Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dissolution of metal oxides in task-specific ionic liquid†
Janine Richter a and
Michael Ruck
a and
Michael Ruck *ab
*ab
aFaculty of Chemistry and Food Chemistry, Technische Universität Dresden, 01062 Dresden, Germany. E-mail: michael.ruck@tu-dresden.de
bMax Planck Institute for Chemical Physics of Solids, 01187 Dresden, Germany
First published on 19th September 2019
Abstract
Due to their typically low reactivity, the activation of metal oxides, as found in ores, earths and minerals, is in general performed by high temperature reactions, which consume much energy. Owing to the prevalence of fossil fuels, this is accompagnied by the generation of large amounts of CO2. Alternatively, ionic liquids (ILs) can be used as solvents for metal oxide dissolution and downstream chemistry at much lower temperatures. The dissolution ability of the dry ionic liquid betainium bis(trifluoromethylsulfonyl)imide, [Hbet][NTf2], was investigated for 30 metal oxides at 175 °C and compared to chloride containing IL [Hbet]2[NTf2]Cl. A general dissolution-promoting effect of chloride anions was found, regarding reaction time as well as the variety of dissolved metal oxides. Up to now, the dissolution in [Hbet]2[NTf2]Cl is limited to basic or amphoteric metal oxides and assumed to be influenced by multiple factors, such as reaction conditions and the lattice energy of the metal oxide as well as its crystal structure. Comprehensive investigations were performed for the dissolution of CuO, which led to the discovery of the water-free complex compound [Cu2(bet)4(NTf2)2][NTf2]2. Proceeding from this compound, a complete exchange of the O-coordination sphere by other ligands was demonstrated, opening up promising possibilities for downstream chemistry.
Introduction
Metals are important resources for many applications, for which reason their extraction from naturally occurring ores, earths and minerals is of great industrial interest. Such natural metal sources often consist of metal oxides alongside concomitant matter (paragenesis). Many of the established metal production processes starting from these resources are based on reactions at high temperatures, typically around 1000 °C, and, thus, are extremely energy consuming and, owing to the prevalence of fossil fuels, CO2 producing.1,2Due to the challenges of climate change and the increasing shortage of non-renewable energy resources, a development towards to more sustainable economic processes is of great importance. Innovative, more efficient ways of extracting metal oxides from their natural mixtures of substances and the subsequent production of metals and diverse metal compounds have to be developed.
During the last decades, ionic liquids (ILs) arouse a great deal of interest due to their promising applications to low temperature reactions. ILs, per definition, are salts with a melting point below 100 °C and favourable properties, such as negligible vapour pressure, high thermal stability or a good solubility of numerous inorganic materials.3
Recently, it was shown that ILs might even be suitable solvents for not readily dissolvable metal oxides. In this context, the presence of coordinating functional groups, as found in so-called task-specific ILs, turned out essential.4–8 In the IL betainium bis(trifluoromethylsulfonyl)imide ([Hbet][NTf2]), interactions to metal cations occur through the carboxyl group at the cation. In a typical dissolution of a metal oxide, this functional group protonates the oxygen atom of the metal oxide yielding water, while the remaining carboxylate group, subsequently, coordinates to the metal cation,4 as illustrated in Fig. 1.
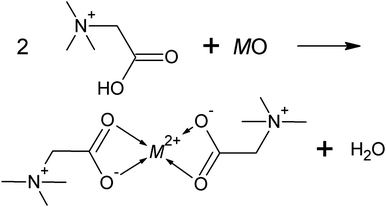 | ||
| Fig. 1 Schematic reaction of a metal oxide and [Hbet][NTf2] resulting in a coordination compound and water (M2+ = metal ion). | ||
Numerous of such metal–betaine complexes were investigated by Nockemann et al., who also noted the crucial role of water as an additional ligand. Thus, the IL [Hbet][NTf2] was merely used as a reactant in an aqueous solution.4,6 Other ILs with a slightly varied cation structure, but the carboxyl group still being present, show a similar reaction behaviour with metal oxides.5
The possibilities of dissolving metal oxides in as far as possible water-free [Hbet][NTf2] was investigated by the group of Binnemans in the course of recycling Nd2Fe14B magnets. 175 °C was identified as the optimal reaction temperature, avoiding the decompositions of the IL, but supporting reaction kinetics at the same time. A significant increase in reaction time in the absence of water as well as a decrease in the coordination number of the metal ion was reported. This is attributed to the [Hbet]+ cation being too sterically demanding to saturate all coordination sites. For this purpose, smaller, additional ligands appear to be necessary, such as water or chloride anions. As demonstrated in a first experiment, the addition of the latter resulted in enhanced dissolution rates.8
Based on these findings, in the present work, the dissolution behaviour of various metal oxides in the IL [Hbet][NTf2] was investigated. Different from the approach of Nockemann et al.4–6 but similar to the work of Binnemans,8 no additional water was used as this usually involves two major problems regarding downstream chemistry: first, with O-coordinated aqua ligands, a leaching of metal ions from potentially resulting complexes or ligand exchange reactions might prove difficult. Second, the relatively small electrochemical window of water often involves the evolution of oxygen and hydrogen gases in electrolysis. Instead, the complex formation ability of metal oxides with betaine was investigated in pure [Hbet][NTf2] and compared to reaction mixtures of the IL and betaine hydrochloride ([Hbet]Cl) as additional ligand source. The long-term goal of these studies is the application to downstream chemistry of metal oxides without the detour over the reduced metal as well as the metal separation from natural sources via leaching and subsequent electrolysis. However, the present work is concerned with the initial step of these problems, the dissolution and complex formation of metal oxides in the ILs [Hbet][NTf2] and [Hbet]2[NTf2]Cl.
Experimental
Chemicals
[Hbet]Cl (99%), Bi2O3 (99%), Cr2O3 (98%), Cu2O (99%), CuO (99.9995%), SnO (p.a.) and SrO (99.5%) were purchased from Alfa Aesar. Al2O3 (p.a.), Nb2O5 (p.a.) and TiO2 (99%) were purchased from Merck. BaO (97%), Fe2O3 (99%), MgO (p.a.) and V2O5 (99.5%) were obtained from Riedel de Haën. Ga2O3 (99.99%), GeO2 (99.999%), MoO3 (99.999%), Sb2O3 (99.6%) and V2O3 (95%) were purchased from ABCR. In2O3 (99.999%) was obtained from Acros. PbO (99%) and PbO2 (p.a.) were purchased from Sigma Aldrich. LiNTf2 (80% solution in water) and trihexyltetradecylphosphonium chloride ([P66614]Cl, 95%) were purchased from Iolitec. NiO (p.a.) originates from VEB Berlin-Chemie, Co3O4 (p.a.) from Chempur and CaO (p.a.) as well as WO3 (p.a.) from Reachim. MnO (99%) and MnO2 (85–90%) were purchased from Sigma-Aldrich. ZnO (p.a.) was obtained from Grillo.ReO3 was synthesised from Re powder (99.5%, Alfa Aesar) by repeated O2 addition and subsequent evacuation in a silica ampoule, resulting in Re2O7. A subsequent chemical transport reaction with I2 from 500 °C to 400 °C yielded ReO3.
PXRD of ThO2 revealed the presence of an unidentified impurity (Fig. S1, ESI†). Similarities with the diffraction pattern of several intermetallic oxide phases suggest (intermediate) products of the ThO2 decay chain. Caution! ThO2 is a weak alpha emitter. All radioactive materials were handled in radioactively controlled facilities that are equipped with personal safety equipment.
All chemicals were used without any further purification, except for CaO, which was dried at 1000 °C after purchase.
Synthesis of [Hbet][NTf2]
The IL [Hbet][NTf2] was synthesized, based on the experimental procedure described in the literature,4 by stirring 0.1 mol [Hbet]Cl dissolved in 50 ml water and LiNTf2 solution equivalent to 0.1 mol for one hour. Subsequently, the IL was separated and washed with ice cold water until testing with AgNO3 solution indicated the absence of chloride ions, typically five times. The IL was dried under vacuum at a rotary evaporator at 60 °C for one hour and at a Schlenk line at 110 °C overnight.Dissolution experiments of metal oxides
Dissolution experiments in the pure IL were performed by heating a metal oxide and [Hbet][NTf2] in the molar ratio of nM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nIL = 1
nIL = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 175 °C for 24 h in a system open to the air.
4 to 175 °C for 24 h in a system open to the air.
For the reaction of Bi2O3 and [Hbet][NTf2] in the absence of air, the starting materials were weighted in an argon-filled glovebox (M. Braun; p(O2)/p0 < 1 ppm, p(H2O)/p0 < 1 ppm). The reaction was performed for 24 h at 175 °C under flowing argon gas. After cooling to room temperature, the sample was handled on air.
