO11: 888.0818 [M + Na]+; found: 888.0814.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc). Subsequently, benzoyl chloride (3.41 mL, 29.4 mmol) and a catalytic amount of N,N-dimethyl-4-aminopyridine were added to the reaction mixture at 0 °C. After stirring for 1 h at rt, the completion of the reaction was confirmed by TLC (5
1 n-hexane/EtOAc). Subsequently, benzoyl chloride (3.41 mL, 29.4 mmol) and a catalytic amount of N,N-dimethyl-4-aminopyridine were added to the reaction mixture at 0 °C. After stirring for 1 h at rt, the completion of the reaction was confirmed by TLC (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc). The reaction was quenched by the addition of dry methanol at 0 °C. Solvents were removed by co-evaporation with toluene, and then the residue was diluted with CHCl3, washed with 2 M HCl, H2O, satd aq NaHCO3, and brine. The organic layer was subsequently dried over Na2SO4, filtered, and concentrated. The resulting residue was purified by silica gel column chromatography (8
1 n-hexane/EtOAc). The reaction was quenched by the addition of dry methanol at 0 °C. Solvents were removed by co-evaporation with toluene, and then the residue was diluted with CHCl3, washed with 2 M HCl, H2O, satd aq NaHCO3, and brine. The organic layer was subsequently dried over Na2SO4, filtered, and concentrated. The resulting residue was purified by silica gel column chromatography (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) to give 17 (2.00 g, 88%) as white foamy material. [α]D +96.5 (c 1.0 in CHCl3); 1H NMR (500 MHz, CDCl3): δ 8.14–7.21 (m, 15H, Ph), 5.93 (t, 1H, J1,2 = J2,3 = 10.0 Hz, H-2), 5.22 (dd, 1H, J3,4 = 3.0 Hz, H-3), 4.95 (d, 1H, H-1), 4.89 (d, 1H, H-4), 4.34 (d, 1H, Jgem = 12.0 Hz, H-6a), 4.30 (dd, 1H, J5,6b = 2.0 Hz, H-6b), 3.66 (s, 1H, H-5), 1.16 (s, 9H, tBu), 0.96 (s, 9H, tBu); 13C NMR (125 MHz, CDCl3): δ 166.1, 165.5, 133.8, 133.3, 133.2, 132.5, 129.8, 129.8, 129.6, 129.4, 128.9, 128.4, 128.4, 127.8, 87.5, 75.5, 75.0, 70.5, 68.1, 67.1, 27.5, 27.5, 23.3, 20.7. HRMS (ESI-TOF): m/z calcd for C34H40O7SSi: 643.2156 [M + Na]+; found: 643.2155.
1 n-hexane/EtOAc) to give 17 (2.00 g, 88%) as white foamy material. [α]D +96.5 (c 1.0 in CHCl3); 1H NMR (500 MHz, CDCl3): δ 8.14–7.21 (m, 15H, Ph), 5.93 (t, 1H, J1,2 = J2,3 = 10.0 Hz, H-2), 5.22 (dd, 1H, J3,4 = 3.0 Hz, H-3), 4.95 (d, 1H, H-1), 4.89 (d, 1H, H-4), 4.34 (d, 1H, Jgem = 12.0 Hz, H-6a), 4.30 (dd, 1H, J5,6b = 2.0 Hz, H-6b), 3.66 (s, 1H, H-5), 1.16 (s, 9H, tBu), 0.96 (s, 9H, tBu); 13C NMR (125 MHz, CDCl3): δ 166.1, 165.5, 133.8, 133.3, 133.2, 132.5, 129.8, 129.8, 129.6, 129.4, 128.9, 128.4, 128.4, 127.8, 87.5, 75.5, 75.0, 70.5, 68.1, 67.1, 27.5, 27.5, 23.3, 20.7. HRMS (ESI-TOF): m/z calcd for C34H40O7SSi: 643.2156 [M + Na]+; found: 643.2155.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetone–H2O) was added N-bromosuccinimide (1.43 g, 8.05 mmol) at 0 °C. After stirring for 15 min at rt, the completion of the reaction was confirmed by TLC (2
1 acetone–H2O) was added N-bromosuccinimide (1.43 g, 8.05 mmol) at 0 °C. After stirring for 15 min at rt, the completion of the reaction was confirmed by TLC (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc). The reaction mixture was diluted with EtOAc, washed with satd aq Na2SO3 and brine. The organic layer was subsequently dried over Na2SO4, filtered, and concentrated. The resulting residue was purified by silica gel column chromatography (7
1 n-hexane/EtOAc). The reaction mixture was diluted with EtOAc, washed with satd aq Na2SO3 and brine. The organic layer was subsequently dried over Na2SO4, filtered, and concentrated. The resulting residue was purified by silica gel column chromatography (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) to give the lactol compound (746 mg, 88%). The lactol (600 mg, 1.14 mmol) was then dissolved in CH2Cl2 (11.4 mL). To the solution were added CCl3CN (1.14 mL, 11.4 mmol) and 1,8-diazabicyclo[5.4.0]undec-7-ene (136 μL, 0.912 mmol) at 0 °C. After stirring for 6 h at 0 °C as the reaction was monitored by TLC (1
1 n-hexane/EtOAc) to give the lactol compound (746 mg, 88%). The lactol (600 mg, 1.14 mmol) was then dissolved in CH2Cl2 (11.4 mL). To the solution were added CCl3CN (1.14 mL, 11.4 mmol) and 1,8-diazabicyclo[5.4.0]undec-7-ene (136 μL, 0.912 mmol) at 0 °C. After stirring for 6 h at 0 °C as the reaction was monitored by TLC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc), the reaction mixture was concentrated. The resulting residue was purified by silica gel column chromatography (15
1 n-hexane/EtOAc), the reaction mixture was concentrated. The resulting residue was purified by silica gel column chromatography (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 2
1 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) to give 18 (711 mg, 93%, α/β = 1/1.5) as white foamy material. 18α: 1H NMR (500 MHz, CDCl3): δ 8.56 (s, 1H, NH), 8.03–7.35 (m, 10H, Ph), 6.79 (d, 1H, J1,2 = 3.5 Hz, H-1), 6.04 (dd, 1H, J2,3 = 10.5 Hz, H-2), 5.69 (dd, 1H, J3,4 = 3.0 Hz, H-3), 5.01 (d, 1H, H-4), 4.34 (dd, 1H, J5,6a = 2.0 Hz, Jgem = 12.5 Hz, H-6a), 4.30 (dd, 1H, J5,6b = 1.5 Hz, H-6b), 4.17 (s, 1H, H-5), 1.15 (s, 9H, tBu), 0.98 (s, 9H, tBu); 13C NMR (125 MHz, CDCl3): δ 166.2, 165.7, 160.8, 133.4, 133.3, 129.9, 129.7, 129.6, 129.0, 128.4, 124.8, 94.6, 91.0, 70.9, 70.7, 69.8, 67.3, 66.6, 27.5, 27.2, 23.3, 20.8. HRMS (ESI-TOF): m/z calcd for C30H36Cl3NO7Si: 694.1168 [M + Na]+; found: 694.1172.
