Open Access Article
Open Access ArticleAn injectable molecular hydrogel assembled by antimicrobial peptide PAF26 for antimicrobial application
Fengyi
Cao
 *,
Lin
Mei
*,
Lin
Mei
 ,
Genxing
Zhu
,
Genxing
Zhu
 ,
Meng
Song
and
Xueli
Zhang
,
Meng
Song
and
Xueli
Zhang
School of Materials and Chemical Engineering, Zhongyuan University of Technology, Zhengzhou 450007, P. R. China. E-mail: caofengyi0513@126.com; Tel: +86 371 69975784
First published on 1st October 2019
Abstract
Wound infection is a crucial factor that inhibits wound recovery. A feasible measure to solve this problem is using antimicrobial biomaterials to suppress the microbial growth. In this work, an amphipathic antimicrobial peptide (Ac-RKKWFW-NH2, PAF26) was investigated to form the antimicrobial hydrogel. Triggered by pH, PAF26 peptide could self-assemble into a hydrogel, and the hydrogel formed was injectable and exhibited shear-thinning ability. Antimicrobial experiments demonstrated that the self-assembled hydrogel had an outstanding antimicrobial ability against pathogenic microbes such as Candida albicans, Staphylococcus aureus, and Escherichia coli via destroying the cell membrane structure. Thus, this study provides a novel method for preparing an injectable antimicrobial peptide hydrogel for antimicrobial therapies.
Introduction
In the past decades, new kinds of antimicrobial wound dressings, such as functional cotton gauzes,1,2 hydrocolloids,3,4 and hydrogels,5–7 have been studied for inhibiting wound infections. Among them, hydrogels, due to their high swelling ratio, are the most promising biomaterial for accelerating the wound healing process.8 The hydrogel is a kind of biomaterial, which is made from natural or artificial polymers. However, its antimicrobial ability is not satisfied. To improve the antimicrobial ability, antimicrobial agents, including antimicrobial drugs,9,10 metal ions (such as Ag+, Zn2+, and Cu2+),11–13 or antimicrobial peptides,14,15 have been introduced.Antimicrobial peptides (AMPs), primarily present in silkworms, are an important class of components for protecting the host against microbial infections. By now, it has been widely found in natural organisms, and more than 1200 different AMPs are known.16 AMPs are reported to exhibit wide spectrum of antibacterial ability, good biocompatibility, and low drug-resistance,17,18 which made them potential candidates as antimicrobial agents. Generally, AMPs have a length of 4–100 amino acids with amphipathic structure, and most are cationic peptides.
The molecular hydrogel, a novel kind of hydrogel, has been extensively studied in recent years due to its big potential for cell culture, drug delivery, and regenerative medicine.19–21 Unlike conventional hydrogel, the molecular hydrogel is formed by a molecular self-assembly in water, without the process of cross-linking or drug loading. Moreover, the molecular hydrogel has the ability of self-delivery and could realize a higher local density at the target sites. The self-assembled molecules include drugs,22 peptides,23–25 sugar-based compounds,26 and amino acid-derivatives,27 which could work as both hydrogelator and active “drug”. For example, Yang and his group28 used two kinds of anti-inflammatory molecules and a uranyl ion chelating ligand to form the supramolecular hydrogel. The hydrogel could self-deliver and be used for simulated uranium wound treatments.
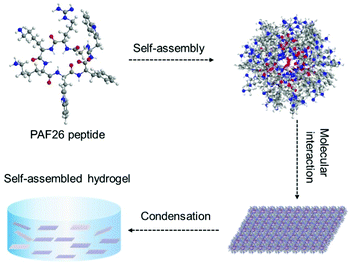
In this study, a hexapeptide PAF26 (Scheme 1) was observed to form the self-assembled molecular hydrogel. With the amphipathic structure, the cationic peptide could self-assemble into a molecular hydrogel triggered by pH. Due to the antimicrobial ability of PAF26 peptide,29,30 when contacted with pathogenic microbes, the hydrogel could destroy the cell-wall and kill the microbes.
Experimental section
Reagents and materials
The PAF26 peptide: Ac-RKKWFW-NH2 (molecular weight: 991.19 g mol−1, GC purity ≥ 95%) was purchased from Shanghai GL Biochem Ltd. (Shanghai, China). Sodium hydroxide (AR) was purchased from Sinopharm chemical reagent Co., Ltd. (Shanghai, China). Potassium bromide (SP) was purchased from Shanghai Yi En chemical technology Co., Ltd. Candida albicans (ATCC10231), Staphylococcus aureus (ATCC6538), and Escherichia coli (ATCC25922) were purchased from Shanghai Luwei technology company (Shanghai, China). Sabouraud dextrose broth (SDB), sabouraud dextrose agar (SDA), casein soya bean digest broth (TSB), and tryptic soy agar (TSA) were purchased from Guangdong Huankai Microbial Sci. & Tech. Co., Ltd. (Guangzhou, China).Synthesis of PAF26 peptide self-assembled hydrogel
Firstly, 40 mg PAF26 peptide was dissolved in 1 mL distilled water. Then, the pH was adjusted by adding 0.5 M NaOH solution in 5–10 μL increments. When the pH reached a value of 7.5, the solution was kept at that pH for several minutes to form the self-assembled hydrogel, and then, the self-assembled hydrogel was stored at RT (for at least 1 day) for the following studies.Rheology test
The rheological property of the self-assembled hydrogel was evaluated using a control-strain rheometer (Anton Paar MCR302, Austria). Frequency sweep test was conducted at a strain, which was within the linear viscoelastic region (1% strain). The frequency was varied from 0.1 to 10 Hz, and the temperature was set as 37 °C (the temperature of the human body). Then, the storage modulus (G′) and loss modulus (G′′) were recorded. The viscosity of the self-assembled hydrogel was examined at the shear rate, which was varied from 0.1 to 1 s−1 at 25 °C (the temperature of injection).Scanning electron microscopy (SEM)
The microscopic structure of the self-assembled hydrogel was evaluated by scanning electron microscopy. Once self-assembled into a hydrogel, the self-assembled hydrogel was placed in liquid nitrogen to freeze and then lyophilized by a freeze-dryer (LGJ-10, China). In addition, the self-assembled hydrogel, after being stored at room temperature for 24 h, was also lyophilized. Then, the lyophilized hydrogels were observed under a scanning electron microscope (FEI Quanta 250 FEG, USA).Circular dichroism
The solid circular dichroism of the self-assembled hydrogel was analyzed using a spectropolarimeter (JASCOJ-815, Japan), with accumulations of 4 s in every 1 nm in the wavelength range of 190–450 nm.Fourier transform infrared spectroscopy (FT-IR)
2 mg of the lyophilized self-assembled hydrogel was mixed with 100–200 mg of KBr. Then, the mixture was ground and repressed into a piece. The FTIR spectra were then collected on a spectrophotometer (Nicolet iS50, USA).Ultraviolet-visible absorbance spectroscopy
A series of PAF26 peptide solutions at different concentrations: 0.2, 0.5, 1, 2, and 40 mg mL−1 were prepared, and the ultraviolet-visible absorbance spectra were recorded on an ultraviolet-visible spectrophotometer (UV1800PC, China). In addition, the ultraviolet-visible absorbance spectra of the self-assembled hydrogel were also recorded.Fluorescence spectroscopy
A series of PAF26 peptide solutions at different concentrations: 0.2, 0.5, 1, 2, and 40 mg mL−1 were prepared, and the fluorescence spectra were recorded on a fluorescence spectrophotometer (F-7100, Japan); λex = 310 nm, λem = 315–500 nm. Moreover, the fluorescence spectra of the self-assembled hydrogel were also recorded.Antimicrobial activity evaluation
Before antimicrobial experiments, the samples: self-assembled hydrogel and agar culture medium as control (for Candida albicans, SDA culture medium was chosen; for Staphylococcus aureus and Escherichia coli, TSA culture medium was chosen), were sterilized for 1 h using UV light.To evaluate the antimicrobial activity, the pathogenic microbes: Candida albicans (fungus), Staphylococcus aureus (Gram-positive), and Escherichia coli (Gram-negative) were chosen. The microbial solutions were prepared in the following way: firstly, the Candida albicans was incubated in SDB culture medium at 26 °C, and the Staphylococcus aureus and Escherichia coli were incubated in TSB culture medium at 37 °C. After incubating them overnight, 0.2 mL of Candida albicans, Staphylococcus aureus, and Escherichia coli microbial medium were each seeded in 30 mL fresh culture medium and further cultured. When the optical density (OD) value of the microbial solution at 600 nm reached 0.1, the antimicrobial experiments were conducted as below.
Results and discussion
Self-assembled molecular hydrogel
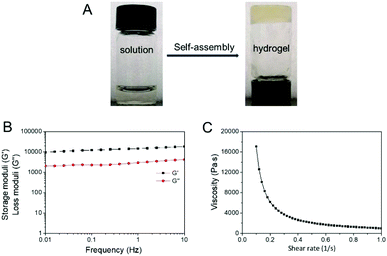
PAF26 peptide is a hexapeptide with an amphipathic structure. After dissolving it in distilled water, an acidic and transparent solution of PAF26 was obtained. On the other hand, when the pH value was adjusted to 7.5, the solution was found to transform into a semi-transparent hydrogel (Fig. 1A). Storage moduli (G′) of the semi-transparent hydrogel was observed to be greater than the loss moduli (G′′) (Fig. 1B), which in turn confirmed that the self-assembled molecular hydrogel possessed the characteristic of a viscoelastic solid.Further, the viscosity test found that the viscosity of the self-assembled molecular hydrogel significantly dropped with an increasing shear rate (Fig. 1C), thereby showing shear-thinning ability, which indicated that the self-assembled molecular hydrogel was injectable and could be used through a syringe for clinical applications.31
Mechanism of the self-assembled hydrogel
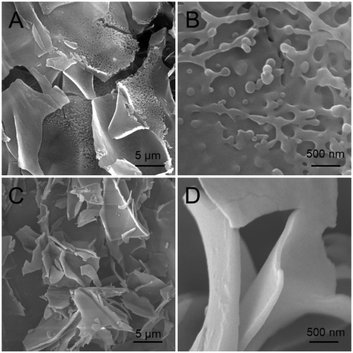
The self-assembly of the amphipathic peptide usually depends on the intermolecular forces and hydrophobic effect. The chemical structure of PAF26 peptide consists of three hydrophobic amino acids and three hydrophilic cationic amino acids. The SEM images showed that when primarily formed, the self-assembled hydrogel had a micro-sheet structure with nanoparticles on the surface (Fig. 2A and B), while after storing for 24 h at room temperature, the self-assembled hydrogel had a uniform micro-sheet structure (Fig. 2C and D).Fourier transform infrared spectroscopy (FT-IR) and circular dichroism (CD) were conducted to evaluate the secondary structure transformation of the self-assembled peptide. According to the FT-IR spectrum (Fig. 3A), the adsorption band of amide-I at 1633 cm−1 seemed to be the characteristic absorbance of the β-sheet conformation; the adsorption band of amide-II at 1677 cm−1 indicated the β-sheet or β-turn conformation structure of the self-assembled hydrogel.32 In addition, the adsorption band of N–H at 3290 cm−1 confirmed that the peptide was self-assembled via intermolecular backbone–backbone hydrogen bonding. In the solid circular dichroism spectrum (Fig. 3B), a strong positive peak was found at 195 nm, and a broad negative peak was found at 230 nm, confirming the β-sheet structure of the self-assembled hydrogel, while the negative peak at 199 nm confirmed that there is still some random coil in the hydrogel.
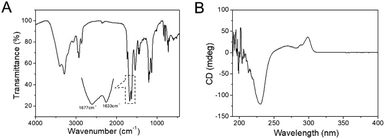 | ||
| Fig. 3 (A) FT-IR spectra of the freeze-dried self-assembled hydrogel; (B) the circular dichroism (CD) spectra of the self-assembled hydrogel. | ||
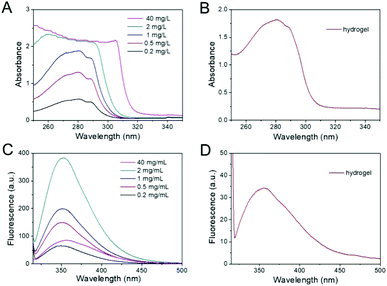
The UV-vis absorbance and fluorescence emission spectra were used to evaluate the effect of aromatic–aromatic π–π stacking interactions in the self-assembly of the PAF26 peptide. When the concentration of PAF26 peptide was increased from 0.2 to 1 mg mL−1, the maximum absorption peak was only subtly shifted (Fig. 4A), while at 2 mg mL−1 and 40 mg mL−1 (though at 40 mg mL−1, due to the high concentration, the absorbance from 250–310 nm could not make sense), the maximum absorption peak was red-shifted, thereby confirming that upon reaching a higher concentration, the PAF26 peptide could interact via aromatic–aromatic π–π stacking interactions.33 Further, the UV-vis absorbance spectra of the self-assembled hydrogel confirmed a red-shift of the maximum absorption peak in the self-assembled hydrogel (Fig. 4B). On the other hand, in the fluorescence spectra, the central peak shifted from 350 to 360 nm with an increase in the concentration of PAF26 peptide from 0.2 to 1 mg mL−1 (Fig. 4C). Similar to the PAF26 peptide, the central peak of the self-assembled hydrogel also displayed an obvious red-shift to 360 nm (Fig. 4D).
As shown in Scheme 2, it could be supposed that when the pH value shifted to 7.5, the PAF26 peptide was forced by aromatic–aromatic π–π stacking interactions to have a secondary structure of β-sheet, which formed the self-assembled micelles, and then the micelles got linked to the catenulate microfiber, polymerized to microsheet, and finally formed the self-assembled hydrogel.
Antimicrobial activity study
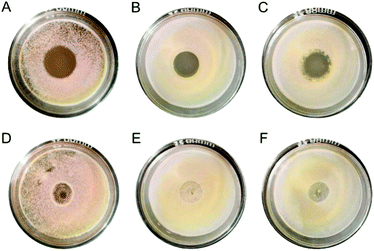
The antimicrobial activities of the self-assembled hydrogel were evaluated against various pathogenic microbes such as Candida albicans, Staphylococcus aureus, and Escherichia coli. When introduced to microbial culture dishes, the self-assembled hydrogel could inhibit the growth of Candida albicans, Staphylococcus aureus, and Escherichia coli in situ (Fig. 5A–C).Meanwhile, the hydrogel could permeate and inhibit microbial growth even outside. For Candida albicans, the diameter of the inhibition zone was 2.00 ± 0.06 cm; for Staphylococcus aureus, the diameter of the inhibition zone was 1.53 ± 0.21 cm; and for Escherichia coli, the diameter of the inhibition zone was 2.01 ± 0.06 cm. On the contrary, as a control, the agar culture medium could hardly inhibit the microbial growth (Fig. 5D–F).
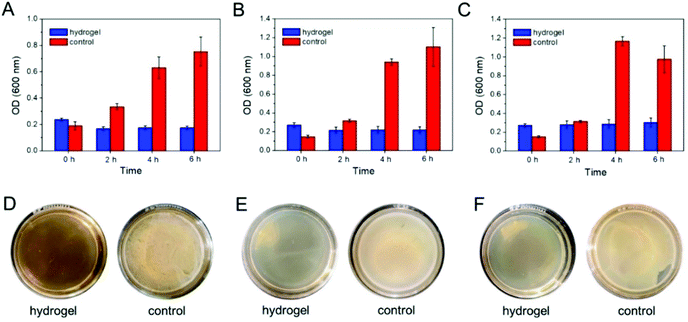
Besides, Fig. 6A–C showed that when co-cultured, the optical densities of Candida albicans, Staphylococcus aureus, and Escherichia coli on an agar culture medium were increased with an increase in the incubation time, which confirmed that the pathogenic microbes were in good condition. Whereas, in the case of the self-assembled hydrogel, the optical densities exhibited almost no change, thereby demonstrating that the self-assembled hydrogel could suppress the microbial growth. Moreover, after being co-cultured with the self-assembled hydrogel, no obvious microbial colonies were found on the dishes, which established that the killing efficiency of the hydrogel was about 100% (Fig. 6D–F).
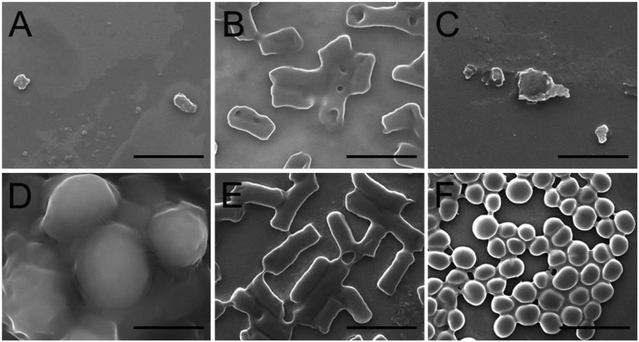
The mechanism of the antimicrobial effect was supposed to be the destruction of the cell membrane. Therefore, the morphologies of Candida albicans, Staphylococcus aureus, and Escherichia coli were investigated by SEM. As shown in Fig. 7, the microbial cells treated with an agar culture medium as control were smooth and retained their respective morphologies: Candida albicans and Staphylococcus aureus exhibited a round shape, and Escherichia coli showed a rod-like shape. In contrast, the serious surface roughness and shape changes were observed for the microbial cells exposed to the self-assembled hydrogel, thereby supporting that the antimicrobial effect of the hydrogel was due to its interaction with the microbial cell wall.
Conclusions
In this work, the self-assembled hydrogel was synthesized from amphipathic PAF26 peptide, with the synthesis being triggered by pH. The self-assembly was forced by the hydrophobicity and aromatic–aromatic π–π stacking interactions of the PAF26 peptide to form β-sheet conformations, thereby resulting in a micro-sheet structure. In addition, the self-assembled hydrogel showed shear-thinning ability and was injectable. Further, the antimicrobial studies confirmed that the self-assembled hydrogel possessed good antimicrobial potential against fungus, Gram-positive and Gram-negative pathogenic microbes. Thus, this study gives a novel method for the application of antimicrobial peptide for antimicrobial therapies.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51703260, 51703259), and Program for Science and Technology of Henan Province (No. 182102310635).Notes and references
- A. P. Gomes, J. F. Mano, J. A. Queiroz and I. C. Gouveia, Carbohydr. Polym., 2015, 127, 451 CrossRef CAS.
- Y. Yuan, H. Wu, H. F. Lu, Y. R. Zheng, J. Y. Ying and Y. G. Zhang, Chem. Commun., 2019, 55, 699 RSC.
- C. G. Otoni, M. R. de Moura, F. A. Aouada, G. P. Camilloto, R. S. Cruz, M. V. Lorevice, N. de F. F. Soares and L. H. C. Mattoso, Food Hydrocolloids, 2014, 41, 188 CrossRef CAS.
- A. H. León, F. Hebal, C. Stake, K. Baldwin and K. A. Barsness, Int. Wound J., 2019, 16, 41 CrossRef.
- P. Li, Y. F. Poon, W. F. Li, H. Y. Zhu, S. H. Yeap, Y. Cao, X. B. Qi, C. C. Zhou, M. Lamrani, R. W. Beuerman, E. T. Kang, Y. G. Mu, C. M. Li, M. W. Chang, S. S. J. Leong and M. B. Chan-Park, Nat. Mater., 2011, 10, 149 CrossRef CAS PubMed.
- S. D. Mu, W. T. Liu, L. Zhao, Y. R. Long and H. B. Gu, Polymer, 2019, 169, 80 CrossRef CAS.
- X. Y. Qiu, P. Y. Yuan, K. Lei, L. Wang, Y. K. Bai, S. Y. Liu and X. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 17018 CrossRef.
- W. R. Leow, X. Liu, Y. L. Wu, P. Z. Guo, X. J. Loh and X. D. Chen, Chem. Soc. Rev., 2018, 47, 6917 RSC.
- Y. Zhang, J. H. Zhang, M. Chen, H. Gong, S. Thamphiwatana, L. Eckmann, W. W. Gao and L. F. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 18367 CrossRef CAS.
- L. Wang, X. Li, T. Sun, Y. H. Tsou, H. Chen and X. Y. Xu, Macromol. Biosci., 2018, 18, 1700325 CrossRef.
- Y. C. Guo, S. Wang, H. H. Du, X. L. Chen and H. Fei, Biomacromolecules, 2018, 20, 558 CrossRef.
- I. A. Neacsu, L. V. Arsenie, R. Trusca, I. L. Ardelean, N. Mihailescu, I. N. Mihailescu, C. Ristoscu, C. Bleotu, A. Ficai and E. Andronescu, Nanomaterials, 2019, 9, 692 CrossRef CAS.
- P. Li, Z. P. Feng, Z. Y. Yu, Y. Chen, P. W. Li, Z. M. Yang, S. D. Li and S. H. Jin, Int. J. Biol. Macromol., 2019, 133, 67 CrossRef CAS.
- D. A. Salick, J. K. Kretsinger, D. J. Pochan and J. P. Schneider, J. Am. Chem. Soc., 2007, 129, 14793 CrossRef CAS.
- H. Sun, Y. X. Hong, Y. J. Xi, Y. J. Zou, J. Y. Gao and J. Z. Du, Biomacromolecules, 2018, 19, 1701 CrossRef CAS.
- M. A. Fox, J. E. Thwaite, D. O. Ulaeto, T. P. Atkins and H. S. Atkins, Peptides, 2012, 33, 197 CrossRef CAS.
- K. Hilpert, R. Volkmer-Engert, T. Walter and R. E. Hancock, Nat. Biotechnol., 2005, 23, 1008 CrossRef CAS.
- V. W. L. Ng, J. M. W. Chan, H. Sardon, R. J. Ono, J. M. García, Y. Y. Yang and J. L. Hedrick, Adv. Drug Delivery Rev., 2014, 78, 46 CrossRef CAS PubMed.
- H. Wang and Z. Yang, Nanoscale, 2012, 4, 5259 RSC.
- F. Zhao, M. L. Ma and B. Xu, Chem. Soc. Rev., 2009, 38, 883 RSC.
- B. G. Xing, C. W. Yu, K. H. Chow, P. L. Ho, D. G. Fu and B. Xu, J. Am. Chem. Soc., 2002, 124, 14846 CrossRef CAS.
- J. L. Li, R. R. Xing, S. Bai and X. H. Yan, Soft Matter, 2019, 15, 1704 RSC.
- H. L. Jin, C. Wan, Z. Zou, G. F. Zhao, L. L. Zhang, Y. Y. Geng, T. Chen, A. Huang, F. G. Jiang, J. P. Feng, J. F. Lovell, J. Chen, G. Wu and K. Y. Yang, ACS Nano, 2018, 12, 3295 CrossRef CAS.
- L. Y. Zhao, Q. L. Zou and X. H. Yan, Bull. Chem. Soc. Jpn., 2019, 92, 70 CrossRef CAS.
- K. Fukunaga, H. Tsutsumi and H. Mihara, Bull. Chem. Soc. Jpn., 2019, 92, 391 CrossRef CAS.
- S. Akama, T. Maki and M. Yamanaka, Chem. Commun., 2018, 54, 8814 RSC.
- P. Xing, H. Chen, H. Xiang and Y. L. Zhao, Adv. Mater., 2018, 30, 1705633 CrossRef.
- Z. M. Yang, K. M. Xu, L. Wang, H. W. Gu, H. Wei, M. J. Zhang and B. Xu, Chem. Commun., 2005, 4414 RSC.
- A. Muñoz, M. Gandaí, E. Harries, L. Carmona, N. D. Read and J. F. Marcos, Fungal Biol. Rev., 2013, 26, 146 CrossRef.
- L. Mendive-Tapia, C. Zhao, A. R. Akram, S. Preciado, F. Albericio, M. Lee, A. Serrels, N. Kielland, N. D. Read, R. Lavilla and M. Vendrell, Nat. Commun., 2016, 7, 10940 CrossRef CAS.
- Y. Li, K. Fukushima, D. J. Coady, A. C. Engler, S. Q. Liu, Y. Huang, J. S. Cho, Y. Guo, L. S. Miller, J. P. Tan, P. L. Ee, W. M. Fan, Y. Y. Yang and J. L. Hedrick, Angew. Chem., Int. Ed., 2013, 52, 674–678 CrossRef CAS.
- S. Y. Qin, H. F. Jiang, X. J. Liu, Y. Pei, H. Cheng, Y. X. Sun and X. Z. Zhang, Soft Matter, 2014, 10, 947 RSC.
- S. Y. Qin, S. S. Xu, R. X. Zhuo and X. Z. Zhang, Langmuir, 2011, 28, 2083 CrossRef.
| This journal is © The Royal Society of Chemistry 2019 |