Chloride containing mixtures were prepared from a metal oxide, [Hbet][NTf2] and [Hbet]Cl in the molar ratio of nM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n[Hbet][NTf2]
n[Hbet][NTf2]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n[Hbet]Cl = 1
n[Hbet]Cl = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and heated likewise.
2 and heated likewise.
For several metal oxides, mixtures with varying molar ratios or reaction times were investigated. Details are listed in Table S4, ESI.†
Work-up of the CuO samples was performed by the addition of acetone, thus separating CuO. The product yield η was determined by weighting the unreacted CuO and using the following formula.
The product could not be obtained phase-pure, as no solvent solely dissolving or not dissolving this complex compound was found. Nevertheless, the product was suitable to perform ligand exchange experiments.
Calculation of lattice energies and U/x values
Lattice energies U of the metal oxides MxOy were calculated according to literature9 with a Born–Haber cycle as phrased in the following equation.ΔHf standard molar enthalpy of formation of MxOy, ΔHs enthalpy of sublimation of M, Ii ionisation energy (i = 1, 2, 3, …), B bond enthalpy of O2, EAi electron affinity of O (i = 1, 2).
For metals whose enthalpy of sublimation was not tabulated, the sum of the enthalpy of fusion (ΔHm) and the enthalpy of vaporisation (ΔHv) was used. The resulting slight error is <2%.9 Furthermore, the ionisation energy of the Co(II,III) mixed oxide Co3O4 was calculated according to 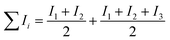 .
.
U/x was calculated by dividing the lattice energy by the number of metal atoms in the chemical formula.
ΔHf values were obtained from Thermochemical Data of Pure Substances,10 ΔHs, ΔHm, ΔHv and B from Lange's Handbook of Chemistry11 and Ii and EAi values from the NIST online database.12
Ligand exchange experiments
Approximately 50 mg of the reaction product of CuO and [Hbet][NTf2] was stirred in 1 g [P66614]Cl at 60 °C for 19 h.Characterisation
Powder X-ray diffraction (PXRD) was performed on two similarly constructed X'Pert Pro and Empyrean diffractometers (PANanalytical) at 296 K equipped with a curved Ge(111) monochromator in Bragg–Brentano geometry using Cu-Kα1 radiation (λ = 154.0598 pm). Rietveld refinement was performed with TOPAS13 by using the fundamental parameter approach and Le Bail method for modelling preferred orientation.Infrared (IR) measurements were performed on a Bruker Vertex 70 FTIR spectrometer with attenuated total reflection (ATR) accessory in a radiation range from 500 cm−1 to 4000 cm−1 and 32 scans per measurement. Data analysis was performed with the programme OPUS.14
For Raman spectroscopy, a FT-Raman spectrometer Bruker RFS 100 was used with an excitation wavelength of 1064 nm and 50 to 200 scans per measurement. Due to the strong dispersion of radiation in coloured substances and the resulting high background, additional scans were taken for these samples. Analysis of the data obtained was performed with the programme OPUS.14
NMR samples were prepared by dissolving a spatula tip of the samples in deuterated dimethylsulfoxide (DMSO-d6) as deuterium lock and filling a NMR tube to a height of approximately 3 cm. 1H NMR spectra were recorded on a Bruker Avance WB NMR 300 MHz spectrometer with the resonance frequency 300 MHz.
The collection of diffraction intensities of single-crystals (SCXRD) for crystal structure analysis took place on a single-crystal X-ray diffractometer SuperNova (Rigaku Oxford Diffraction) with Cu-Kα radiation (λ = 154.184 pm) at 100 K under flowing nitrogen gas. Empirical multi-scan absorption corrections were applied to the data. Structure solution was performed with the programme SHELXT15 in OLEX2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 via intrinsic phasing. For structure refinement, the method of full-matrix least squares on Fo2 with the programme SHELXL15 in OLEX2
16 via intrinsic phasing. For structure refinement, the method of full-matrix least squares on Fo2 with the programme SHELXL15 in OLEX2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 was used. Non-hydrogen atoms were refined with anisotropic displacement parameters, while hydrogen atoms were refined with riding coordinates and displacement parameters. The crystal structure was plotted in the programme Diamond 4.5.2.17 The full crystallographic data is deposited at the Cambridge Crystallographic Data Centre, deposit number CCDC 1947513.†
16 was used. Non-hydrogen atoms were refined with anisotropic displacement parameters, while hydrogen atoms were refined with riding coordinates and displacement parameters. The crystal structure was plotted in the programme Diamond 4.5.2.17 The full crystallographic data is deposited at the Cambridge Crystallographic Data Centre, deposit number CCDC 1947513.†
For energy dispersive X-ray spectroscopy (EDX), a drop of a sample dispersed in acetone was placed on a silicon wafer fixed on a SEM sample holder and stored in a vacuum chamber for three days. The studies were performed on a Hitachi SU8020 scanning electron microscope with an Oxford Silicon Drift Detector (SDD) X-MaxN. An accelerating voltage of 25 kV was used.
Results and discussion
Dissolution of metal oxides in water-free [Hbet][NTf2]
After synthesis of the IL, it became apparent from PXRD studies that the IL consists of a mixture of approximately 66% [Hbet][NTf2] and 34% [(Hbet)3(bet)][NTf2]3. The powder X-ray diffractograms as well as the Rietveld refinement are shown in Fig. S2 and S3, ESI.† The fact of roughly one sixth of the cations being unprotonated betaine might affect the maximum amount of a metal oxide dissolvable in the IL. However, as the experiments performed in this work are of rather qualitative than quantitative nature, the resulting error is considered insignificant. In the following, the synthesized IL is referred to as [Hbet][NTf2].For all dissolution experiments in the pure IL, a mixture of a metal oxide and [Hbet][NTf2] was heated to 175 °C for 24 h in a system open to the air to allow the evaporation of the arising water. Metal oxides were chosen in order to cover a wide range of different properties regarding the position of the metal in the periodic table, its oxidation state as well as the basicity of the oxide. An overview about their dissolution behaviour in [Hbet][NTf2] as well as [Hbet]2[NTf2]Cl is given in Table 1. The tested metal oxides included Al2O3, BaO, Bi2O3, CaO, Co3O4, Cr2O3, Cu2O, CuO, Fe2O3, Ga2O3, GeO2, In2O3, MgO, MnO, MnO2, MoO3, Nb2O5, NiO, PbO, PbO2, ReO3, Sb2O3, SnO, SrO, ThO2, TiO2, V2O3, V2O5, WO3 and ZnO. In order to provide enough ligands for complex formation, but at the same time minimise interfering signals of the unreacted IL, a typical metal ion-IL ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 was chosen. With this set-up, BaO, Bi2O3, CaO, Cu2O, CuO, MgO, MnO, PbO, PbO2, SrO, V2O3 and ZnO could successfully be dissolved in the IL, while Al2O3, Cr2O3, Fe2O3, Ga2O3, GeO2, In2O3, MnO2, MoO3, Nb2O5, NiO, ReO3, Sb2O3, ThO2, TiO2 and WO3 visually resulted in suspensions of the reagents. Only the liquid of the SnO sample was coloured brown, however, the 1H NMR spectrum shown in Fig. S9, ESI† reveals an intact IL. The colour of the liquid might result from very fine, dispersed SnO particles. An overview about the products obtained is given in Table S1, ESI.† In agreement with this, all reflections in the PXRD patterns of the latter samples could be attributed to the starting materials (see Fig. S4 and S5, ESI†). In contrast to this, the eight soluble metal oxides BaO, CaO, MgO, MnO, PbO, PbO2, SrO and ZnO gave homogenous solutions or pastes, whereby the high viscosity of the latter products could be lowered by using a higher amount of [Hbet][NTf2]. Apart from the relatively low-viscosity liquids of the CaO and MgO samples, crystallisation occurred during cooling or upon application on the PXRD sample holder, giving completely unidentified reflection patterns. Reflections at low 2θ angles suggest a large unit cell that might result from metal–betaine complex compounds.
