Open Access Article
Open Access ArticleMicrowave-assisted iodine-catalyzed oxidative coupling of dibenzyl(difurfuryl)disulfides with amines: a rapid and efficient protocol for thioamides†
Jinyang Chen a,
Lan Meia,
Jialing Liua,
Chuntao Zhonga,
Binfang Yuan
a,
Lan Meia,
Jialing Liua,
Chuntao Zhonga,
Binfang Yuan *a and
Qiang Li
*a and
Qiang Li b
b
aCollege of Chemistry and Chemical Engineering, Yangtze Normal University, Fuling, Chongqing, 408000, P. R. China. E-mail: 6781022@163.com
bInstitution of Functional Organic Molecules and Materials, School of Chemistry and Chemical Engineering, Liaocheng University, No. 1, Hunan Street, Liaocheng, Shandong 252059, P. R. China
First published on 10th September 2019
Abstract
An efficient protocol for synthesis of thioamides was developed via the microwave-assisted iodine-catalyzed oxidative coupling of dibenzyl(difurfuryl)disulfides with amines. This process is scalable and tolerates a wide spectrum of amines to deliver the corresponding products in moderate to excellent yields in 10 minutes, providing a cheap and rapid approach to thioamides.
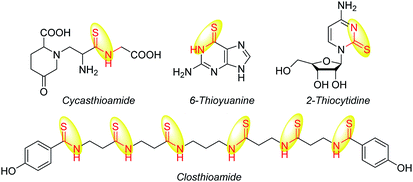
Thioamides have become an attractive synthetic goal in organic chemistry,1 because thioamide skeletons are widely present in drugs and natural products,2 such as cycasthioamide,3 6-thioyuanine,4 2-thiocytidine5 and closthioamide2a (Scheme 1), and their application as useful precursors and versatile building blocks for construction of a range of heterocyclic compounds has also been studied.1a,6 In addition, thioamides also can be used as a synthetic isostere for amides in peptide backbones,7 and their application as directing groups has also been reported.8,1c Furthermore, the application of thioamides in developing novel a fluorophore/quencher pair for monitoring the unfolding of a small protein was also explored.9
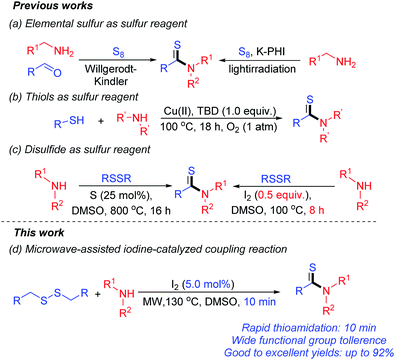
Because of their wide application, efforts are devoted toward their generation.10 The Willgerodt–Kindler reaction is a well-known method for construction of thioamides by using aldehydes and secondary amines as starting materials.11 However, this thioamidation always suffers from the problems of low conversions and harsh conditions. Though, modified variations in Willgerodt–Kindler reaction were also reported,1a,12 the use of excessive amounts of elemental sulphur makes it a less economical method. Lawesson's reagent was also used as common sulfur reagent for synthesis of thioamides,13 however, this reaction was also occur under harsh conditions. Another thioamidation, where elemental sulfur was used as sulfur reagent, was also reported by Savateev's groups via a photoinitiated reaction (Scheme 2a).11a However, this transformation was only suitable for special thioamides with the same structure of amines. Thiols can also be used as sulfur reagent for construction of thioamides,13b,c but its pungent smell makes this reaction difficult to carry out (Scheme 2b). Recently, Nguyen's group described a novel process to thioamides by using sulfur as catalyst.14 This method was suitable for a large range of amines for giving the corresponding products in good to excellent yields at 80 °C for a long time of 16 h.
Other methods for thioamides were also reported,15 but challenges still exist in developing more convenient and efficient ways to these important scaffolds. In our previous work, thioamides were synthesized via the iodine-promoted thioamidation of several of amines.16 However, this reaction must be performed under high temperature, for a long time (100 °C, 8.0 h), and 0.5 equiv. of I2 must to be used to promote the thioamidation effectively (Scheme 2c).
As one of the efficient and clean procedures in modern synthetic organic chemistry, microwave-assisted organic synthesis (MAOS) has aroused wide interest among scientists, which are suited to the increased demands in industry with the advantages of short reaction times and expanded reaction range.17 Herein, we reported an efficient method for synthesis of thioamides via the microwave-assisted iodine-catalyzed oxidative coupling of dibenzyl(furan-2-ylmethyl) disulfides with amines (Scheme 2d).
We initiated our studies with the reaction of dibenzyldisulfide (1a) with N-methylpiperazine (2a) catalyzed by 10 mol% of I2 in DMSO under microwave radiation (100 °C) for 10 minutes, and the desired product (3a) was obtained in the yield of 36% (Table 1, entry 1). The reaction temperature affected the reaction obviously, and highest yield was obtained when the temperature was increased to 130 °C for 10 minutes (Table 1, entry 4). But no increase in yield was observed when the temperature was increased to 140 °C (Table 1, entry 5) and the reaction time to 15 minutes (Table 1, entry 8). Next, a series of solvents (such as DMSO, DMF, 1,4-dioxane, THF, CH3CN, HOAc, chlorobenzene and toluene, or solvent-free) were also examined to promote the reaction (Table 1, entries 7–14), and results showed that DMSO is the best solvent, affording the desired product (3a) in the yield of 88% in 10 minutes (Table 1, entry 4). Then we explored the influence of the catalyst on the thioamidation, and results showed that the amount of 5.0 mol% of I2 was enough to promote the reaction effectively (88%, Table 1, entry 15). The yield of desired product decreased to 73% and 78% respectively, even extending the reaction time to 15 minutes, when 3.0 mol% of I2 was used. In addition, no increase in yield was observed, when the ratio of reactants (2a/1a) was increased to 3.0 (Table 1, entry 18). Only 18% of desired product was obtained, when the reaction was performed in the presence of 5.0 mol% of I2 in DMSO at 130 °C for 10 minutes without microwave irradiation (Table 1, entry 18). After extensive screening, we were glad to find that the reaction of dibenzyldisulfide (1a) with N-methylpiperazine (2a) in DMSO catalyzed by 5.0 mol% of I2 and assisted by microwave radiation provided the desired product (3a) with an excellent yield of 88% within 10 min (Table 1, entry 15).
| Entry | I2 (mol%) | Solvent | Temp. (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: dibenzyldisulfide 1a (0.2 mmol), N-methylpiperazine 2a (0.4 mmol), I2, solvent (2.0 mL).b GC yields based on dibenzyldisulfide 1a.c Isolated yields based on dibenzyldisulfide 1a.d 0.6 mmol of N-methylpiperazine 2a was used.e Without microwave radiation. | |||||
| 1 | I2 (10 mol%) | DMSO | 100 | 10 | 36% |
| 2 | I2 (10 mol%) | DMSO | 110 | 10 | 48% |
| 3 | I2 (10 mol%) | DMSO | 120 | 10 | 65% |
| 4 | I2 (10 mol%) | DMSO | 130 | 10 | 88% |
| 5 | I2 (10 mol%) | DMSO | 140 | 10 | 88% |
| 6 | I2 (10 mol%) | DMSO | 130 | 15 | 88% |
| 7 | I2 (10 mol%) | DMF | 130 | 10 | 74% |
| 8 | I2 (10 mol%) | 1,4-Dioxane | 130 | 10 | 67% |
| 9 | I2 (10 mol%) | THF | 130 | 10 | 65% |
| 10 | I2 (10 mol%) | CH3CN | 130 | 10 | 70% |
| 11 | I2 (10 mol%) | HOAc | 130 | 10 | 62% |
| 12 | I2 (10 mol%) | Chlorobenzene | 130 | 10 | 74% |
| 13 | I2 (10 mol%) | Toluene | 130 | 10 | 63% |
| 14 | I2 (10 mol%) | Solvent-free | 130 | 10 | 21% |
| 15 | I2 (5 mol%) | DMSO | 130 | 10 | 88% (86%)c |
| 16 | I2 (3 mol%) | DMSO | 130 | 10 | 73% |
| 17 | I2 (3 mol%) | DMSO | 130 | 15 | 78% |
| 18d | I2 (5 mol%) | DMSO | 130 | 10 | 88% |
| 19e | I2 (5 mol%) | DMSO | 130 | 10 | 18% |
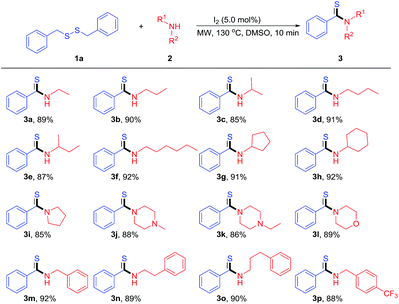
With the optimal conditions in hand, the scope of the amines and disulfides were investigated, and results were summarized in Table 2. As shown in Table 2, we can see that both aliphatic amines and aralkyl amines could efficiently undergo oxidative coupling effectively, affording corresponding products in good to excellent yields. The length and the steric hindrance of the alkyl affected the reactions slightly, and gave corresponding products with an excellent yield of 85–92% (3a–3h). The optimal conditions were also suitable for other N-containing heterocyclic amines (such as pyrrolidine, morpholine, 1-methylpiperazine and 1-ethylpiperazine), and corresponding products were obtained in the yield of 85–89% (3i–3l). Good yield was also obtained, when dibenzyldisulfide (1a) was treated with benzylamine (2-phenylethan-1-amine or 3-phenylpropan-1-amine) under the optimal conditions (3m–3p, 88–92%), and the strong electron-withdrawing group (CF3) presenting at the ring of the benzyl amine affected the thioamidation slightly, giving the desired product 3p in the yield of 88%.
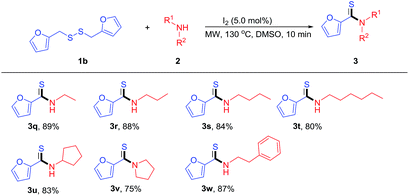
In subsequent studies, we examined the reaction of various amines with difurfuryl disulfide under the optimal conditions, and the results were summarized in Table 3. Analyzing Table 3, we can see that the thioamidation of difurfuryldisulfide with both alkylamines and heterocyclic amines gave desired products in moderate to good yields (3q–3w, 75–88%). And the steric hindrance (n-hexyl or cyclopentyl) of the aliphatic group affects the reaction slightly (3t and 3u, 80% and 83%). To our delight, phenethyl amine was also prone to this thioamidation, for giving the desired product (3w) in the yield of 87%.
The thioamidation can also be carried out on a larger scale reaction, and the desired product (3a) were obtained in the yields of 87%, when 5 mmol of dibenzyldisulfide (1a) was treated with 10 mmol of N-methylpiperazine (2a) under the standard conditions (Scheme 3a). To shed light on the mechanism of the reaction, dibenzyldisulfide (1a) was treated with N-methylpiperazine (2a) under standard conditions by using 2.0 equiv. of TEMPO or BHT as radical scavengers (Scheme 3b), and desired product 3a was obtained in the yields of 86% and 80% respectively, suggesting that no single-electron transfer process was involved through the whole reaction. In addition, thioamides have been used as important intermediates for the construction of heterocycles and other compounds containing both nitrogen and sulfur in their backbones (Scheme 3c).18
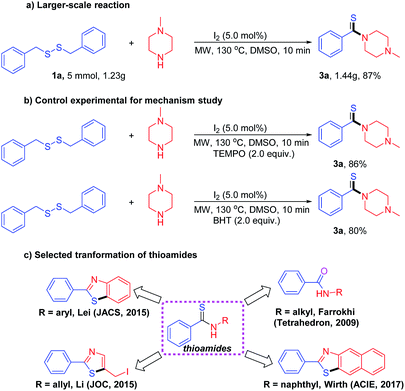 | ||
| Scheme 3 (a) Larger-scale synthesis of 3a. (b) Control experimental for mechanism study. (c) Selected transformation of thioamides. | ||
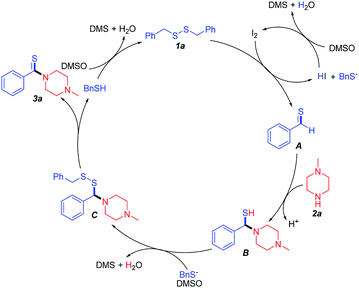
On the basis of the above experimental results and previous works,16,19 a possible mechanism has been depicted in Scheme 4. The first step of the thioamidation is the generation of the intermediate A by the reaction of dibenzyldisulfide (1a) with I2, with concomitant loss of HI and BnS−. Then intermediate A react with N-methylpiperazine (2a) to yield intermediate B, which was converted to species C via coupling with BnS− in the presence of DMSO. Finally, species C decomposed to desired product (3a) and BnSH, which was then converted to dibenzyldisulfide (1a) via oxidation reaction. During the whole reaction, the catalyst (I2) was regenerated by the cycle of HI in the presence of DMSO, which catalyzed the thioamidation effectively.
Conclusions
In summary, we have developed a rapid and efficient protocol for the synthesis of thioamides via the microwave-assisted iodine-catalyzed oxidative coupling of dibenzyl(difurfuryl) disulfides with amines at 130 °C for 10 minutes. A broad range of amines were tolerated, and all the desired products could be obtained in good to excellent yields. Comparing with the previous methods, the present strategy has the advantages of high efficiency, simple operation, rapid reaction and less catalyst, providing a convenient way to thioamides, which are key intermediate for synthesis of other heterocycles compounds.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (21902014) and the Basic and Frontier Research Project of Chongqing (Cstc2018jcyjAX0051) for the funding support.Notes and references
-
(a) T. S. Jagodziński, Chem. Rev., 2003, 103, 197 CrossRef PubMed
; (b) T. Bretschneider, E.-M. Franken, U. Görgens, M. Fusslein, A. Hense and J. Kluth, US Pat., US9428487B2, 2016
; (c) P. Jain, P. Verma, G. Xia and J.-Q. Yu, Nat. Chem., 2017, 9, 140 CrossRef CAS PubMed
; (d) X.-Y. Qian, S.-Q. Li, J. Song and H.-C. Xu, ACS Catal., 2017, 7, 2730 CrossRef CAS
; (e) R. W. Newberry, B. VanVeller and R. T. Raines, Chem. Commun., 2015, 51, 9624 RSC
; (f) N. Mahanta, D. M. Szantai-Kis, E. J. Petersson and D. A. Mitchell, ACS Chem. Biol., 2019, 14, 142 CrossRef CAS PubMed
.
-
(a) T. Lincke, S. Behnken, K. Ishida, M. Roth and C. Hertweck, Angew. Chem., Int. Ed., 2010, 49, 2011 CrossRef CAS PubMed
; (b) C. J. Schwalen, G. A. Hudson, B. Kille and D. A. Mitchell, J. Am. Chem. Soc., 2018, 140, 9494 CrossRef CAS PubMed
; (c) S. Coyne, C. Chizzali, M. N. A. Khalil, A. Litomska, K. Richter, L. Beerhues and C. Hertweck, Angew. Chem., Int. Ed., 2013, 52, 10564 CrossRef CAS PubMed
; (d) G. E. Kenney, L. M. K. Dassama, M.-E. Pandelia, A. S. Gizzi, R. J. Martinie, P. Gao, C. J. DeHart, L. F. Schachner, O. S. Skinner, S. Y. Ro, X. Zhu, M. Sadek, P. M. Thomas, S. C. Almo, J. M. Bollinger Jr, C. Krebs, N. L. Kelleher and A. C. Rosenzweig, Science, 2018, 359, 1411 CrossRef CAS PubMed
; (e) S. A. Abas, M. B. Hossain, D. van der Helm, F. J. Schmitz, M. Laney, R. Cabuslay and R. C. Schatzman, J. Org. Chem., 1996, 61, 2709 CrossRef CAS PubMed
.
- S. Banala and R. D. Süssmuth, ChemBioChem, 2010, 11, 1335 CrossRef CAS PubMed
.
- A. J. Van Der Vlies, U. Hasegawa and J. A. Hubbell, Mol. Pharmaceutics, 2012, 9, 2812 CrossRef CAS PubMed
.
- M.-K. Chung, C. M. Hebling, J. W. Jorgenson, K. Severin, S. J. Lee and M. R. Gagné, J. Am. Chem. Soc., 2008, 130, 11819 CrossRef CAS PubMed
.
-
(a) A. Padwa, D. J. Austin, M. Ishida, C. L. Muller, S. S. Murphree and P. E. Yeske, J. Org. Chem., 1992, 57, 1161 CrossRef CAS
; (b) A. S. Hamman and B. E. Bayoumy, Collect. Czech. Chem. Commun., 1985, 50, 71 CrossRef
; (c) C. R. Kelly, I. Gebhard and N. Wicnienski, J. Org. Chem., 1986, 51, 4590 CrossRef
.
- J. H. Miwa, L. Pallivathucal, S. Gowda and K. E. Lee, Org. Lett., 2002, 4, 4655 CrossRef CAS PubMed
.
- P. W. Tan, A. M. Mak, M. B. Sullivan, D. J. Dixon and J. Seayad, Angew. Chem., Int. Ed., 2017, 56, 16550 CrossRef CAS PubMed
.
-
(a) J. M. Goldberg, S. Batjargal and E. J. Petersson, J. Am. Chem. Soc., 2010, 132, 14718 CrossRef CAS PubMed
; (b) W. Lin, X. Cao, Y. Ding, L. Yuan and L. Long, Chem. Commun., 2010, 46, 3529 RSC
; (c) J. M. Goldberg, R. F. Wissner, A. M. Klein and E. J. Petersson, Chem. Commun., 2012, 48, 1550 RSC
; (d) J. M. Goldberg, S. Batjargal, B. S. Chen and E. J. Petersson, J. Am. Chem. Soc., 2013, 135, 18651 CrossRef CAS PubMed
; (e) J. M. Goldberg, X. Chen, N. Meinhardt, D. C. Greenbaum and E. J. Petersson, J. Am. Chem. Soc., 2014, 136, 2086 CrossRef CAS PubMed
; (f) C. Liu, T. Barrett, X. Chen, J. Ferrie and E. J. Petersson, ChemBioChem, 2019, 20, 2059 CrossRef CAS PubMed
.
-
(a) B. Kurpil, B. Kumru, T. Heil, M. Antonietti and A. Savateev, Green Chem., 2018, 20, 838 RSC
; (b) Y. A. Tayade, A. D. Jangale and D. S. Dalal, ChemistrySelect, 2018, 3, 8895 CrossRef CAS
; (c) C. T. Brain, A. Hallett and S. Y. Ko, J. Org. Chem., 1997, 62, 3808 CrossRef CAS
; (d) J. Wei, Y. Li and X. Jiang, Org. Lett., 2016, 18, 340 CrossRef CAS PubMed
; (e) K. Kumar, D. Konar, S. Goyal, M. Gangar, M. Chouhan, R. K. Rawal and V. A. Nair, ChemistrySelect, 2016, 1, 3228 CrossRef CAS
.
-
(a) R. N. Hurd and G. DeLaMater, Chem. Rev., 1961, 61, 45 CrossRef CAS
; (b) R. Wegler, E. Kuhle and W. Schafer, Angew. Chem., Int. Ed., 1958, 70, 351 CrossRef CAS
; (c) H. R. Darabi, K. Aghapoor and M. Tajbakhsh, Tetrahedron Lett., 2004, 45, 4167 CrossRef
.
-
(a) D. L. Priebbenow and C. Bolm, Chem. Soc. Rev., 2013, 42, 7870 RSC
; (b) K. Aghapoor, H. R. Darabi and K. Tabar-Heydar, Phosphorus, Sulfur Silicon Relat. Elem., 2002, 177, 1183 CrossRef CAS
; (c) K. Okamoto, T. Yamamoto and T. Kanbara, Synlett, 2007, 2687 CAS
; (d) T. Cuntreddi, R. Vanjari and K. N. Singh, Tetrahedron, 2014, 70, 3887 CrossRef
.
-
(a) D. C. Smith, S. W. Lee and P. L. Fuchs, J. Org. Chem., 1994, 59, 348 CrossRef CAS
; (b) F. M. Moghaddam and M. Ghaffarzadeh, Synth. Commun., 2001, 31, 317 CrossRef CAS
; (c) Z. Kaleta, G. Tárkányi, Á. Gömöry, F. Kálmán, T. Nagy and T. Soós, Org. Lett., 2006, 8, 1093 CrossRef CAS PubMed
; (d) Z. Kaleta, B. T. Makowski, T. Soós and R. Dembinski, Org. Lett., 2006, 8, 1625 CrossRef CAS PubMed
.
- T. B. Nguyen, L. P. Anh Nguyen and T. T. T. Nguyen, Adv. Synth. Catal., 2019, 361, 1787 CrossRef CAS
.
-
(a) N. D. Koduri, H. Scott, B. Hileman, J. D. Cox, M. Coffin, L. Glicksberg and S. R. Hussaini, Org. Lett., 2012, 142, 440 CrossRef PubMed
; (b) S. P. Pathare, P. S. Chaudhari and K. G. Akamanchi, Appl. Catal., A, 2012, 425, 125 CrossRef
; (c) Z. Zhou, J.-T. Yu, Y. Zhou, Y. Jiang and J. Cheng, Org. Chem. Front., 2017, 4, 413 RSC
; (d) M. F. Aly and R. Grigg, Tetrahedron, 1988, 44, 7271 CrossRef CAS
; (e) X. Li, Q. Pan, R. Hu, X. Wang, Z. Yang and S. Han, Asian J. Org. Chem., 2016, 5, 1353 CrossRef CAS
; (f) N. Borthakur and A. Goswami, Tetrahedron Lett., 1995, 36, 6745 CrossRef CAS
; (g) S. Kumar, R. Vanjari, T. Guntreddi and K. N. Singh, Tetrahedron, 2016, 72, 2012 CrossRef CAS
.
- S. Chen, Y. Li, J. Chen, X. Xu, L. Su, Z. Tang, C.-T. Au and R. Qiu, Synlett, 2016, 27, 2339 CrossRef CAS
.
-
(a) C. Bordoni, C. M. Cima, E. Azzali, G. Costantino and A. Brancale, RSC Adv., 2019, 9, 20113 RSC
; (b) A. M. Rodríguez, P. Prieto, D. R. Martín and J. I. García, ChemistryOpen, 2015, 4, 308 CrossRef PubMed
; (c) R. Rahaman, N. Devi, K. Sarma and P. Barman, RSC Adv., 2016, 6, 10873 RSC
; (d) S. K. Bhatia, V. Samdhian and B. Kaur, J. Heterocycl. Chem., 2018, 55, 935 CrossRef CAS
; (e) A. Kokel, C. Schäfer and B. Török, Green Chem., 2017, 19, 3729 RSC
.
-
(a) K. Bahrami, M. M. Khodaei and A. Farrokhi, Tetrahedron, 2009, 65, 7658 CrossRef CAS
; (b) G.-Q. Liu, C.-H. Yang and Y.-M. Li, J. Org. Chem., 2015, 80, 11339 CrossRef CAS PubMed
; (c) G. Zhang, C. Liu, H. Yi, Q. Meng, C. Biao, H. Chen, J.-X. Jian, L.-A. Wu and A. Lei, J. Am. Chem. Soc., 2015, 137, 9273 CrossRef CAS PubMed
; (d) A. A. Folgueiras-Amador, K. Philipps, S. Guilbaud, J. Poelakker and T. Wirth, Angew. Chem., Int. Ed., 2017, 56, 15446 CrossRef CAS PubMed
.
-
(a) M. Wang, J.-C. Xiang, Y. Cheng, Y.-D. Wu and A.-X. Wu, Org. Lett., 2016, 18, 524 CrossRef CAS PubMed
; (b) X. Wang, M. Ji, S. Lim and H.-Y. Jang, J. Org. Chem., 2014, 79, 7256 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra05939c |
| This journal is © The Royal Society of Chemistry 2019 |