1 n-hexane/EtOAc) to give 18 (711 mg, 93%, α/β = 1/1.5) as white foamy material. 18α: 1H NMR (500 MHz, CDCl3): δ 8.56 (s, 1H, NH), 8.03–7.35 (m, 10H, Ph), 6.79 (d, 1H, J1,2 = 3.5 Hz, H-1), 6.04 (dd, 1H, J2,3 = 10.5 Hz, H-2), 5.69 (dd, 1H, J3,4 = 3.0 Hz, H-3), 5.01 (d, 1H, H-4), 4.34 (dd, 1H, J5,6a = 2.0 Hz, Jgem = 12.5 Hz, H-6a), 4.30 (dd, 1H, J5,6b = 1.5 Hz, H-6b), 4.17 (s, 1H, H-5), 1.15 (s, 9H, tBu), 0.98 (s, 9H, tBu); 13C NMR (125 MHz, CDCl3): δ 166.2, 165.7, 160.8, 133.4, 133.3, 129.9, 129.7, 129.6, 129.0, 128.4, 124.8, 94.6, 91.0, 70.9, 70.7, 69.8, 67.3, 66.6, 27.5, 27.2, 23.3, 20.8. HRMS (ESI-TOF): m/z calcd for C30H36Cl3NO7Si: 694.1168 [M + Na]+; found: 694.1172.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc). The reaction was quenched by the addition of satd aq NaHCO3 at 0 °C. The precipitate was filtered through Celite pad and the pad was washed with EtOAc. The combined filtrate and washings were washed with satd aq NaHCO3, 2 M HCl, and brine. The organic layer was subsequently dried over Na2SO4, filtered, and concentrated. The resulting residue was purified by silica gel column chromatography (9
1 n-hexane/EtOAc). The reaction was quenched by the addition of satd aq NaHCO3 at 0 °C. The precipitate was filtered through Celite pad and the pad was washed with EtOAc. The combined filtrate and washings were washed with satd aq NaHCO3, 2 M HCl, and brine. The organic layer was subsequently dried over Na2SO4, filtered, and concentrated. The resulting residue was purified by silica gel column chromatography (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) to give 19 (229 mg, 96%, α/β = 20/1) as purple powder. 19α: 1H NMR (500 MHz, CDCl3): δ 8.23 (d, 1H, 3Jortho = 8.5 Hz, Ar), 8.05–7.36 (m, 11H, Ar), 7.16 (s, 1H,
1 n-hexane/EtOAc) to give 19 (229 mg, 96%, α/β = 20/1) as purple powder. 19α: 1H NMR (500 MHz, CDCl3): δ 8.23 (d, 1H, 3Jortho = 8.5 Hz, Ar), 8.05–7.36 (m, 11H, Ar), 7.16 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–N), 5.96 (dd, 1H, J1,2 = 3.5 Hz, J2,3 = 10.5 Hz, H-2), 5.94 (d, 1H, H-1), 5.90 (dd, 1H, J3,4 = 3.0 Hz, H-3), 5.03 (d, 1H, H-4), 4.35 (dd, 1H, J5,6a = 2.0 Hz, Jgem = 13.0 Hz, H-6a), 4.22 (dd, 1H, J5,6b = 1.5 Hz, H-6b), 4.10 (s, 1H, H-5), 2.56 (s, 3H, Ac), 1.19 (s, 9H, tBu), 1.00 (s, 9H, tBu); 13C NMR (125 MHz, CDCl3): δ 168.1, 166.2, 166.0, 140.2, 133.6, 133.5, 133.2, 130.6, 129.9, 129.7, 129.0, 128.5, 128.5, 128.4, 125.4, 122.3, 118.4, 116.2, 107.9, 97.5, 77.2, 70.9, 70.7, 68.3, 68.1, 66.8, 27.6, 27.4, 24.0, 23.3, 20.8. HRMS (ESI-TOF): m/z calcd for C38H41BrClNO9Si: 820.1315 [M + Na]+; found: 820.1315.
CH–N), 5.96 (dd, 1H, J1,2 = 3.5 Hz, J2,3 = 10.5 Hz, H-2), 5.94 (d, 1H, H-1), 5.90 (dd, 1H, J3,4 = 3.0 Hz, H-3), 5.03 (d, 1H, H-4), 4.35 (dd, 1H, J5,6a = 2.0 Hz, Jgem = 13.0 Hz, H-6a), 4.22 (dd, 1H, J5,6b = 1.5 Hz, H-6b), 4.10 (s, 1H, H-5), 2.56 (s, 3H, Ac), 1.19 (s, 9H, tBu), 1.00 (s, 9H, tBu); 13C NMR (125 MHz, CDCl3): δ 168.1, 166.2, 166.0, 140.2, 133.6, 133.5, 133.2, 130.6, 129.9, 129.7, 129.0, 128.5, 128.5, 128.4, 125.4, 122.3, 118.4, 116.2, 107.9, 97.5, 77.2, 70.9, 70.7, 68.3, 68.1, 66.8, 27.6, 27.4, 24.0, 23.3, 20.8. HRMS (ESI-TOF): m/z calcd for C38H41BrClNO9Si: 820.1315 [M + Na]+; found: 820.1315.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeOH–THF) was added sodium methoxide (1.0 M solution in MeOH, 77 μL, 76.8 μmol) at 0 °C. After stirring for 1.5 h at rt as the reaction was monitored by TLC (1
1 MeOH–THF) was added sodium methoxide (1.0 M solution in MeOH, 77 μL, 76.8 μmol) at 0 °C. After stirring for 1.5 h at rt as the reaction was monitored by TLC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc), the reaction was neutralized with Muromac (H+) resin. The resin was filtered off and washed with MeOH. The filtrate and washings were concentrated. The resulting residue was purified by silica gel column chromatography (1
1 n-hexane/EtOAc), the reaction was neutralized with Muromac (H+) resin. The resin was filtered off and washed with MeOH. The filtrate and washings were concentrated. The resulting residue was purified by silica gel column chromatography (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) to give 20 (225 mg, 88%) as white powder. [α]D +106.7 (c 1.0 in CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.87 (br s, 1H, NH), 7.37 (d, 1H, 3Jortho = 8.5 Hz, Ar), 7.10 (d, 1H, JC
1 n-hexane/EtOAc) to give 20 (225 mg, 88%) as white powder. [α]D +106.7 (c 1.0 in CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.87 (br s, 1H, NH), 7.37 (d, 1H, 3Jortho = 8.5 Hz, Ar), 7.10 (d, 1H, JC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH,NH = 3.0 Hz, C
CH,NH = 3.0 Hz, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.09 (d, 1H, Ar), 5.46 (d, 1H, J1,2 = 3.0 Hz, H-1), 4.56 (d, 1H, J3,4 = 1.5 Hz, H-4), 4.30 (dd, 1H, J5,6a = 2.0 Hz, Jgem = 12.5 Hz, H-6a), 4.17 (dd, 1H, J5,6b = 1.5 Hz, H-6b), 4.04–3.97 (m, 2H, H-2, H-3), 3.95 (s, 1H, H-5), 2.67 (m, 1H, OH), 2.45 (m, 1H, OH), 1.08 (s, 9H, tBu), 1.06 (s, 9H, tBu); 13C NMR (125 MHz, CDCl3): δ 136.7, 133.4, 127.2, 124.3, 118.2, 113.5, 111.4, 110.3, 100.7, 77.2, 73.2, 71.5, 70.1, 68.2, 66.8, 27.6, 27.2, 23.4, 20.7. HRMS (ESI-TOF): m/z calcd for C38H41BrClNO9Si: 570.0685 [M + Na]+; found: 570.0688.
CH), 7.09 (d, 1H, Ar), 5.46 (d, 1H, J1,2 = 3.0 Hz, H-1), 4.56 (d, 1H, J3,4 = 1.5 Hz, H-4), 4.30 (dd, 1H, J5,6a = 2.0 Hz, Jgem = 12.5 Hz, H-6a), 4.17 (dd, 1H, J5,6b = 1.5 Hz, H-6b), 4.04–3.97 (m, 2H, H-2, H-3), 3.95 (s, 1H, H-5), 2.67 (m, 1H, OH), 2.45 (m, 1H, OH), 1.08 (s, 9H, tBu), 1.06 (s, 9H, tBu); 13C NMR (125 MHz, CDCl3): δ 136.7, 133.4, 127.2, 124.3, 118.2, 113.5, 111.4, 110.3, 100.7, 77.2, 73.2, 71.5, 70.1, 68.2, 66.8, 27.6, 27.2, 23.4, 20.7. HRMS (ESI-TOF): m/z calcd for C38H41BrClNO9Si: 570.0685 [M + Na]+; found: 570.0688.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CHCl3/MeOH). Solvents were removed by co-evaporation with toluene. The resulting residue was purified by silica gel column chromatography on Iatrobeads 6RS-8060 (10
1 CHCl3/MeOH). Solvents were removed by co-evaporation with toluene. The resulting residue was purified by silica gel column chromatography on Iatrobeads 6RS-8060 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1→ 5
1→ 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CHCl3/MeOH) to give 9 (150 mg, 93%) as light sky blue powder. [α]D +117.2 (c 1.0 in CHCl3); 1H NMR (500 MHz, CD3OD): δ 7.28 (d, 1H, 3Jortho = 9.0 Hz, Ar), 7.25 (s, 1H, C
1 CHCl3/MeOH) to give 9 (150 mg, 93%) as light sky blue powder. [α]D +117.2 (c 1.0 in CHCl3); 1H NMR (500 MHz, CD3OD): δ 7.28 (d, 1H, 3Jortho = 9.0 Hz, Ar), 7.25 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.14 (d, 1H, Ar), 5.38 (d, 1H, J1,2 = 3.5 Hz, H-1), 4.07 (t, 1H, J5,6a = J5,6b = 6.0 Hz, H-5), 4.05–4.00 (m, 2H, H-3, H-4), 3.97 (dd, 1H, J2,3 = 10.0 Hz, H-2), 3.73 (d, 2H, H-6a, H-6b); 13C NMR (125 MHz, CD3OD): δ 137.1, 135.1, 127.1, 125.0, 119.6, 114.1, 113.4, 112.7, 101.9, 73.4, 71.6, 71.0, 70.4, 62.7, 49.9. HRMS (ESI-TOF): m/z calcd for C14H15BrClNO6: 429.9663 [M + Na]+; found: 429.9668.
CH), 7.14 (d, 1H, Ar), 5.38 (d, 1H, J1,2 = 3.5 Hz, H-1), 4.07 (t, 1H, J5,6a = J5,6b = 6.0 Hz, H-5), 4.05–4.00 (m, 2H, H-3, H-4), 3.97 (dd, 1H, J2,3 = 10.0 Hz, H-2), 3.73 (d, 2H, H-6a, H-6b); 13C NMR (125 MHz, CD3OD): δ 137.1, 135.1, 127.1, 125.0, 119.6, 114.1, 113.4, 112.7, 101.9, 73.4, 71.6, 71.0, 70.4, 62.7, 49.9. HRMS (ESI-TOF): m/z calcd for C14H15BrClNO6: 429.9663 [M + Na]+; found: 429.9668.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported in part by JSPS KAKENHI Grant Number JP18K05455 (to A. I.). We thank Prof. Dr Thiem and Dr Böttcher (University of Hamburg) for technical support on the synthesis of indoxyl derivatives.Notes and references
- J. A. Kiernan, Biotech. Histochem., 2007, 82, 73–103 CrossRef CAS PubMed.
- R. J. Barrnett and A. M. Seligman, Science, 1951, 114, 579–582 CrossRef CAS PubMed.
- (a) S. Saito, S. Sumita, Y. Kanda and Y. Sasaki, Tetrahedron Lett., 1992, 33, 7381–7384 CrossRef CAS; (b) S. Saito, S. Sumita, Y. Kanda and Y. Sasaki, Chem. Pharm. Bull., 1994, 42, 1016–1027 CrossRef CAS PubMed.
- For review see: S. Böttcher and J. Thiem, Trends Carbohydr. Res., 2014, 6, 1–10 Search PubMed and references therein.
- N-Acetyl-indoxyl acceptor is more likely present in the keto form in non-polar and aprotic solvents, which was confirmed in 1H NMR spectrum measured in this study (see ESI†).
- S. Böttcher, M. Hederos, E. Champion, G. Dékány and J. Thiem, Org. Lett., 2013, 15, 3766–3769 CrossRef PubMed.
- (a) S. Böttcher and J. Thiem, Eur. J. Org. Chem., 2014, 564–574 CrossRef; (b) S. Böttcher and J. Thiem, RSC Adv., 2014, 4, 10856–10861 RSC.
- X. Wei, Q. Wu, J. Zhang, Y. Zhang, W. Guo, M. Chen, Q. Gu, Z. Cai and M. Lu, Chem. Commun., 2017, 53, 103–106 RSC.
- W. Berlin and B. Sauer, Anal. Biochem., 1996, 243, 171–175 CrossRef CAS PubMed.
- L. Marmuse, M. Asther, E. Fabre, D. Navarro, L. Lesage-Meessen, M. Asther, M. O'Donohue, S. Fort and H. Driguez, Org. Biomol. Chem., 2008, 6, 1208–1214 RSC.
- (a) F. B. Anderson and D. H. Leaback, Tetrahedron, 1961, 12, 236–239 CrossRef CAS; (b) J. P. Horwitz, J. Chua, R. J. Curby, A. J. Tomson, M. A. Da Rooge, B. E. Fisher, J. Mauricio and I. Klundt, J. Med. Chem., 1964, 7, 574–575 CrossRef CAS PubMed.
- M. E. van Dort, K. C. Lee, C. A. Hamilton, A. Rehemtulla and B. D. Ross, Mol. Imaging, 2008, 7, 187–197 CrossRef CAS PubMed.
- K. Yoshida, N. Iino and I. Koga, Chem. Pharm. Bull., 1975, 32, 1759–1763 CrossRef PubMed.
- S. Kaneko, M. Kitaoka, A. Kuno and K. Hayashi, Biosci., Biotechnol., Biochem., 2000, 64, 741–745 CrossRef CAS PubMed.
- Essentials of Glycobiology, ed. A. Varki, R. D. Cummings, J. D. Esko, P. Stanley, G. W. Hart, M. Aebi, A. G. Darvill, T. Kinoshita, N. H. Packer, J. H. Prestegard, R. L. Schnaar and P. H. Seeberger, Cold Spring Harbor Laboratory Press, New York, 3rd edn, 2017 Search PubMed.
- M. Yde and C. K. De Bruyne, Carbohydr. Res., 1973, 26, 227–229 CrossRef CAS.
- (a) A. Imamura, H. Ando, S. Korogi, G. Tanabe, O. Muraoka, H. Ishida and M. Kiso, Tetrahedron Lett., 2003, 44, 6725–6728 CrossRef CAS; (b) A. Imamura, A. Kimura, H. Ando, H. Ishida and M. Kiso, Chem.: Eur. J., 2006, 12, 8862–8870 CrossRef CAS PubMed; (c) A. Imamura, N. Matsuzawa, S. Sakai, T. Udagawa, S. Nakashima, H. Ando, H. Ishida and M. Kiso, J. Org. Chem., 2016, 81, 9086–9104 CrossRef CAS PubMed.
- (a) R. R. Schmidt and J. Michel, Angew. Chem., Int. Ed. Engl., 1980, 19, 731–732 CrossRef; (b) R. R. Schmidt, Angew. Chem., Int. Ed. Engl., 1986, 25, 212–235 CrossRef.
- S. Rio, J.-M. Beau and J.-C. Jacquinet, Carbohydr. Res., 1991, 219, 71–90 CrossRef CAS PubMed.
- X. Liu, S. Ren, Q. Gao, C. Hu, Y. Li and N. Ding, Org. Lett., 2018, 20, 5186–5189 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: NMR data for all new compounds. See DOI: 10.1039/c9ra05797h |
| This journal is © The Royal Society of Chemistry 2019 |