4 was chosen. With this set-up, BaO, Bi2O3, CaO, Cu2O, CuO, MgO, MnO, PbO, PbO2, SrO, V2O3 and ZnO could successfully be dissolved in the IL, while Al2O3, Cr2O3, Fe2O3, Ga2O3, GeO2, In2O3, MnO2, MoO3, Nb2O5, NiO, ReO3, Sb2O3, ThO2, TiO2 and WO3 visually resulted in suspensions of the reagents. Only the liquid of the SnO sample was coloured brown, however, the 1H NMR spectrum shown in Fig. S9, ESI† reveals an intact IL. The colour of the liquid might result from very fine, dispersed SnO particles. An overview about the products obtained is given in Table S1, ESI.† In agreement with this, all reflections in the PXRD patterns of the latter samples could be attributed to the starting materials (see Fig. S4 and S5, ESI†). In contrast to this, the eight soluble metal oxides BaO, CaO, MgO, MnO, PbO, PbO2, SrO and ZnO gave homogenous solutions or pastes, whereby the high viscosity of the latter products could be lowered by using a higher amount of [Hbet][NTf2]. Apart from the relatively low-viscosity liquids of the CaO and MgO samples, crystallisation occurred during cooling or upon application on the PXRD sample holder, giving completely unidentified reflection patterns. Reflections at low 2θ angles suggest a large unit cell that might result from metal–betaine complex compounds.
| Metal oxide | U [kJ mol−1] | U/x [kJ mol−1] | Solubility in [Hbet][NTf2] | Solubility in [Hbet]2[NTf2]Cl |
|---|---|---|---|---|
| Al2O3 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 464 464 |
7732 | Negligible | Negligible |
| BaO | 3121 | 3121 | Full | Full (BaCl2 precipitation) |
| Bi2O3 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318 318 |
6659 | Low (in Ar flow) | Full (BiOCl precipitation) |
| CaO | 3486 | 3486 | Full | Full |
| Co3O4 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 067 067 |
6022 | Low (reduction) | Full |
| Cr2O3 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 252 252 |
7626 | Negligible | Negligible |
| Cu2O | 3290 | 1645 | High | Full (CuCl precipitation) |
| CuO | 4150 | 4150 | High | Full |
| Fe2O3 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 078 078 |
7539 | Negligible | Low |
| Ga2O3 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 511 511 |
7756 | Negligible | Negligible |
| GeO2 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852 852 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 852 852 |
Negligible | Negligible |
| In2O3 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 439 439 |
7220 | Negligible | Negligible |
| MgO | 3889 | 3889 | Full | Full |
| MnO | 3798 | 3798 | Full | Full |
| MnO2 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 075 075 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 075 075 |
Negligible | Full |
| MoO3 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 909 909 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 909 909 |
Low | Low |
| Nb2O5 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030 030 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 015 015 |
Negligible | Negligible |
| NiO | 4077 | 4077 | Negligible | Low |
| PbO | 3532 | 3532 | Full | Full (PbCl2 precipitation) |
| PbO2 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706 706 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706 706 |
Full | Full (PbCl4 formation) |
| ReO3 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 433 433 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 433 433 |
Negligible | Negligible |
| Sb2O3 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 760 |
6880 | Negligible | High |
| SnO | 3662 | 3662 | Negligible | Full |
| SrO | 3322 | 3322 | Full | Full |
| ThO2 | 9967 | 9967 | Negligible | High |
| TiO2 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 114 114 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 114 114 |
Negligible | Negligible |
| V2O3 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 890 890 |
7445 | Full | High |
| V2O5 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 738 738 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 369 369 |
Low (reduction) | Full |
| WO3 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 312 312 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 312 312 |
Negligible | Negligible |
| ZnO | 4074 | 4074 | Full | Full |
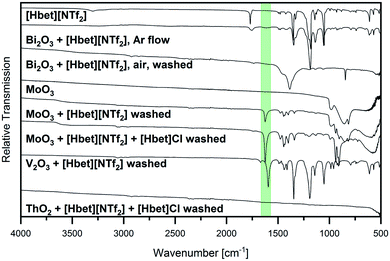
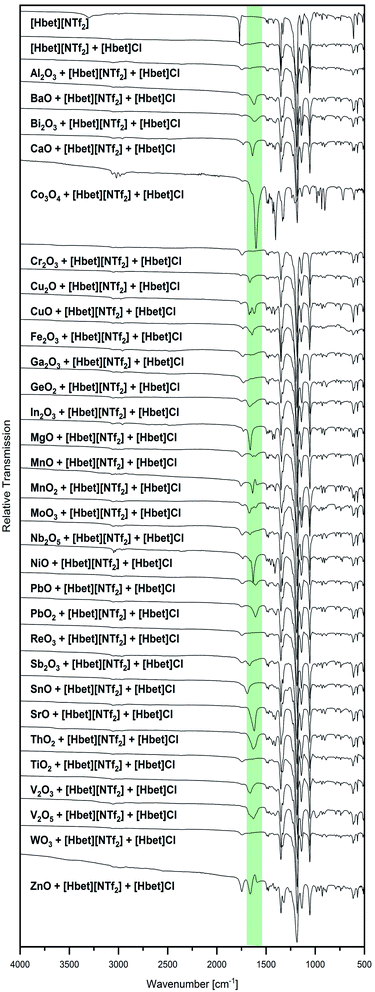
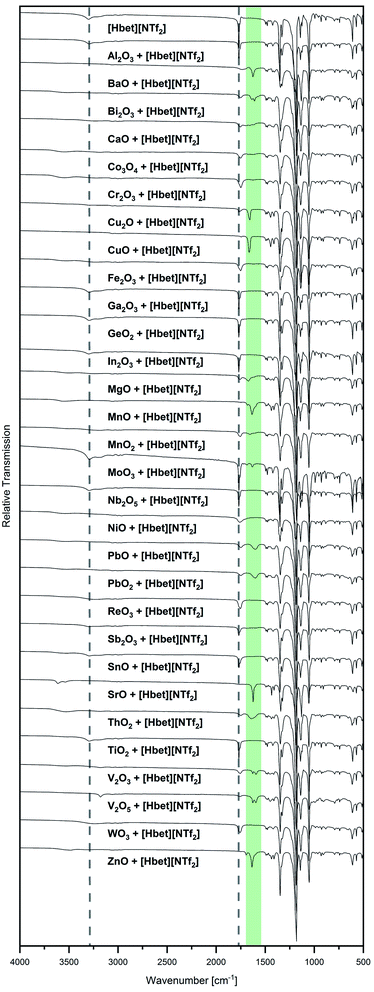
Besides visual examination and PXRD, evidence for complex formation is given by IR spectroscopy, where two bands attributed to the [Hbet]+ cation are a good indicator: the asymmetric stretching vibration of the carboxyl OH (νas OH) at 3301 cm−1 and the asymmetric stretching vibration of COO (νas COO) at 1770 cm−1 in the spectrum of the pure IL, which are marked with dotted lines in Fig. 2. Upon coordination, νas OH disappears due to the deprotonation of the carboxyl group. Meanwhile, a shift of νas COO to lower wavenumbers can be observed as the excitation of this vibration needs less energy when the carboxylate group binds to a heavier metal atom instead of a proton. In agreement with visual observations and PXRD patterns, no shift of νas COO is found for the samples of Al2O3, Co3O4, Cr2O3, Fe2O3, Ga2O3, GeO2, In2O3, MnO2, Nb2O5, NiO, ReO3, Sb2O3, SnO, TiO2 and WO3, indicating no significant dissolution of these metal oxides in [Hbet][NTf2]. As highlighted in green, a shift of this band as well as the disappearance of νas OH is observed for BaO, Bi2O3, CaO, Cu2O, CuO, MgO, MnO, PbO, PbO2, SrO, V2O3 and ZnO. The lack of correlation between the metal atom mass and the wavenumber of νas COO is attributed to different possible coordination modes of betaine, i.e. mono-, bidentate or bridging.
The IR spectra of several samples suggest the presence of metal–betaine complexes, however, precipitations occur. For CuO, no complete reaction was achieved, but small amounts of the starting material were present in a blue solution from which blue crystals precipitated. The same blue compound was obtained from Cu2O, as identified by PXRD, indicating the oxidation of copper(I). Similarly, the V2O3 sample contained a greenish blue powder in a colourless liquid. In order to clarify the nature of the solid, it was washed with acetone, hence, removing the IL. IR spectroscopy suggests the presence of V3+–betaine complexes due to the bands typical for betaine and [NTf2]− but νas COO shifted to 1595 cm−1 (highlighted in green in Fig. 3). Furthermore, from Bi2O3, a white powder in a colourless liquid was obtained. After washing with acetone, it was identified as (BiO)2CO3 by PXRD (Fig. S5, ESI†). Bi2O3 is known to readily react with the acid anhydride CO2 to (BiO)2CO3,18 however, [Hbet][NTf2], apparently, significantly catalyses this reaction. In agreement with this, the IR spectrum in Fig. 3 is typical for this compound according to literature,18 indicating the absence of solid bismuth(III)–betaine complex compounds. The repetition of the reaction under argon flow reveals a significantly lower reactivity of the reactants. Meeting general expectations, no reaction with CO2 takes place, but a suspension of a yellow powder in a colourless liquid forms. By PXRD, only reflections corresponding to the reagent Bi2O3 are found, which already contained some (BiO)2CO3 (Fig. S6, ESI†). However, the IR spectrum, displayed in Fig. 3, shows two νas COO bands at 1755 cm−1 and 1619 cm−1. This suggests a majority of unreacted IL alongside with a small amount of betaine coordinated to bismuth. Therefore, Bi2O3 is assumed to have a low solubility in [Hbet][NTf2]. The fast reaction on air might be ascribed to the precipitation of (BiO)2(CO3) shifting the reaction to the product side.
Some kind of special behaviour was also observed for Co3O4 and V2O5. Very small amounts of both oxides dissolve in the IL, yielding a violet, respectively green or sometimes brown liquid. As both metal oxides are strong oxidising agents,2 their partly dissolution is attributed to the oxidation of the IL. This is supported by the NMR spectra displayed in Fig S9,† where the occurrence of additional signals compared to pure [Hbet][NTf2] suggests the partial decomposition of the IL. In agreement with this, the PXRD pattern of the V2O5 sample product, shows several unidentified reflections besides the unreacted metal oxide. These might result from crystallised decomposition fragments of the IL. IR bands of low intensity at 1630 cm−1 and 1598 cm−1 are also attributed to the decomposition of the IL or small amounts of vanadium(III)–betaine complexes. However, it also has to be taken into account, that the synthesis of [Hbet][NTf2] does not guarantee the complete absence of chloride. Therefore, also the oxidation of chloride impurities to chlorine by cobalt(III) and vanadium(V) might play a role in the dissolution of the metal oxides.
Several low intensity IR bands in the range highlighted in green in Fig. 2 can also be found for the sample of MoO3, despite the unreacted appearance of the product as well as PXRD only identifying the reagents. IR spectroscopy of the remaining white powder washed with acetone gives evidence for the presence of molybdenum–betaine complexes, as shown in Fig. 3. The low intensity bands in the range 1300 cm−1 to 1500 cm−1 suggest the presence of betaine, while the nonappearance of the bands at lower wavenumbers might indicate the absence of [NTf2]− anions. Furthermore, the occurrence of νas COO at 1626 cm−1, but no band at 1770 cm−1 gives evidence for the presence of betaine only in a coordinated state. Accordingly, MoO3 is suspected to show a low solubility in [Hbet][NTf2]. As a reason for this behaviour deviating from other MO3 type metal oxides, such as WO3 and ReO3, the crystal structure might serve. In contrast to the latter salts, MoO3 is built from layers of MoO6 octahedrons, which are expected to be affected by dissolution significantly easier than 3D cross-linked structures.
Similarly, the sample of ThO2 appears like a suspension of the reagents, but the IR spectrum shows a band at 1640 cm−1. However, PXRD of the starting material reveals some minor reflections besides the main pattern of ThO2, which could not be assigned to a phase and might result from the decay chain of ThO2 (Fig. S2, ESI†). Therefore, it is assumed that ThO2 does not dissolve in [Hbet][NTf2], but merely the impurities.
In order to substantiate these experimental observations, the dissolved, respectively undissolved metal oxides were investigated for common properties. A method to estimate the solubility of metal oxides in water-saturated [Hbet][NTf2] was recently suggested by Fan et al. who proposed a correlation between the lattice energy of a metal oxide and its solubility in the IL. Therefore, they introduced the U/x value of a metal oxide MxOy where U is the lattice energy. For U/x < 7000 kJ mol−1, a good solubility of metal oxides was found while U/x > 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kJ mol−1 indicated metal oxides insoluble in water-saturated [Hbet][NTf2]. Corresponding to their method,9 we calculated the lattice energies for all metal oxides examined experimentally by using the Born–Haber cycle. The resulting U/x values are listed in Table 1, detailed information about the data used for calculation can be found in Table S2, ESI.† In agreement with the assumptions of Fan et al., the U/x value corresponds to the experimental results in most cases. Thus, also for systems of water-free [Hbet][NTf2], the dissolution of metal oxides can be expected for U/x < 7000 kJ mol−1. However, there are some exceptions from this general rule.
000 kJ mol−1 indicated metal oxides insoluble in water-saturated [Hbet][NTf2]. Corresponding to their method,9 we calculated the lattice energies for all metal oxides examined experimentally by using the Born–Haber cycle. The resulting U/x values are listed in Table 1, detailed information about the data used for calculation can be found in Table S2, ESI.† In agreement with the assumptions of Fan et al., the U/x value corresponds to the experimental results in most cases. Thus, also for systems of water-free [Hbet][NTf2], the dissolution of metal oxides can be expected for U/x < 7000 kJ mol−1. However, there are some exceptions from this general rule.
So no sign for dissolution is found for NiO despite its low lattice energy. Furthermore, Sb2O3 and V2O3 both possess U/x values around 7000 kJ mol−1, Co3O4 even lower with 6022 kJ mol−1, but only V2O3 with the highest U/x dissolves in [Hbet][NTf2]. Fan et al. also pointed out that there is no solubility for MO2 type metal oxides due to their typically high lattice energies. However, in our experiments, PbO2 formed a clear solution with the IL. Similarly, the partly dissolution of MoO3 is unexpected according to this rule, because of the high lattice energy. These deviations are in agreement with the report by Jayachandran et al. who claim that an increased temperature allows a significant dissolution of PuO2 in water-saturated [Hbet][NTf2],19 although predicted insoluble by Fan et al. with U/x = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 381 kJ mol−1.9
381 kJ mol−1.9
From these observations it is concluded that the lattice energies of metal oxides, respectively their U/x values, merely allow a rough assessment of their solubility in [Hbet][NTf2]. The assumed reason is the lattice energy not being the only factor influencing solubility. Hence, the reaction conditions, such as temperature might have a significant influence, as assumed by Jayachandran et al.19 As illustrated by the example of MoO3 (layered structure) in contrast to WO3 and ReO3 (3D frameworks), the crystal structure of the starting materials might also have an effect on their disintegration. Furthermore, the product side of the reaction should not be neglected as complex formation constants as well as a possible change in the coordination number of the metal ion could affect the dissolution behaviour. A significant influence on the dissolution, in our opinion, also originates from the acidity or basicity of the metal oxide. Apparently, in the here performed experiments only relatively basic or amphoteric oxides readily react with the IL, meeting general expectations as [Hbet]+ is an acidic cation. However, these suggestions are based on qualitative observations that should be supported by measuring data. Yet, to our knowledge, no comprehensive table about the acid–base properties of metal oxides exists to provide such evidence.
Dissolution of CuO and downstream chemistry
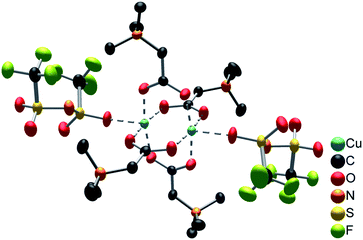
The compound [Cu2(bet)4(NTf2)2][NTf2]2 consists of cationic [Cu2(bet)4(NTf2)2]2+ complexes and non-coordinating [NTf2]− anions, as shown in Fig. S7, ESI.† The centroids of the complex cations (inversion centres) are located on the corners and in the centre of the unit cell. The dinuclear complex cation [Cu2(bet)4(NTf2)2]2+, displayed in Fig. 4, has a paddle-wheel structure with two Cu2+ ions coordinated by four μ-bridging betaine zwitterions via the carboxylate group. The distorted octahedral geometry is completed by Cu–Cu interactions (d(Cu–Cu) = 262.7 pm) and weakly O-coordinating [NTf2]− anions at the axial positions. The coordinating oxygen atoms of betaine form an almost planar square around each copper(II) ion in equal distance (194.5–196.5 pm). The cis angles between two adjacent betaine ligands (Obet–Cu–Obet) range from 88.6° to 90.6° while the trans angles between two opposing ligands amount to 168.8° indicating a considerable deviation from planarity. Perpendicular to this plane, a [NTf2]− anion coordinates via an oxygen atom. The distance of 215.8 pm being 10% longer than the distance between copper(II) and the coordinating oxygen atom of betaine, indicates weaker interactions.
The very similar complex cation [Cu2(bet)4(H2O)2]2+ was found in the compound [Cu2(bet)4(H2O)2][NTf2]6 by Nockemann et al. with two H2O molecules coordinating to copper(II) instead of the [NTf2]− anions.4 The distance between two copper(II) ions in this compound (265.7 pm) is just slightly larger than in [Cu2(bet)4(NTf2)2]2+, while the distance between copper(II) and the coordinating oxygen atom of betaine lies in the same range (195.8–197.3 pm). Weaker interactions are observed between copper(II) and the coordinating aqua ligand (d(Cu–OH2O) = 213.3 pm).4 Also the structure-related complex [Cu2(betmMor)4(NTf2)2]2+ (betmMor = N-carboxy-methyl-N-methylmorpholinium) in the compound [Cu2(betMor)4][NTf2]4 represents a paddle-wheel complex with four betmMor ligands μ-bridging two copper(II) ions and, perpendicular to this plane, two O-coordinating [NTf2]− anions. The bridging betMor ligand is structurally related to betaine with two methyl groups on N replaced by a heterocyclic morpholine function. The interatomic distances (d(Cu–Cu) = 262.8 pm, d(Cu–ObetmMor) = 196.1 pm and d(Cu–O[NTf2]−) = 218.3 pm)5 are in good agreement with the complex found in this work.
Further, similar [Cu2(bet)4]4+ paddle-wheel structures were reported, whereby the Cu2+ coordination sphere is completed by chloride and bromide anions instead of [NTf2]−. Examples are the complex [Cu2(bet)4Cl2]2+ in the compound [Cu2(bet)4Cl2]Cl2·4H2O20 and [Cu2(bet)4Br2]2+ in the compounds [Cu2(bet)4Br2]Br2·2H2O21 and [Cu2(bet)4Br2][CuBr4]·H2O.22 The coordination of the strong chloride and bromide ligands results in a relatively large distance between the two copper(II) ions (276.6 pm, 276.8 pm, 278.3 pm, respectively).20–22 This supports the assumption of weaker interactions between copper(II) and the [NTf2]− ligand in [Cu2(bet)4(NTf2)2]2+ with a significantly shorter d(Cu–Cu).
Many further examples of copper paddle-wheel structures showing the same characteristics, such as the Cu–Cu distance, the distance ratio of coordination bonds and a distorted octahedral geometry, are known in literature.23,24
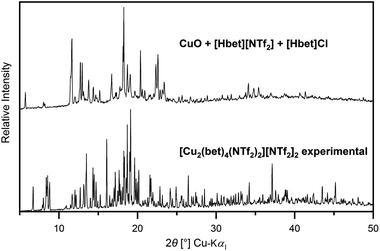
A comparison of the experimental PXRD pattern of the CuO–[Hbet][NTf2] reaction product to the one simulated from single-crystal data of [Cu2(bet)4(NTf2)2][NTf2]2 gives evidence for the phase impurity of the synthesis product, as shown in Fig. S8, ESI.† All theoretical reflections turn up in the experimental pattern, whereby the shift to lower 2θ values meets the expectations due to the temperature difference of PXRD (RT) and SCXRD (100 K) measurements. By Rietveld refinement, a room temperature unit cell was obtained with 0.9%, 1.5% and 1.6% enlarged a, b and c lattice parameters, respectively (a = 1459.0 pm, b = 1470.2 pm, c = 1522.9 pm, β = 95.3°).
Besides, apparently, another unidentified phase is present, as numerous reflections cannot be ascribed to any known phase. Attempts to determine a possible unit cell were not successful, while a microscopic separation by crystal morphology also is not possible due to irregular crystal shapes. However, due to the low 2θ angles of several reflections, indicating a similarly large unit cell, another unknown copper–betaine compound phase should be considered.
Stirring a mixture of the blue complex compound and [P66614]Cl, an immediate yellow colouring of the liquid as well as an discolouration of the blue powder after several hours is observed. For a closer investigation of the processes taking place, a crystal of [Cu2(bet)4(NTf2)2][NTf2]2 was covered with one drop of [P66614]Cl, revealing a discolouration of the crystal beginning from the surface accompanied by the yellow colouring of the IL in the surroundings, as shown in Fig. 5. The remaining colourless crystal was identified as [Hbet]Cl by SCXRD. It crystallises in the monoclinic space group P21/c with the cell parameters a = 743.6 pm, b = 898.4 pm, c = 1150.0 pm and β = 97.0° at 100 K, in agreement with literature.25,26
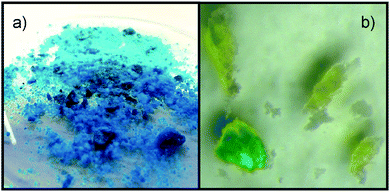 | ||
| Fig. 5 Images of (a) [Cu2(bet)4(NTf2)2][NTf2]2 and (b) the discolouration in [P66614]Cl accompanied by the yellowing of the surrounding IL. | ||
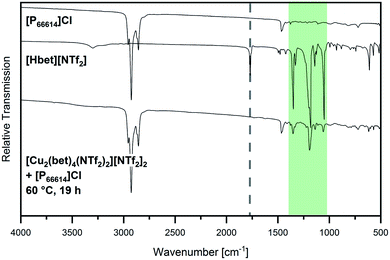
The consequent conclusion of [Hbet]+ cations not being dissolved in [P66614]Cl is supported by IR spectroscopy, as shown in Fig. 6. Clearly observable are the main peaks of [P66614]Cl, but also the most intensive signals of [Hbet][NTf2] are visible in the spectrum of the sample (highlighted in green). According to literature27–30 all of them are attributed to vibrational modes of the [NTf2]− anion (see Table S3, ESI†), while νas COO at 1770 cm−1 (respectively the shifted signal of coordinated [Hbet]+ at 1655 cm−1) is not observed despite its medium intensity.
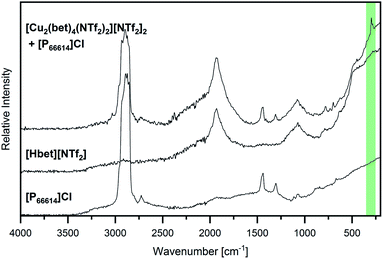
Further evidence of copper(II) being leached from the [Cu2(bet)4(NTf2)2]2+ complex is given by the Raman spectrum displayed in Fig. 7. A band at 297 cm−1 suggests a Cu–Cl stretching vibration (νs CuCl, highlighted in green),31,32 while no signal is found around 385 cm−1, where Cu–O vibrations would be expected.31 Furthermore, Suffren et al. reported a correlation of the band position of νs CuCl and the trans angles in [CuCl4]2− complex anions, whereby they found a shift to higher wavenumbers with increasing tetrahedral distortion over a square planar geometry.33 Therefore, the experimental Raman band at a relatively high wavenumber is in good agreement with the yellow colour of the solution suggesting a distorted tetrahedral geometry for [CuCl4]2−. A distorted tetrahedron is the high-temperature phase of the thermochromic [CuCl4]2−. Despite being the electrostatically more favoured geometry, it is assumed that the square-planar RT phase is stabilized by hydrogen bonds at lower temperatures.34 As in an environment of [P66614]+ cations, hydrogen bonds to [CuCl4]2− are not likely, the distorted tetrahedral geometry of this complex anion seems reasonable.
Altogether, these investigations suggest that copper(II) ions are leached from the Cu–betaine complex and form [CuCl4]2− complex anions in a distorted tetrahedral geometry. Thus, a complete exchange of the O-coordination sphere around copper(II) by chloride could be shown, opening promising possibilities for downstream chemistry starting from metal oxides.
The role of chloride for the dissolution of metal oxides
The role of a strong nucleophile as potential additional ligand in the dissolution of metal oxides was investigated by the addition of [Hbet]Cl, thus introducing chloride anions without any other, possibly interfering cations. Typically, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of [Hbet][NTf2] and [Hbet]Cl was used. Samples with the ratio of metal cations to [Hbet]2[NTf2]Cl of 1
1 mixture of [Hbet][NTf2] and [Hbet]Cl was used. Samples with the ratio of metal cations to [Hbet]2[NTf2]Cl of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 were investigated under the same conditions like in the previous experiments. However, 1H NMR spectroscopy gives evidence for the decomposition of the IL by a significant increase in the number of signals observed. In the spectrum of [Hbet][NTf2] displayed in Fig. S9, ESI,† the peaks at 3.1 ppm and 4.1 ppm are attributed to the protons of the CH3 and CH2 groups of betaine, respectively. Another signal of the carboxyl proton is not observed which is attributed to its low intensity and the expected broadness.35 Due to the complexity of the spectrum obtained from heated [Hbet]2[NTf2]Cl, no attribution to specific decomposition fragments was realised, yet. Concomitant in the IR spectrum of the resulting brown liquid (Fig. 8), the sharp, medium-intensity νas COO band at 1770 cm−1 transforms into two low intensity bands at 1743 cm−1 and 1666 cm−1. The first band is attributed to νas COO of undecomposed [Hbet]+, while the second band is assumed to result from a not yet identified decomposition fragment. In Fig. S9, ESI,† 1H NMR spectra of several samples give evidence for the decomposition of the IL taking place in mixtures with metal oxides as well. Further studies of several systems indicate, that this can be reduced by increasing the amount of [Hbet][NTf2] as well as by decreasing the reaction time.
2 were investigated under the same conditions like in the previous experiments. However, 1H NMR spectroscopy gives evidence for the decomposition of the IL by a significant increase in the number of signals observed. In the spectrum of [Hbet][NTf2] displayed in Fig. S9, ESI,† the peaks at 3.1 ppm and 4.1 ppm are attributed to the protons of the CH3 and CH2 groups of betaine, respectively. Another signal of the carboxyl proton is not observed which is attributed to its low intensity and the expected broadness.35 Due to the complexity of the spectrum obtained from heated [Hbet]2[NTf2]Cl, no attribution to specific decomposition fragments was realised, yet. Concomitant in the IR spectrum of the resulting brown liquid (Fig. 8), the sharp, medium-intensity νas COO band at 1770 cm−1 transforms into two low intensity bands at 1743 cm−1 and 1666 cm−1. The first band is attributed to νas COO of undecomposed [Hbet]+, while the second band is assumed to result from a not yet identified decomposition fragment. In Fig. S9, ESI,† 1H NMR spectra of several samples give evidence for the decomposition of the IL taking place in mixtures with metal oxides as well. Further studies of several systems indicate, that this can be reduced by increasing the amount of [Hbet][NTf2] as well as by decreasing the reaction time.
The influence of chloride anions on the dissolution of a metal oxide was investigated in detail on the example of CuO. As a result of this, a general dissolution promoting effect of chloride can be stated, however, the specific impact strongly depends on the amount of [Hbet]Cl added.
On one side, the addition of chloride in small amounts (0.3 mol% based on the total number of counter ions of [Hbet]+) reveals a strong catalytic effect. Thus, in a reaction for 24 h, the yield of [Cu2(bet)4(NTf2)2][NTf2]2 could be increased from 13% to 66% by the addition of [Hbet]Cl. Such a promoting effect of chloride anions is not uncommon, e.g. was a similar outcome observed for the synthesis of Cu3−xP from copper and red phosphorous, whereby the product yield could significantly be increased by the addition of a few mol% of [P66614]Cl to [P66614][NTf2].36
While the ideal reaction time for the sample with 0.3 mol% chloride amounts to 24 h, by the addition of equimolar amounts of [Hbet]Cl, i.e. [Hbet]2[NTf2]Cl, it reduces to only 4 h. However, besides an effect on the reaction time, also an influence on the product phase is observed. As shown in Fig. 9, the PXRD pattern does not correspond to the pattern of [Cu2(bet)4(NTf2)2][NTf2]2. Instead, the low 2θ angles of the numerous unidentified reflections suggest a large unit cell that might result from another copper–betaine complex compound. Evidence for this is found in the IR spectrum displayed in Fig. 8. A low intensity band at 1753 cm−1 might result from a small amount of uncoordinated [Hbet]+, while another band at 1669 cm−1 might be conform with the respective band in the [Cu2(bet)4(NTf2)2][NTf2]2 spectrum resulting from bridging coordination of betaine. However, two additional bands at 1655 cm−1 and 1618 cm−1 are solely found in the spectrum of the chloride-rich sample and might indicate another coordination of betaine to copper(II), i.e. monodentate or bidentate. Thus, the positive effect of chloride anions on the reaction rate, as suggested by the group of Binnemans,8 can be confirmed. Hence, this property is not unique to water, but H2O in principle appears to be substitutable by other small molecules or ions.
In order to investigate whether chloride ions are only suitable to decrease reaction time or might also support the dissolution of so far insoluble metal oxides, their effect in other reaction systems was examined. In agreement with the previous findings, significantly more metal oxides compared to the pure IL could be dissolved by the addition of chloride. Thus, in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
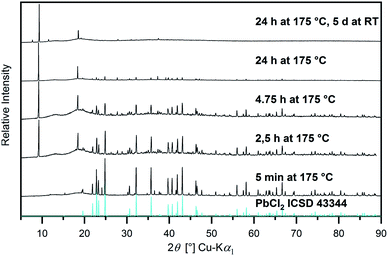
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of [Hbet][NTf2] and [Hbet]Cl, Co3O4, Fe2O3, MnO2, NiO, Sb2O3, SnO, ThO2, and V2O5 apparently form metal–betaine complexes to some extent, which does not occur in the pure IL. Additionally, also BaO, Bi2O3, CaO, Cu2O, CuO, MgO, MnO, MoO3, PbO, PbO2, SrO, V2O3 and ZnO appear to react with [Hbet][NTf2] in the presence of chloride, as well. Information about the products obtained are given in Table S4, ESI.† While Sb2O3, SrO and ZnO give clear solutions with [Hbet][NTf2] in the presence of [Hbet]Cl, for the CaO and MgO samples, solid products were obtained and for MnO, a paste. By further dilution with [Hbet][NTf2], the three metal oxides dissolved and yielded clear solutions as well. In contrast to this, for the samples of BaO and Cu2O, the powders present in the product suspensions were identified as the respective chloride salts by PXRD, while for Bi2O3, BiOCl was found. Apparently, the interactions of the metal ions with chloride are favoured over betaine, leading to precipitation following dissolution of the oxide. The reaction to chloride salts is, furthermore, found for the two lead oxides, however, they appear to be unstable as time-resolved PXRD measurements reveal (Fig. 10). Thus, for the sample of PbO a white precipitation occurs immediately after the melting of the IL and, after 5 minutes reaction time, PbCl2 is identified as the only crystalline phase by PXRD. With increasing time, the PbCl2 phase disappears, giving way for another, yet unidentified phase. This change of composition continues during storage of the sample at room temperature. It is conceivable that a partly ligand exchange reaction takes place, yielding Pb–Cl–betaine complexes or the decomposition products of the IL (Fig. S9, ESI†) might play a role in these observations. Similarly, for the sample of PbO2, a yellow colouring of the molten IL takes place, suggesting the presence of PbCl4. According to the literature,2 this metastable liquid decomposes into solid PbCl2 and Cl2 gas above 50 °C. However, PbCl2 could not be found by PXRD, which is attributed to further reactions according to the PbO sample. Several experiments in the PbO and PbO2 systems showed that a relatively large variety of diffraction patterns with unidentified phases is obtained, whereby no rational rule could be determined as causal, yet. The numerous different phases occurring during the reactions should be investigated by further experiments.
1 mixture of [Hbet][NTf2] and [Hbet]Cl, Co3O4, Fe2O3, MnO2, NiO, Sb2O3, SnO, ThO2, and V2O5 apparently form metal–betaine complexes to some extent, which does not occur in the pure IL. Additionally, also BaO, Bi2O3, CaO, Cu2O, CuO, MgO, MnO, MoO3, PbO, PbO2, SrO, V2O3 and ZnO appear to react with [Hbet][NTf2] in the presence of chloride, as well. Information about the products obtained are given in Table S4, ESI.† While Sb2O3, SrO and ZnO give clear solutions with [Hbet][NTf2] in the presence of [Hbet]Cl, for the CaO and MgO samples, solid products were obtained and for MnO, a paste. By further dilution with [Hbet][NTf2], the three metal oxides dissolved and yielded clear solutions as well. In contrast to this, for the samples of BaO and Cu2O, the powders present in the product suspensions were identified as the respective chloride salts by PXRD, while for Bi2O3, BiOCl was found. Apparently, the interactions of the metal ions with chloride are favoured over betaine, leading to precipitation following dissolution of the oxide. The reaction to chloride salts is, furthermore, found for the two lead oxides, however, they appear to be unstable as time-resolved PXRD measurements reveal (Fig. 10). Thus, for the sample of PbO a white precipitation occurs immediately after the melting of the IL and, after 5 minutes reaction time, PbCl2 is identified as the only crystalline phase by PXRD. With increasing time, the PbCl2 phase disappears, giving way for another, yet unidentified phase. This change of composition continues during storage of the sample at room temperature. It is conceivable that a partly ligand exchange reaction takes place, yielding Pb–Cl–betaine complexes or the decomposition products of the IL (Fig. S9, ESI†) might play a role in these observations. Similarly, for the sample of PbO2, a yellow colouring of the molten IL takes place, suggesting the presence of PbCl4. According to the literature,2 this metastable liquid decomposes into solid PbCl2 and Cl2 gas above 50 °C. However, PbCl2 could not be found by PXRD, which is attributed to further reactions according to the PbO sample. Several experiments in the PbO and PbO2 systems showed that a relatively large variety of diffraction patterns with unidentified phases is obtained, whereby no rational rule could be determined as causal, yet. The numerous different phases occurring during the reactions should be investigated by further experiments.
Despite the precipitation of chloride salts in several cases, the IR spectra display a νas COO band shifted to lower wavenumbers towards the pure IL (highlighted in green in Fig. 8). This suggest the presence of some amount of metal ions coordinated by betaine for these samples. The same assumption is made for MnO2, where an unidentified solid precipitation was obtained in terms of numerous colourless crystals in a brown liquid. The crystal colour clearly deviating from the black reagent suggests dissolution.
Furthermore, for the mentioned metal oxides, assumed dissolved, the reagent oxide cannot be identified by PXRD, as shown in Fig. S10 and S11, ESI.† This is different from Fe2O3, NiO, V2O3 and ThO2. From Fe2O3 a red powder identified as Fe2O3 by PXRD was obtained in a brown liquid. Additional unidentified reflections are attributed to decomposition fragments of the IL in agreement with the 1H NMR spectrum in Fig. S9, ESI.† However, two bands in the IR spectrum at 1737 cm−1 and 1641 cm−1 suggest the occurrence of uncoordinated as well as coordinated betaine. The presence of dissolved iron(III) in the separated liquid phase was confirmed by testing with aqueous KSCN and NaF solutions. Therefore, a low dissolution for Fe2O3 is assumed. For the solid NiO sample product, a medium intensity IR band at 1632 cm−1 suggests the coordination of betaine. Further evidence for the partly dissolution of NiO is given by the addition of acetone, which according to our experience is a suitable solvent for some metal–betaine complexes, but not for the respective oxides. Thus, a green liquid is obtained, indicating the presence of dissolved nickel(II) ions, alongside with green NiO powder. Similarly, for V2O3, a suspension of a brown paste and a black powder is obtained, which is identified as V2O3 by PXRD. Changing the reagent ratio to nV![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n[Hbet][NTf2]
n[Hbet][NTf2]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n[Hbet]Cl = 1
n[Hbet]Cl = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 yields a clear solution with only a few black particles. Corresponding to this, the IR spectrum shows a band at 1662 cm−1 suggesting the coordination of betaine to vanadium(III).
1 yields a clear solution with only a few black particles. Corresponding to this, the IR spectrum shows a band at 1662 cm−1 suggesting the coordination of betaine to vanadium(III).
In contrast to this, ThO2 appears to react to a white, amorphous powder. Only ThO2 is identified by PXRD, which is attributed to a small amount of black particles in the sample. Hence, no complete reaction was achieved (neither with triple the amount of IL). However, EDX gives evidence for the white powder being an organometallic thorium compound with Th, O and C in an approximate ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (Fig. S12 and Table S5, ESI†). As only semiquantitative measurement conditions were applied and H atoms cannot be detected by EDX, no assumptions of the precise composition of this compound should be made. However, as no N is detected, the presence of thorium–betaine complexes appears unlikely. Instead, the powder might be formed by thorium and a decomposition product of the IL. This is in agreement with the IR spectra of the powder washed with acetone, as shown in Fig. 3. No bands are observed for the washed powder indicating the absence of betaine as well as [NTf2]− ions. Further experiments in this system should be performed in order to investigate whether the precipitation of a thorium compound can be avoided by suppressing the decomposition of the IL and to clarify the nature of this white powder.
10 (Fig. S12 and Table S5, ESI†). As only semiquantitative measurement conditions were applied and H atoms cannot be detected by EDX, no assumptions of the precise composition of this compound should be made. However, as no N is detected, the presence of thorium–betaine complexes appears unlikely. Instead, the powder might be formed by thorium and a decomposition product of the IL. This is in agreement with the IR spectra of the powder washed with acetone, as shown in Fig. 3. No bands are observed for the washed powder indicating the absence of betaine as well as [NTf2]− ions. Further experiments in this system should be performed in order to investigate whether the precipitation of a thorium compound can be avoided by suppressing the decomposition of the IL and to clarify the nature of this white powder.
Similar to the reaction in a chloride-free system, also the dissolution of very small amounts of MoO3 is suspected due to several bands in the highlighted range in the IR spectrum. As PXRD indicates the presence of unreacted MoO3, the product was washed with acetone and again studied by IR spectroscopy, giving evidence for molybdenum–betaine complexes, as shown in Fig. 3. Hence, a low solubility of MoO3 is assumed in the presence of chloride as well as in the pure IL.
For other products, such as Al2O3, Ga2O3, GeO2, Nb2O5, ReO3, TiO2 and WO3, the reagent metal oxide is present in a brown liquid and no dissolution is assumed. The respective IR spectra typically correspond to uncoordinated betaine in a [Hbet][NTf2]–[Hbet]Cl mixture and PXRD patterns show unidentified reflections. This is attributed to decomposition fragments of the IL crystallising. For the presumably undissolved metal oxide In2O3, the two bands in the IR spectrum at 1743 cm−1 and 1666 cm−1 are present in intensity proportions deviating from metal-free [Hbet]2[NTf2]Cl. This is attributed to varied amounts of decomposition fragments of the IL, but as the band positions are similar, no complex formation is assumed. In contrast to this, in the Cr2O3 sample, the metal oxide is present in a colourless liquid. However, the 1H NMR spectrum in Fig. S9, ESI† reveals the partly decomposition of the IL. The IR spectrum does not suggest any dissolution.
Altogether, again, a general trend is observed, that very acidic metal oxides do not react with [Hbet]+, but only basic or amphoteric ones. However, as V2O5 and Co3O4 form an exception from this trend, another dissolution mechanism was identified as causal. Although the black powder in the V2O5 sample could not be identified by PXRD, it clearly is not the yellow reagent V2O5. Instead, a green colour in solution usually is associated with vanadium(III),2 suggesting a redox reaction. The IR spectra shows only one broad band at 1628 cm−1 that might result from betaine coordinated to vanadium(III). As V2O5 is known to oxidize chloride to chlorine,2 the reduction of vanadium(V) to vanadium(III) by the oxidation of chloride is conceivable. However, the 1H NMR spectrum in Fig. S9, ESI† also suggests the decomposition of the IL.
Similarly, the dissolution of Co3O4 is attributed to a redox reaction. Many small, blue crystals were obtained as reaction product. As CoCl2 could be ruled out by a different PXRD pattern and differing physical properties (less hygroscopic, different dissolution behaviour in acetone), the small crystals are assumed to consist of cobalt–betaine complexes. The blue colour, respectively the violet colour of an aqueous solution, suggest an oxidation state of cobalt(II), as cobalt(III) would be expected to form green aqua-complexes.37 This is in agreement with the known oxidising effect of cobalt(III) in the presence of chloride by the formation of chlorine,2 but the 1H NMR spectrum also suggests the decomposition of the IL. Therefore, the nature of the reductant of V2O5 and Co3O4 is uncertain, yet.
Summarising, for the here studied metal oxides, the presence of chloride in several cases has a positive effect on the dissolution. So in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of [Hbet][NTf2] and [Hbet]Cl, Co3O4, Fe2O3, MnO2, NiO, Sb2O3, SnO, ThO2, and V2O5 apparently form metal–betaine complexes, which does not occur in the pure IL. Furthermore, complex formation is assumed for BaO, Bi2O3, CaO, Cu2O, CuO, MgO, MnO, MoO3, PbO, PbO2, SrO, V2O3 and ZnO in the pure IL as well as in the presence of chloride. Altogether, the addition of chloride ions enables not only the dissolution of more metal oxides than in pure [Hbet][NTf2], but, as studied in detail for the CuO, system, also has a decreasing effect on the reaction time and might affect the product phase. This reaction-promoting effect of small co-ligands is in agreement with numerous investigations of other research groups.4,7,8,38 However, not only water molecules can be such co-ligands, but the extensive investigations in this work give evidence for the applicability of chloride ions. Nevertheless, it has to be considered, that several metal ions form not readily soluble chloride salts after dissolution, which might complicate downstream chemistry. Altogether, the dissolution of metal oxides in task-specific reaction systems seems possible. Further investigations have to be performed to determine whether the chloride ions act as permanent ligands in metal–betaine complexes or exhibit a catalytic effect. Furthermore, experiments with different small ions or molecules as potential co-ligands will be interesting.
1 mixture of [Hbet][NTf2] and [Hbet]Cl, Co3O4, Fe2O3, MnO2, NiO, Sb2O3, SnO, ThO2, and V2O5 apparently form metal–betaine complexes, which does not occur in the pure IL. Furthermore, complex formation is assumed for BaO, Bi2O3, CaO, Cu2O, CuO, MgO, MnO, MoO3, PbO, PbO2, SrO, V2O3 and ZnO in the pure IL as well as in the presence of chloride. Altogether, the addition of chloride ions enables not only the dissolution of more metal oxides than in pure [Hbet][NTf2], but, as studied in detail for the CuO, system, also has a decreasing effect on the reaction time and might affect the product phase. This reaction-promoting effect of small co-ligands is in agreement with numerous investigations of other research groups.4,7,8,38 However, not only water molecules can be such co-ligands, but the extensive investigations in this work give evidence for the applicability of chloride ions. Nevertheless, it has to be considered, that several metal ions form not readily soluble chloride salts after dissolution, which might complicate downstream chemistry. Altogether, the dissolution of metal oxides in task-specific reaction systems seems possible. Further investigations have to be performed to determine whether the chloride ions act as permanent ligands in metal–betaine complexes or exhibit a catalytic effect. Furthermore, experiments with different small ions or molecules as potential co-ligands will be interesting.
Conclusions
[Hbet][NTf2] is a suitable IL for the dissolution of various, basic or amphoteric metal oxides by the formation of metal–betaine complexes. The dissolution is assumed to be influenced by a combination of various factors, whereas the basicity is considered important, but also the lattice energy of the metal oxide, the crystal structure and the reaction temperature play a role. Furthermore, a decrease of the reaction rate in the absence of water can be confirmed. The addition of chloride ions improves the solubility of metal oxides. Thereby, the amount of chloride in the reaction mixture appears to not only have an effect on the reaction time by means of a catalyst, but also to affect the product phase.Closer investigations of the reaction of CuO in [Hbet][NTf2] revealed the new complex compound [Cu2(bet)4(NTf2)2][NTf2]2. Regarding downstream chemistry, it was shown that by addition of this compound to the IL [P66614]Cl a ligand exchange reaction takes place, yielding [CuCl4]2− anions. Thus, a proof of concept for the dissolution of a metal oxide in [Hbet][NTf2] with a subsequent complete replacement of the O-coordination sphere by other ligands was provided. This opens up a new, low-temperature approach for the transformation of metal oxides into chemicals in demand, that further research might make applicable to naturally occurring ores, earths and minerals.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We wish to thank Dr Jens Hunger for the SCXRD measurement, Tobias Pietsch for the Raman and NMR measurements and Mai Lê Anh for the EDX measurements. This research was supported by the German Research Foundation (DFG) within the framework of the Priority Program SPP 1708.Notes and references
- D. Bernhardt, J. F. Reilly II, Mineral Commodity Summaries, U.S. Geological Survey, 2019 Search PubMed.
- A. F. Holleman, E. Wiberg and N. Wiberg, Anorganische Chemie, De Gruyter, Berlin Boston, 103rd edn, 2017 Search PubMed.
- Ionic liquids in synthesis, ed. P. Wasserscheid, Wiley-VCH, Weinheim, 2002 Search PubMed.
- P. Nockemann, B. Thijs, S. Pittois, J. Thoen, C. Glorieux, K. Van Hecke, L. Van Meervelt, B. Kirchner and K. Binnemans, J. Phys. Chem. B, 2006, 110, 20978–20992 CrossRef CAS PubMed.
- P. Nockemann, B. Thijs, T. N. Parac-Vogt, K. Van Hecke, L. Van Meervelt, B. Tinant, I. Hartenbach, T. Schleid, V. T. Ngan, M. T. Nguyen and K. Binnemans, Inorg. Chem., 2008, 47, 9987–9999 CrossRef CAS PubMed.
- P. Nockemann, B. Thijs, K. V. Hecke, L. V. Meervelt and K. Binnemans, Cryst. Growth Des., 2008, 8, 1353–1363 CrossRef CAS.
- D. Dupont and K. Binnemans, Green Chem., 2015, 17, 2150–2163 RSC.
- M. Orefice, K. Binnemans and T. Vander Hoogerstraete, RSC Adv., 2018, 8, 9299–9310 RSC.
- F.-L. Fan, Z. Qin, S.-W. Cao, C.-M. Tan, Q.-G. Huang, D.-S. Chen, J.-R. Wang, X.-J. Yin, C. Xu and X.-G. Feng, Inorg. Chem., 2019, 58, 603–609 CrossRef CAS PubMed.
- I. Barin, Thermochemical Data of Pure Substances, VCH, Weinheim, 3rd edn, 1995 Search PubMed.
- J. G. Speight, Lange's handbook of chemistry, McGraw-Hill, New York, 16th edn, 2005 Search PubMed.
- A. Kramida, Y. Ralchenko and J. Reader, NIST Atomic Spectra Database 5.6.1, National Institute of Standards and Technology, Gaithersburg, 2018 Search PubMed.
- A. Coelho, TOPAS, Academic V5, Brisbane Australia, 2012 Search PubMed.
- OPUS 6.5, Bruker Optik GmbH, Ettlingen, Germany, 2009 Search PubMed.
- G. M. Sheldrick, Shelx-97, Program for Crystal Structure Determination, University of Göttingen, Germany, 1997 Search PubMed.
- OLEX2 v1.2, OlexSys Ltd, Durham , UK, 2014 Search PubMed.
- K. Brandenburg, Diamond v. 4.5.2, Crystal and Molecular Structure Visualization, Crystal Impact GbR., Bonn, Germany, 2018 Search PubMed.
- J.-L. Ortiz-Quiñonez, I. Zumeta-Dubé, D. Díaz, N. Nava-Etzana, E. Cruz-Zaragoza and P. Santiago-Jacinto, Inorg. Chem., 2017, 56, 3394–3403 CrossRef PubMed.
- K. Jayachandran, R. Gupta, K. R. S. Chandrakumar, D. Goswami, D. M. Noronha, S. Paul and S. Kannan, Chem. Commun., 2019, 55, 1474–1477 RSC.
- M. R. Silva, J. A. Paixão, A. M. Beja, L. A. da Veiga and J. Martín-Gil, J. Chem. Crystallogr., 2001, 31, 167–171 CrossRef CAS.
- L. Wiehl, J. Schreuer and A. Stojic, Z. Kristallogr. - New Cryst. Struct., 2006, 221, 527–528 CAS.
- J. Schreuer, L. Wiehl, J. Biehler and P. Hofmann, Z. Kristallogr. - New Cryst. Struct., 2006, 221, 529–531 CAS.
- A. N. Wein, R. Cordeiro, N. Owens, H. Olivier, K. I. Hardcastle and J. F. Eichler, J. Fluorine Chem., 2009, 130, 197–203 CrossRef CAS.
- J. Soldevila-Sanmartín, J. A. Ayllón, T. Calvet, M. Font-Bardia, C. Domingo and J. Pons, Inorg. Chem. Commun., 2016, 71, 90–93 CrossRef.
- M. S. Fischer, D. H. Templeton and A. Zalkin, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1970, 26, 1392–1397 CrossRef CAS.
- K. Venkatasubramanian, Indian J. Phys., A, 1985, 58, 40 Search PubMed.
- M. Viertorinne, J. Valkonen, I. Pitkänen, M. Mathlouthi and J. Nurmi, J. Mol. Struct., 1999, 477, 23–29 CrossRef CAS.
- K. Hanke, M. Kaufmann, G. Schwaab, M. Havenith, C. T. Wolke, O. Gorlova, M. A. Johnson, B. P. Kar, W. Sander and E. Sanchez-Garcia, Phys. Chem. Chem. Phys., 2015, 17, 8518–8529 RSC.
- M. Szafran, A. Katrusiak, Z. Dega-Szafran and I. Kowalczyk, J. Mol. Struct., 2013, 1031, 49–55 CrossRef CAS.
- M. M. Ilczyszyn and M. Ilczyszyn, J. Raman Spectrosc., 2003, 34, 693–704 CrossRef CAS.
- D. P. Strommen, A.-M. Giroud-godquin, P. Maldivi, J.-C. Marchon and B. Marchon, Liq. Cryst., 1987, 2, 689–699 CrossRef CAS.
- I. R. Beattie, T. R. Gilson and G. A. Ozin, J. Chem. Soc. A, 1969, 534–541 RSC.
- Y. Suffren, F.-G. Rollet and C. Reber, Comments Inorg. Chem., 2011, 32, 246–276 CrossRef CAS.
- D. R. Bloomquist, M. R. Pressprich and R. D. Willett, J. Am. Chem. Soc., 1988, 110, 7391–7398 CrossRef CAS.
- J. Dimitric-Markovic, U. Mioc, J. Baranac and Z. Nedic, J. Serb. Chem. Soc., 2001, 66, 451–462 CrossRef CAS.
- A. Wolff, T. Doert, J. Hunger, M. Kaiser, J. Pallmann, R. Reinhold, S. Yogendra, L. Giebeler, J. Sichelschmidt, W. Schnelle, R. Whiteside, H. Q. N. Gunaratne, P. Nockemann, J. J. Weigand, E. Brunner and M. Ruck, Chem. Mater., 2018, 30, 7111–7123 CrossRef CAS.
- G. Wangila and R. B. Jordan, Inorg. Chim. Acta, 2003, 343, 347–350 CrossRef CAS.
- P. Davris, D. Marinos, E. Balomenos, A. Alexandri, M. Gregou, D. Panias and I. Paspaliaris, Hydrometallurgy, 2018, 175, 20–27 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Information on reagents and products, crystal structure of [Cu2(bet)4(NTf2)2][NTf2]2, IR band assignment of [Hbet][NTf2], U/x values of metal oxides, EDX of a ThO2 sample. CCDC 1947513. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra06423k |
| This journal is © The Royal Society of Chemistry 2019 |

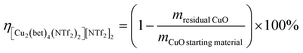
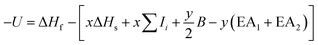
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif)