Open Access Article
Open Access ArticleA new way for lead–boron resin composite modification: SiO2 coated lead powders by a sol–gel method
Lingcheng
Ma
 a,
Ying
Liu
ab,
Jun
Li
*ab,
Na
Jin
ab,
Cheng
Li
a,
Xue
Zhou
a and
Jiaxu
Ma
a
a,
Ying
Liu
ab,
Jun
Li
*ab,
Na
Jin
ab,
Cheng
Li
a,
Xue
Zhou
a and
Jiaxu
Ma
a
aCollege of Materials Science and Engineering, Sichuan University, Chengdu 610065, P. R. China. E-mail: abthonyli@163.com
bKey Laboratory of Advanced Special Materials and Technology Ministry of Education, Chengdu 610065, P. R. China
First published on 30th September 2019
Abstract
In order to improve the composition distribution and flame retardancy of composites, the effects of silicon dioxide (SiO2) coatings with different contents on physical properties of lead (Pb) powders and composites were investigated in this research. SiO2 coated Pb powders (SiO2@Pb) with contents of 0, 0.237, 0.486, 0.683, 0.967 wt% were synthesized by a sol–gel method, then mixed with boron carbide (B4C) powders and boron phenolic resins (BPRs) to prepare SiO2@Pb/B4C/BPRs composites by molding. SiO2 coating on the surface of Pb powders in flakes or islands increases the specific surface area and oxidation temperature of the SiO2@Pb powders. For the SiO2@Pb/B4C/BPRs composites, the composition uniformity of composites is improved due to the reduction of the true density difference value between fillers (Pb, B4C), which is beneficial for the physical properties of the composites. Furthermore, the mechanical properties and thermal conductivity increase with the addition of SiO2 content, achieving a maximum value at 0.237 wt%, and then decrease gradually with a further increase of SiO2 content. Moreover, SiO2 coatings improve the limit oxygen index (LOI) of the composites and reduce the cracks of composites after burning. Composites with the SiO2 content of 0.486 wt% have optimal comprehensive physical properties, where the tensile strength, bending strength, impact toughness are 42.5 MPa, 72.4 MPa, 6.5 kJ m−2, respectively and the LOI is 41.8%.
1. Introduction
Nuclear shielding composites are important materials used for blocking γ rays and neutron radiation to ensure safety of personnel and machines.1–5 Lead-boron polyethylene (PBPE) is widely used for nuclear shielding. The resins have good moderating effect on fast neutrons, while boron atoms can absorb thermal neutrons effectively and Pb atoms have effective shielding effect on γ rays.6–8 However, the volume ratio of fillers (Pb, B4C) of lead–boron resins composites is over 30% to ensure the nuclear shielding performance. There is inevitable component segregation in the composites due to the huge density difference between fillers, which has adverse effects on the comprehensive physical properties of composites and restricts their widespread application.Up to now, the improvement of the physical properties of resin matrix composites is mainly by adding aids, such as adding flame retardants to improve the flame resistance properties of composites, adding glass fibers to increase the mechanical properties of composites, etc.9,10 Regrettably, the addition of aids is generally large, which will affect composition and properties of composites and some aids (halogen flame retardants) may produce toxic gases, which are rarely used.11–16 So far, many efforts are devoted to the study of aids but there is little literature refers to improving the physical properties and component homogeneity of composites by surface treatment of fillers.
SiO2 is used as a potential environmental flame retardant to improve the flame resistance of composites. Some literatures indicate that SiO2 forms SiC layers with the residual carbon layers or glassy SiO2 layers to improve the compactness of carbon layers, which prevents the fire spread and heat transfer.17,18 However, there are few literatures on its flame resistance mechanism in the composites containing fillers with low melting point such as Pb powders.
This paper presented the study of coating SiO2 shell on the surface of Pb powders (SiO2@Pb) by sol–gel method.19 Consequently, a type of resin matrix shielding composites with high flame retardant and excellent mechanical properties was obtained with SiO2@Pb powders, B4C powders and BPRs. The effects of SiO2 coatings on physical properties of powders and composites were investigated in this research. The study can provide a new way, which is modifying fillers surface, to improve the properties of composites.
2. Experimental section
2.1 Materials
Boron phenolic resins (BPRs), lead powders (Pb), boron carbide powders (B4C), tetraethoxysilane (TEOS), NH4OH, silane coupling agent (KH550) and other used reagents are all commercially available. All of the reagents used in these experiments are analytical grade.2.2 Synthesis of SiO2@Pb powders
Fig. 1a shows the schematic diagram of the synthesis of SiO2@Pb powders. In this work, the SiO2@Pb powders with different content of SiO2 were synthesized by sol–gel.20–22 The Pb powders and NH4OH were added into alcohol–water solution (volume ratio of alcohol to water was 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with mechanical stirring. Then the TEOS was dropped by a constant flow pump with 0.5 mL min−1 under 25 °C. Until the TEOS was dropped completely, the temperature was set to 80 °C to get the SiO2@Pb slurry. Finally put the SiO2@Pb slurry into a vacuum oven under 80 °C to remove all the solvent and get dry SiO2@Pb powders.
1) with mechanical stirring. Then the TEOS was dropped by a constant flow pump with 0.5 mL min−1 under 25 °C. Until the TEOS was dropped completely, the temperature was set to 80 °C to get the SiO2@Pb slurry. Finally put the SiO2@Pb slurry into a vacuum oven under 80 °C to remove all the solvent and get dry SiO2@Pb powders.
 | ||
| Fig. 1 (a) Schematic diagram of the synthesis of SiO2@Pb powders; (b) schematic diagram of the preparation of SiO2@Pb/B4C/BPRs composites. | ||
2.3 Preparation of SiO2@Pb/B4C/BPRs composites
Fig. 1b shows the schematic diagram of the preparation of SiO2@Pb/B4C/BPRs composites. 15 wt% BPRs, 80 wt% SiO2@Pb powders, 5 wt% B4C powders and KH550 were added in alcohol and mixed by mechanical stirring for 4 h in 40 °C. Then put the SiO2@Pb/B4C/BPRs slurry into a vacuum oven under 110 °C and crushed to obtain raw material particles. The raw material particles were put into a hard alloy mould with cold pressing firstly and then hot pressing at 120 °C for 1 h, 150 °C for 1 h and 180 °C for 3 h to get the SiO2@Pb/B4C/BPRs composites.2.4 Measurements
The functional groups on surface of Pb powders was examined by a Nicolet IS10 Fourier transform infrared spectroscopy (FTIR) recorded between 400 and 4000 cm−1. The surface morphology of SiO2@Pb powders was observed by a scanning electron microscopy (SEM) using a field-emission scanning election microscope (FESEM, Inspect F20). The transmission electron microscope (TEM) images was tested by field-emission transmission electron microscopy (FEI Talos F200). The true density of the SiO2@Pb powders was measured by 3H-2000 TD2 fully automatic true density analyser. The specific surface area of the SiO2@Pb powders was measured by a kubo-x1000 BET surface analyser. The mechanical properties of composites were tested by a Universal Material Testing Machine (Instron 4302). Thermogravimetric analysis (TGA) was performed on a DSC1/700 instrument heated from room temperature to 800 °C at a heating rate of 10 °C min−1 in air. The thermal conductive of the composites was tested by a TPS2500S thermal constant analyser. The LOI was performed on a JF-3 digital display oxygen index tester. The density of the composites was measured by an electronic scale (ESJ110-4A) based on Archimedes principle.3. Results and discussion
3.1 Characterization of SiO2@Pb powders
In this research, the theoretical addition of SiO2 is 0.25, 0.5, 0.75, 1 wt% of Pb. Fig. 2a shows the FI-TR spectra of SiO2@Pb powders with different SiO2 theoretical addition. It can be seen that the asymmetrical telescopic vibration absorption peak of Si–O–Si (1041.79 cm−1) and the symmetrical telescopic vibration absorption peak of Si–O (799 cm−1) are indicated, which demonstrates the formation of SiO2. Additionally, with the increase of SiO2 content, the intensity of H–O–H (1630 cm−1) recedes gradually. The phenomenon is for the reason that the SiO2 coatings reduce the water content on the Pb powders surface as hydrophobic layers.23 | ||
| Fig. 2 (a) FI-TR spectra of SiO2@Pb powders with different theoretical addition of SiO2; (b) true density of SiO2@Pb with different theoretical addition of SiO2. | ||
Fig. 2b shows the true density of SiO2@Pb powders with different theoretical addition of SiO2. Average value of true density of SiO2@Pb powders with 0, 0.25, 0.5, 0.75, 1 wt% theoretical addition of SiO2 is 11.252, 11.143, 11.031, 10.944, 10.821 g cm−3. According to the formula (eqn (1)),
 | (1) |
The SEM images and EDS of SiO2@Pb powders are shown in Fig. 3, the result of EDS reveals that Si existed on the surface of Pb powders after surface modification. Combined with the FI-TR spectra, it can be inferred that SiO2 coats on the surface of Pb powders by sol–gel. According to the SEM images, the SiO2 layers increase the surface roughness of Pb powders. In addition, lamellar SiO2 (Fig. 3d) coated on the surface of Pb powders gradually change to islands with the SiO2 content increasing further to 0.967 wt% (Fig. 3f).
 | ||
| Fig. 3 SEM images and EDS of SiO2@Pb powders with different theoretical addition of SiO2: (a and b) Pb; (c and d) 0.486 wt% SiO2@Pb; (e and f) 0.967 wt% SiO2@Pb. | ||
To further characterize the core–shell structure of SiO2@Pb powders, the TEM image and energy spectrum analysis of 0.967 wt% SiO2@Pb powders were obtained as shown in Fig. 4, Fig. 4a is the image of high-angle annular dark field (HAADF), Fig. 4b is the energy spectrum surface scanning image of Pb and Si corresponding to the HAADF imaging mode horizon. HADDF results show that there is obvious contrast difference between the outer and the core of the particles. It can be seen that the bright annular region of the outer ring is SiO2 with smaller relative mass, and the dark circular region is Pb. Besides, the result of elemental surface scanning image is also agreement of that of HADDF, showing Si distributes in the outer ring and Pb concentrates in the central region. Therefore, the 0.967 wt% SiO2@Pb powders show obvious core–shell structure.
 | ||
| Fig. 4 TEM image and energy spectrum analysis of 0.967 wt% SiO2@Pb powders. (a) HAADF-STEM; (b) surface scanning of Pb and Si. | ||
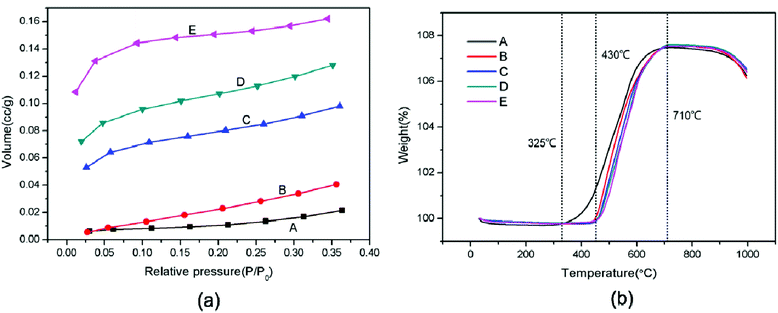
For the roughening of Pb powders after modification, the BET specific surface area of SiO2@Pb powders was also characterized. Fig. 5a is the nitrogen adsorption isotherm of SiO2@Pb powders. With the increase of SiO2 content, the nitrogen adsorption volume of SiO2@Pb powders increases obviously. In addition, the left end of the curves (relative pressure: 0–0.1) approaches the Y axis, which indicates that the interaction force between the SiO2@Pb powders and nitrogen increases gradually. According to the IUPAC classification, the isotherm of Pb is type III, which shows the weak adsorption between Pb powders and nitrogen. After surface modification, the isotherms change to type II or type IV.25,26 It can be inferred that the isotherm is type IV because SiO2 is a disordered mesoporous material.27 The BET specific surface areas of SiO2@Pb powders are 0.0470, 0.1324, 0.2763, 0.3654, 0.4800 m2 g−1, respectively with the increase of SiO2 content.
Fig. 5b shows the TGA curves of SiO2@Pb powders from room temperature to 800 °C in air. After surface modification, the initial oxidation temperature of Pb powders increases from 325 °C to 430 °C. It can be attributed to the lower thermal conductivity of SiO2 (1.03 W m−1 K−1)28 compared with Pb (34.8 W m−1 K−1).29 The SiO2 coatings act as heat insulation layers to insulate the external heat, which increases the initial oxidation temperature and delay the oxidation reaction.
3.2 Characterization of SiO2@Pb/B4C/BPRs composites
For the lead–boron resin matrix composites, component segregation exists in composites inevitably because of the large density difference between fillers, which causes weak shielding zones in composites and damages the physical properties of composites. Thus, the composition uniformity of the 0.486% SiO2@Pb/B4C/BPRs composites was also analysed. Composition homogeneity in composites can be expressed by the dispersion degree of density in each region of composites. The large degree of dispersion indicates that the component segregation in the composites. Fig. 6 shows the dispersion degree of density of Pb/B4C/BPRs composites and 0.486 wt% SiO2@Pb/B4C/BPRs composites in 20 regions. Eqn (2) is the standard deviation calculation formula,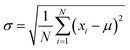 | (2) |
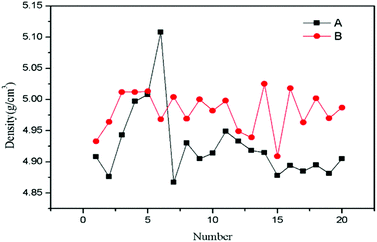 | ||
| Fig. 6 Density of SiO2@Pb/B4C/BPRs composites in 20 regions. A: Pb/B4C/BPRs composites; B: 0.486 wt% SiO2@Pb/B4C/BPRs composites. | ||
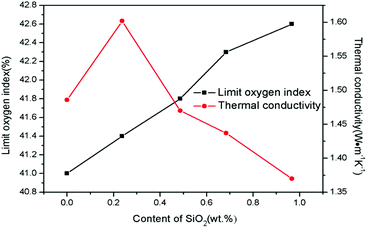
For the further study about the SiO2 coating effect on SiO2@Pb/B4C/BPRs composites, the thermal conductivity and LOI of composites were tested as shown in Fig. 7. The enhancement of interfacial interaction between Pb and BPRs reduces the interfacial thermal resistance and improved the thermal conductivity when the SiO2 content is 0.237 wt%.31 Then, the thermal conductivity decreases gradually with the increase of SiO2 content. Pb powders agglomerating in SiO2@Pb/B4C/BPRs composites form thermal conductivity chains, which improves the thermal conductivity of composites. SiO2 coatings insulate heat transfer due to the low powders in composites, which inhibits Pb powders forming thermal conductivity chains in the composites.
The LOI of the composites was also characterized to observe the effect of SiO2 on the composites flame retardancy. Literatures show SiO2 can improve the flame retardancy of composites by improve the compactness of carbon layers.17,18 The same result is obtained in this study, where the LOI of SiO2@Pb/B4C/BPRs composites increases from 41% to 42.6%. SiO2 coatings on Pb powders surface show new flame-retardant mechanism in this research as following. As shown in Fig. 8a, huge holes appear in the composites with the maximum diameter of holes about 350 μm and composites crack obviously, which promote the flame spreading to the interior of composites. The reason of the phenomenon is that fillers segregation exists in Pb/B4C/BPRs composites. Hence, agglomerated Pb powders melt and flow, which causes holes and cracks in composites. From Fig. 8b, small Pb particles disperse in the interior of composites without agglomeration. Furthermore, there are fewer holes in the composites and the cracking of composites is also improved. It can be attributed to reduction of true density difference value between fillers after coating, which is instrumental in the distribution of Pb powders in composites. With the further increase of SiO2 content, cracks on the surface of composites disappear and solidified Pb blocks appear in the composites (Fig. 8c). This is because the SiO2 coatings inhibit the flow of Pb liquid. The Pb liquid fills the holes and cracks to keep the structural integrity of the composites. Moreover, after solidification, it isolates the contact between oxygen and the resins to improve the composites flame retardancy.
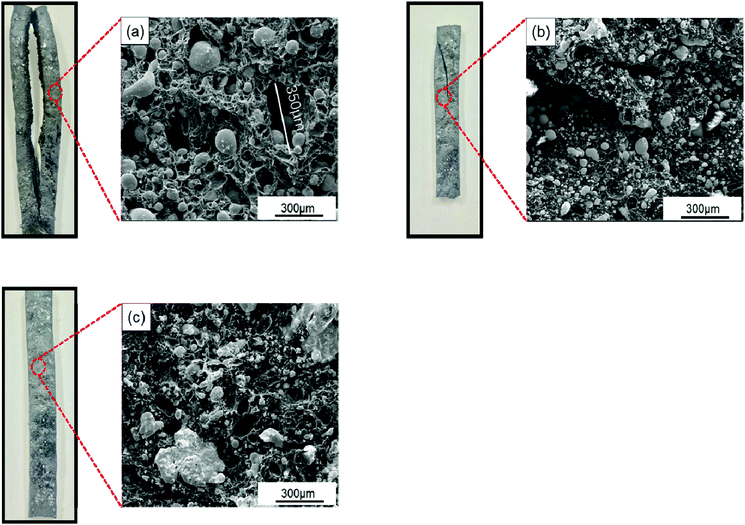 | ||
| Fig. 8 Photographs and SEM images of SiO2@Pb/B4C/BPRs composites after burning with different content of SiO2: (a) Pb, (b) 0.486 wt% SiO2@Pb, (c) 0.967 wt% SiO2@Pb. | ||
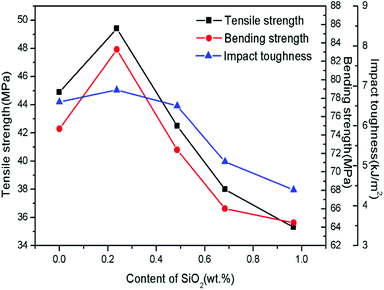
Fig. 9 displays the mechanical properties of SiO2@Pb/B4C/BPRs composites with different SiO2 content. The trend of mechanical properties of Pb/B4C/BPRs composites reaches the maximum when the SiO2 content is 0.237 wt% and then decreased with a further content. There are two main reasons for the improvement of mechanical properties: (1) SiO2 increases the roughness of the surface of Pb powders, which is conducive to resin adhesion on the surface of Pb powders. In addition, OH− groups on the surface of SiO2 enhance the interfacial bond between Pb powders and resin. Therefore, the SiO2 coatings reduces the gap between Pb powders and resin matrix in the microstructure, and improves the mechanical properties of the composites; (2) due to the huge density difference between Pb powders and B4C powders, powders agglomerate in the SiO2@Pb/B4C/BPRs composites. There are few resins in these areas, which make it difficult to adhere to powders. Therefore, it is easy to produce cracks in these areas which lead to material fracture. After surface treatment, the density of Pb powders decrease, which is conducive to the uniform dispersion of Pb powders and B4C powders in the composites. However, with the further increase of SiO2 content, the specific surface area of Pb powders increases from 0.0470 to 0.4800 m2 g−1 after modification. As a result, it is difficult for BPRs to coat on the surface of all powders uniformly and the BPRs coating thickness of powders surface also decreases, which lead to for the formation and propagation of internal cracks in composites. Additionally, elasticity modulus for Pb and SiO2 are 16 GPa (ref. 32) and ∼70 GPa,33 respectively, and elasticity modulus for BPRs is very low. Thus, SiO2 wrapped onto Pb powder surfaces increased the modulus mismatch between the Pb and BPRs. The modulus mismatch makes more prone to fine cracks at the interface under the external force.
4 Conclusions
In the view of the results presented in this work, the conclusions can be drawn as following:(1) SiO2 coated Pb powders (SiO2@Pb) with different content can be synthesized by sol–gel method. The SiO2 coatings on the surface of Pb powders is in flakes or islands. The specific surface area of SiO2@Pb powders increases from 0.0470 to 0.4800 m2 g−1 with the addition of SiO2 content. Moreover, the SiO2 coatings increase the initial oxidation temperature of SiO2@Pb powders from 325 °C to 430 °C as thermal barriers.
(2) The SiO2 coatings improve the interface bonding between Pb powders and BPRs and decrease the density difference between fillers, which reduce the agglomeration of fillers. Thus, the composition uniformity of SiO2@Pb/B4C/BPRs composites is improved after surface treatment of Pb powders, which increases the LOI, mechanical properties and decreases the thermal conductivity of SiO2@Pb/B4C/BPRs composites.
(3) SiO2 coatings on Pb powders surface inhibit the agglomeration of Pb powders and the flow of molten Pb, which are beneficial for reducing voids and cracks in composites. The LOI of SiO2@Pb/B4C/BPRs composites increased from 41% to 42.8% with the increase of SiO2 content.
(4) Analysing the LOI and the mechanical properties of composites, the 0.486 wt% SiO2@Pb/B4C/BPRs composites show the optimal comprehensive physical properties. The mechanical properties of composites prepared with 0.486 wt% SiO2@Pb are 42.5 MPa, 72.4 MPa, 6.5 kJ m−2, respectively and the LOI is 41.6%.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was support by the Program for the Sichuan Key Laboratory of strategic resources application and innovation 2016sfgw001.References
- Y. Jiang, X. Fu, Z. Zhang, W. Du, P. Xie, C. Cheng and R. Fan, J. Alloys Compd., 2019, 804, 305–313 CrossRef CAS.
- Y. Qu, Y. Du, G. Fan, J. Xin, Y. Liu, P. Xie, S. You, Z. Zhang, K. Sun and R. Fan, J. Alloys Compd., 2019, 771, 699–710 CrossRef CAS.
- J. M. DeWitt, E. R. Benton, Y. Uchihori, N. Yasuda, E. V. Benton and A. L. Frank, Radiat. Meas., 2009, 44, 905–908 CrossRef CAS.
- D. Jóźwiak-Niedźwiedzka, K. Gibas, A. M. Brandt, M. A. Glinicki, M. Dąbrowski and P. Denis, Procedia Eng., 2015, 108, 162–169 CrossRef.
- T. Xing, Y. Huang, K. Zhang and J. Wu, RSC Adv., 2014, 4, 53628–53633 RSC.
- J. W. Shin, J.-W. Lee, S. Yu, B. K. Baek, J. P. Hong, Y. Seo, W. N. Kim, S. M. Hong and C. M. Koo, Thermochim. Acta, 2014, 585, 5–9 CrossRef CAS.
- Y. Q. Tan, H. Luo, X. S. Zhou, S. M. Peng and H. B. Zhang, RSC Adv., 2018, 8, 39314–39320 RSC.
- V. Y. Topolov and C. R. Bowen, Mater. Lett., 2015, 142, 265–268 CrossRef CAS.
- L. Li, Z. Wu, S. Jiang, S. Zhang, S. Lu, W. Chen, B. Sun and M. Zhu, Polym. Compos., 2015, 36, 892–896 CrossRef CAS.
- P. Wang, X. Zhang, G. Lim, H. Neo, A. A. Malcolm, Y. Xiang, G. Lu and J. Yang, J. Mater. Sci., 2015, 50, 5978–5992 CrossRef CAS.
- A. Bobovitch, E. Gutman, M. Schenker, L. Utevski and M. Muskatel, Mater. Lett., 1995, 23, 317–320 CrossRef CAS.
- M. A. Khattab, J. Appl. Polym. Sci., 2000, 78, 6 CrossRef.
- X. Wang, M. Q. Romero, X.-Q. Zhang, R. Wang and D.-Y. Wang, RSC Adv., 2015, 5, 10647–10655 RSC.
- C. Wu, W. Wu, H. Qu and J. Xu, Mater. Lett., 2015, 160, 282–285 CrossRef CAS.
- N. Wu and R. Yang, Polym. Adv. Technol., 2011, 22, 495–501 CrossRef CAS.
- J. Zhang, Q. Wu, G. Li, M.-C. Li, X. Sun and D. Ring, RSC Adv., 2017, 7, 24895–24902 RSC.
- J. W. G. Takashi Kashiwagi, K. M. Butler, R. H. Harris, J. R. Shields and A. Asano, Fire Mater., 2000, 24, 277–289 CrossRef.
- H. Zhong, D. Wu, P. Wei, P. Jiang, Q. Li and J. Hao, J. Mater. Sci., 2007, 42, 10106–10112 CrossRef CAS.
- L. Qomariyah, A. F. Arif, W. Widiyastuti, S. Winardi, S. Taniguchi and T. Ogi, RSC Adv., 2018, 8, 26277–26282 RSC.
- P. Xie, Z. Zhang, K. Liu, L. Qian, F. Dang, Y. Liu, R. Fan, X. Wang and S. Dou, Carbon, 2017, 125, 1–8 CrossRef CAS.
- P. Xie, Z. Zhang, Z. Wang, K. Sun and R. Fan, Research, 2019, 2019, 1–11 CrossRef.
- L. Martín-García, S. Ruiz-Gómez, M. Abuín, Y. Montaña, N. Carmona and L. Pérez, RSC Adv., 2015, 5, 97503–97507 RSC.
- S. A. Mahadik, M. S. Kavale, S. K. Mukherjee and A. V. Rao, Appl. Surf. Sci., 2010, 257, 333–339 CrossRef CAS.
- F. Balas, M. Rodríguez-Delgado, C. Otero-Arean, F. Conde, E. Matesanz, L. Esquivias, J. Ramírez-Castellanos, J. Gonzalez-Calbet and M. Vallet-Regí, Solid State Sci., 2007, 9, 351–356 CrossRef CAS.
- IUPAC, Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity, 1985 Search PubMed.
- G. Toussaint, M. A. Rodriguez, R. Cloots, J. Rubio, F. Rubio, B. Vertruyen and C. Henrist, J. Non-Cryst. Solids, 2011, 357, 951–957 CrossRef CAS.
- X. Wang, Y. Pei, M. Lu, X. Lu and X. Du, J. Mater. Sci., 2014, 50, 2113–2121 CrossRef.
- T. Yamane, N. Nagai, S.-i. Katayama and M. Todoki, J. Appl. Phys., 2002, 91 Search PubMed.
- W. Hemminger, Int. J. Thermophys., 1989, 10, 765–777 CrossRef CAS.
- A. K. Suri, C. Subramanian, J. K. Sonber and T. S. R. C. Murthy, Int. Mater. Rev., 2013, 55, 4–40 CrossRef.
- J. Zhao, F. Du, W. Cui, P. Zhu, X. Zhou and X. Xie, Composites, Part A, 2014, 58, 1–6 CrossRef CAS.
- D. L. Waldorf and G. A. Alers, J. Appl. Phys., 1962, 33, 3266–3269 CrossRef CAS.
- C. P. Wong and R. S. Bollampally, J. Appl. Polym. Sci., 1999, 74, 3396–3403 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |