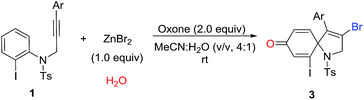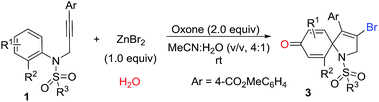Open Access Article
Open Access ArticleZnBr2/Oxone-mediated ipso-cyclization of N-(3-phenylprop-2-yn-1-yl)aniline†
Keke Huanga,
Jia-Ni Lib,
Guanyinsheng Qiu *bd,
Wenlin Xiec and
Jin-Biao Liu
*bd,
Wenlin Xiec and
Jin-Biao Liu *a
*a
aSchool of Metallurgical and Chemical Engineering, Jiangxi University of Science and Technology, 86 Hongqi Road, Ganzhou 341000, China. E-mail: liujinbiao@jxust.edu.cn
bCollege of Biological, Chemical Science and Engineering, Jiaxing University, 118 Jiahang Road, Jiaxing 314001, China. E-mail: qiuguanyinsheng@mail.zjxu.edu.cn
cSchool of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China
dDepartment of Chemistry, Fudan Unversity, Shanghai, 200438, China
First published on 17th October 2019
Abstract
In this work, a selective synthetic strategy towards 1-azaspiro[4.5]deca-3,6,9-trien-8-ones from N-tosyl-N-(prop-2-yn-1-yl)aniline is developed. The transformation proceeds smoothly in a mixed solvent including acetonitrile and water when ZnBr2 and Oxone are employed. Mechanism studies show that the reaction proceeds in a regioselective manner via a radical ipso-cyclization pathway.
Quaternary carbons are ubiquitous in many useful architectures.1 Consequently, tremendous effort has been made towards its synthetic methodology development.2 Among these established achievements, ipso-cyclization of arene served as a powerful tool towards quaternary carbon-containing spirocycles.3 Electrophilic ipso-cyclization was initially developed at the beginning of the new century.4 To date, many unprecendented advances on electrophilic ipso-cyclization have already been witnessed, and a series of spirocyclic compounds were achieved accordingly. In the past years a particular emphasis of synthetic chemists was put on radical ipso-cyclization.5 Compared to electrophilic ipso-cyclization, the radical way was able to be appliable for more substrates with a broader functional group tolerance.
Recently, a formal 6-endo radical cyclization of aryl propiolate was developed to construct substituent-shifted isocoumarin derivatives.6 Mechanism studies suggested the above formal 6-endo-cyclization was constituted by radical α-addition of propiolate, radical ipso-cyclization, and ester migration, which caused the shift of the substituents in core of isocoumarin. To provide a hint on this ipso-cyclization, many elegant examples were developed on electrophilic and/or radical ipso-cyclization of propiolates, leading to spirocycles 1-oxaspiro[4.5]deca-3,6,9-triene-2,8-diones.7
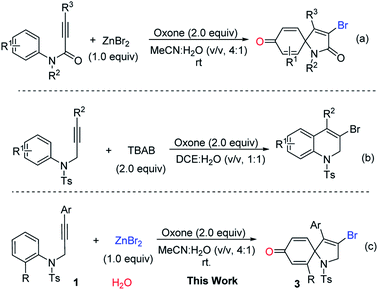
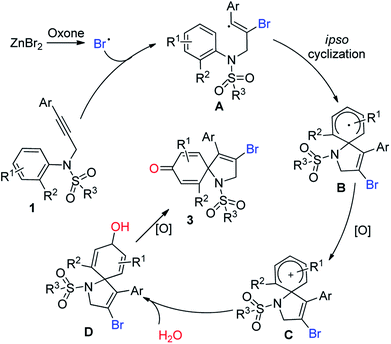
As an important analog of propiolate, N-aryl propiolamides was reasonable to be investigated for ipso-cyclization.8 Pleasingly, electrophilic/radical ipso-cyclization of N-aryl propiolamides was also reached so for. Particularly, for radical pathways, many radical precursors were recognized as efficient reaction partners. One example from our group indicated that bromo radical was also compatible for the ipso-cyclization of N-aryl propiolamides, and a series of bromo-containing 1-azaspiro[4.5]deca-3,6,9-triene-2,8-dione was observed (Scheme 1, eqn (a)).9 As we know, bromo group served as a versatile building block for structural elaboration through these classical transformations with/without metal catalysis. Interestingly, bromo radical was in situ generated in the presence of Oxone and bromo anion. In the process, bromo anion occurred to be oxidized into bromo radical through a single electron oxidization, and triggered the radical ipso-cyclization of N-aryl propiolamides for the synthesis of 1-azaspiro[4.5]deca-3,6,9-triene-2,8-dione as desired.
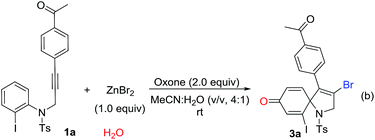
Over the past years, our group was focusing intensively on developing a more efficient and more economic system to realize regioselective transformations of alkynes.10 For instance, we recently reported a base-promoted α-addition of propiolamides, which led to various azetidin-2-ones with high efficiency and good reaction scope.10a In addition, the ortho-cyclizative reaction of ortho-substituent-free N-tosyl-N-(prop-2-yn-1-yl)aniline was reported by our group under Oxone and tetrabutylammonium bromide (TBAB), where released a distinctive compound 3-bromo-1-tosyl-1,2-dihydroquinoline derivatives (Scheme 1, eqn (b)).11 As our continuous interest on alkyne-based regioselective transformations and in light of the findings presented in Scheme 1a and b, we would like to disclose the radical ipso-cyclization of N-(prop-2-yn-1-yl)aniline 1 under mild conditions for the synthesis of 1-azaspiro[4.5]deca-3,6,9-trien-8-one 3 (Scheme 1, eqn (c)). Considering our findings on Oxone chemistry,12 in this paper we envisioned the radical brominative ipso-cyclization of N-tosyl-N-(prop-2-yn-1-yl)aniline could be activated by Oxone and ZnBr2. To reduce the possibility of ortho-cyclization, the reaction of 2-iodo-N-tosyl-N-(3-arylprop-2-yn-1-yl)aniline 1a was herein employed as the model substrate. 2-Iodo-N-tosyl-N-(3-arylprop-2-yn-1-yl)aniline was often used as a dual-functional building block.13
To our delight, a preliminary result from the model reaction of 1a and ZnBr2 was favored to deliver the spirocyclic compound 1-azaspiro[4.5]deca-3,6,9-trien-8-one 3a as expected, and the reaction yield was 70% at room temperature.
Other reaction condition trials on temperature, solvent, and bromo source were optimized accordingly. The results were presented in Table 1. Based on the results, an increase of reaction temperature to 60 °C did not make positive impact on the reaction outcome, just providing the desired product 3a in 62% yield (entry 2, Table 1). A similar yield was observed when the loading of ZnBr2 was used (entry 3, Table 1). The reactions using other solvents, including DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 1
H2O (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and THF
1) and THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 1
H2O (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), gave rise to inferior yields (entries 4 and 5, Table 1). Changing the ratio between MeCN and H2O did not give better yields, suggesting MeCN
1), gave rise to inferior yields (entries 4 and 5, Table 1). Changing the ratio between MeCN and H2O did not give better yields, suggesting MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 4
H2O (v/v = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) being the best choice (entries 6 and 7, Table 1). The use of TBAB, KBr or NaBr as a replacement of ZnBr2 was not favourable for the reaction, resulting in lower yield of the desired product 3a (entries 8–10 Table 1). The use of 2.0 equiv. of TEMPO as a radical scavenger totally shut the model reaction down, probably suggesting the model reaction going through a radical pathway (entry 11, Table 1).
1) being the best choice (entries 6 and 7, Table 1). The use of TBAB, KBr or NaBr as a replacement of ZnBr2 was not favourable for the reaction, resulting in lower yield of the desired product 3a (entries 8–10 Table 1). The use of 2.0 equiv. of TEMPO as a radical scavenger totally shut the model reaction down, probably suggesting the model reaction going through a radical pathway (entry 11, Table 1).
| Entry | Variation of standard conditions | Yield of 3aa,b (%) |
|---|---|---|
| a Isolated yield based on 1a.b Standard conditions: 1a (0.2 mmol), ZnBr2 (1 equiv.), Oxone (2.0 equiv.), solvent (2 mL), rt, overnight; TBAB = n-tetrabutyl ammonium bromide; Oxone = 2KHSO5·KHSO4·K2SO4. | ||
| 1 | — | 70 |
| 2 | 60 °C | 62 |
| 3 | 1.5 equiv. ZnBr2 | 70 |
| 4 | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 1 H2O (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
61 |
| 5 | THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 1 H2O (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
32 |
| 6 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v, 10 H2O (v/v, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
67 |
| 7 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v, 1 H2O (v/v, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
45 |
| 8 | 2.0 equiv. TBAB | 47 |
| 9 | 2.0 equiv. KBr | 41 |
| 10 | 2.0 equiv. NaBr | 38 |
| 11 | 2.0 equiv. TEMPO | 0 |
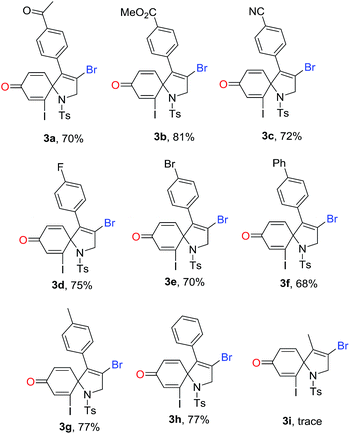
With the optimized conditions in hand, we then explored the reaction generality. The results are illustrated in Table 2. As shown in Table 2, a series of substituted 1-azaspiro[4.5]deca-3,6,9-trien-8-one 3 was achieved in good yields. The substituent Ar effects of aryl alkynes were investigated. The results reveal that both electron-donating groups and electron-deficient groups were compatible for the reactions. For example, the reaction with the substrate containing a 4-cyanophenyl group on Ar group afforded the desired 1-azaspiro[4.5]deca-3,6,9-trien-8-one 3c in a 72% yield, while the substrate having a 4-methylphenyl gave the corresponding product 3g in a 77% yield. Other substituents including 4-acetylphenyl, 4-esterphenyl, 4-fluorophenyl, 4-bromophenyl, and 4-phenylphenyl groups were efficient reaction partners, leading to the corresponding products 3a–3h in 68–81% yields. However, the reaction of 2-iodo-N-tosyl-N-(but-2-yn-1-yl)aniline 1i became complex, and did not offer the desired product 3i. It was reasoned that methyl in alkyne took part in the reaction, causing the reaction complex.
| a Isolated yield based on 1. |
|---|
 |
Subsequently, we explored the tolerance of substituents on ring of aniline (Table 3). Encouragingly, it seemed that R1 substituents could be replaced by methyl and chloro. The corresponding products 3j–3l were obtained in 50–65% yields. For instance, the reaction using the substrate with 5-methyl substituent produced a desired 1-azaspiro[4.5]deca-3,6,9-trien-8-one 3j in a 65% yield, while using the substrate substituted by 5-chlorogave rise to 3k in a 62% yield. Surprising, the reaction was suppressed by the presence of the 3-chloro group in substituent R1, resulting in a decreased yield of 3l in 50% yield. The substituent effect of R2 on the ring of aniline was also exploited. Beside iodo function, it was pleased to find the R2 could be also replaced by bromo and methyl, and the yield of corresponding products 3m and 3n were 75% and 68%, respectively. It was noteworthy that 1-azaspiro[4.5]deca-3,6,9-trien-8-ones 3b and 3n has been synthesized under the standard conditions of Scheme 1b.11 Compared to our previous finding, this results were highlighted by a higher efficiency, indicating a broader reaction scope simultaneously.
| a Isolated yield based on 1. |
|---|
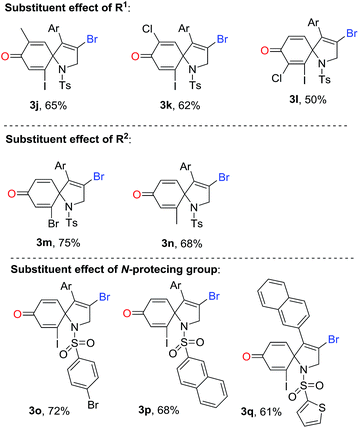 |
Finally, various N-protecting groups were screened. Pleasingly, N-tosyl could be replaced with 4-bromophenylsulfonyl, 2-naphthalenesulfonyl, and 2-thiophenesulfonyl. The desired 1-azaspiro[4.5]deca-3,6,9-trien-8-ones 3o–3q were detected in 61–72% yields. However, when acetyl group or hydrogen atom attached on the nitrogen atom, no desired product was observed.
Enlightened by the above information and the previous results, a plausible mechanism was proposed in Scheme 2. As illustrated in Scheme 2, bromo anion was oxidized into bromo radical,14 which occurred to undergo regioselective radical α-addition to form an intermediate A.15 Radical ipso-cyclization of the intermediate A then took place. Followed by oxidation, the spiro intermediate B was converted into a spirocation intermediate C. Thanks to the use of water as a mixed solvent, herein the intermediate C was trapped by water to afford hydroxyl-linked species D. Oxidation again provided the final targeted molecules 3.
In conclusion, we have developed a selective synthetic strategy towards 1-azaspiro[4.5]deca-3,6,9-trien-8-ones from N-tosyl-N-(prop-2-yn-1-yl)aniline. The transformation proceeded smoothly in a mixed solvent system of MeCN/H2O when ZnBr2 and Oxone was employed as the promoters. Mechanism studies showed that the reaction proceeded in a regioselective manner via a radical ipso-cyclization pathway. It was believed that the products were synthetically versatile since iodo and bromo groups in the product 1-azaspiro[4.5]deca-3,6,9-trien-8-ones was ready to be structurally elaborated through some classical transformations.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial supports from the Natural Science Foundation of China (nos: 21762018, 21961014 and 21772067), the program of Qingjiang Excellent Young Talents of Jiangxi University of Science and Technology (JXUSTQJBJ2018003), China Postdoctoral Science Foundation (2018M630396 for G. Qiu), and Science and Technology Innovation Outstanding Young Talents Program of Jiangxi Province (20192BCBL23009) are gratefully acknowledged.Notes and references
- For selected review, see: E. A. Peterson and L. E. Overman, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 11943 CrossRef CAS.
- For selected reviews on construction of quaternary carbon, see: (a) B. Wang and Y.-Q. Tu, Acc. Chem. Res., 2011, 44, 1207 CrossRef CAS; (b) Z.-L. Song, C.-A. Fan and Y.-Q. Tu, Chem. Rev., 2011, 111, 7523 CrossRef CAS; (c) C.-X. Zhuo, C. Zheng and S.-L. You, Acc. Chem. Res., 2014, 47, 2558 CrossRef CAS; (d) L. Pouysegu, D. Deffieux and S. Quideau, Tetrahedron, 2010, 66, 2235 CrossRef CAS; (e) W. Sun, G. Li, L. Hong and R. Wang, Org. Biomol. Chem., 2016, 14, 2164 RSC; (f) Q. Ding, X. Zhou and R. Fan, Org. Biomol. Chem., 2014, 12, 4807 RSC; (g) Y. Duan, S. Jiang, Y. Han, B. Sun and C. Zhang, Chin. J. Org. Chem., 2016, 36, 1973 CrossRef CAS.
- For reviews, see: (a) R. Song and Y. Xie, Chin. J. Chem., 2017, 35, 280 CrossRef CAS; (b) L. Shi, W. Zhang, S. Chen, L. Lu, R. Fan, J. Tan and C. Zheng, Curr. Org. Chem., 2018, 15, 904 CAS; (c) E. Vessally, M. Babazadeh, K. Didehban, A. Hosseinian and L. Edjlali, Curr. Org. Chem., 2018, 22, 286 CrossRef CAS; (d) C. R. Reddy, S. K. Prajapti, K. Warudikar, R. Ranjan and B. B. Rao, Org. Biomol. Chem., 2017, 15, 3130 RSC; (e) G. Liu, H. Liu, G. Qiu, S. Pu and J. Wu, Chem. Commun., 2012, 48, 7049 RSC; (f) S. Ni, J. Zhou, H. Mei and J. Han, Tetrahedron Lett., 2018, 59, 1309 CrossRef CAS.
- X. Zhang and R. C. Larock, J. Am. Chem. Soc., 2005, 127, 12230 CrossRef CAS.
- (a) T. Lanza, R. Leardini, M. Minozzi, D. Nanni, P. Spagnolo and G. Zanardi, Angew. Chem., Int. Ed., 2008, 47, 9439 CrossRef CAS PubMed; (b) Y. Zhou, X. Zhang, Y. Zhang, L. Ruan, J. Zhang, D. Zhang-Negrerie and Y. Du, Org. Lett., 2017, 19, 150 CrossRef CAS.
- For recent selected examples on ipso-cyclization of alkynoate, see: (a) Z. Li, X. Wang, L. Wang and P. Li, Tetrahedron, 2019, 75, 1044 CrossRef; (b) H. Ren, M. Zhang and Q. Ai, Tetrahedron, 2018, 74, 4435 CrossRef CAS; (c) M.-J. Bu, G.-P. Lu and C. Cai, Catal. Commun., 2018, 114, 70 CrossRef CAS; (d) C. Pan, Y. Chen, S. Song and J. Yu, J. Org. Chem., 2016, 81, 12065 CrossRef CAS; (e) S. Ni, L. Zhang, W. Zhang, H. Mei, J. Han and Y. Pan, J. Org. Chem., 2016, 81, 9470 CrossRef CAS; (f) R. Yan, J. Huang, J. Luo, P. Wen, G. Huang and Y. Liang, Synlett, 2010, 1071 CAS; (g) S. Ni, Y. Zhang, C. Xie, H. Mei, J. Han and Y. Pan, Org. Lett., 2015, 17, 5524 CrossRef CAS; (h) K. Yan, D. Yang, W. Wei, F. Wang, Y. Shuai, Q. Li and H. Wang, J. Org. Chem., 2015, 80, 1550 CrossRef CAS PubMed; (i) X. Mi, C. Wang, M. Huang, Y. Wu and Y. Wu, J. Org. Chem., 2015, 80, 148 CrossRef CAS; (j) Y.-F. Zeng, D.-H. Tan, Y. Chen, W.-X. Lv, X.-G. Liu, Q. Li and H. Wang, Org. Chem. Front., 2015, 2, 1511 RSC; (k) W. Xie, Y. Wu, J. Zhang, Q. Mei, Y. Zhang, N. Zhu, R. Liu and H. Zhang, Eur. J. Med. Chem., 2018, 145, 35 CrossRef CAS.
- For selected example, see: M. D. Aparece and P. A. Vadola, Org. Lett., 2014, 16, 6008 CrossRef CAS.
- For selected examples, see (a) T.-S. Jiang, X.-G. Zhang and J.-H. Li, Synthesis, 2009, 18, 3029 Search PubMed; (b) L. Zhu, Y. Xiong and C. Li, J. Org. Chem., 2015, 80, 628 CrossRef CAS; (c) L.-J. Wu, F. Tan, M. Li, R.-J. Song and J.-H. Li, Org. Chem. Front., 2017, 4, 350 RSC; (d) X.-H. Yang, X.-H. Ouyang, W.-T. Wei, R.-J. Song and J.-H. Li, Adv. Synth. Catal., 2015, 357, 1161 CrossRef CAS.
- Y. He and G. Qiu, Org. Biomol. Chem., 2017, 15, 3485 RSC.
- (a) L. Zhang, L. Ma, H. Zhou, J. Yao, X. Li and G. Qiu, Org. Lett., 2018, 20, 2407 CrossRef CAS; (b) S.-T. Yuan, H. Zhou, L. Gao, J.-B. Liu and G. Qiu, Org. Lett., 2018, 20, 562 CrossRef CAS; (c) D. Chen, Y. Shan, J. Li, J. You, X. Sun and G. Qiu, Org. Lett., 2019, 21, 4044 CrossRef CAS; (d) Y.-C. Wang, R.-X. Wang, G. Qiu, H. Zhou, W. Xie and J.-B. Liu, Org. Chem. Front., 2019, 6, 2471 RSC; (e) R. Liu, M. Li, W. Xie, H. Zhou, Y. Zhang and G. Qiu, J. Org. Chem., 2019, 84, 11763 CrossRef CAS; (f) G. Qiu, M. Mamboury, Q. Wang and J. Zhu, Angew. Chem., Int. Ed., 2016, 55, 15377 CrossRef CAS; (g) G. Qiu, X. Qiu, J. Liu and J. Wu, Adv. Synth. Catal., 2013, 355, 2441 CrossRef CAS.
- M. Yang, X. Hu, B. Ouyang, W. Xie and J.-B. Liu, Tetrahedron, 2019, 75, 3516 CrossRef CAS.
- For a review and some results on Oxone chemistry, see: (a) H. Husain, I. R. Green and I. Ahmed, Chem. Rev., 2013, 113, 3329 CrossRef; (b) G. Qiu, Z.-F. Chen, W. Xie and H. Zhou, Eur. J. Org. Chem., 2019, 4327 CrossRef CAS; (c) Y. Zheng, M. Liu, G. Qiu, W. Xie and J. Wu, Tetrahedron, 2019, 75, 1663 CrossRef CAS; (d) Y.-H. Wang, G. Qiu, H. Zhou, W. Xie and J.-B. Liu, Tetrahedron, 2019, 75, 3850 CrossRef CAS; (e) Y.-H. Wang, B. Ouyang, G. Qiu, W. Xie and J.-B. Liu, Org. Biomol. Chem., 2019, 17, 4335 RSC; (f) L.-H. Lu, S.-J. Zhou, M. Sun, J.-L. Chen, W. Xia, X. Yu, X. Xu and W.-M. He, ACS Sustainable Chem. Eng., 2019, 7, 1574 CrossRef CAS; (g) L. Y. Xie, S. Peng, F. Liu, G.-R. Chen, W. Xia, X. Yu, W.-F. Li, Z. Cao and W.-M. He, Org. Chem. Front., 2018, 5, 2604 RSC; (h) L.-H. Lu, S.-J. Zhou, W.-B. He, W. Xia, P. Chen, X. Yu, X. Xu and W.-M. He, Org. Biomol. Chem., 2018, 16, 9064 RSC; (i) L.-Y. Xie, S. Peng, T.-G. Fan, Y.-F. Liu, M. Sun, L.-L. Jiang, X.-X. Wang, Z. Cao and W.-M. He, Sci. China: Chem., 2019, 62, 460 CrossRef CAS.
- For a dual-functional synthon, see: (a) C. Wu, Z. Wang, Z. Hu, F. Zeng, X.-Y. Zhang, Z. Cao, Z. Tang, W.-M. He and X.-H. Xu, Org. Biomol. Chem., 2018, 16, 3177 RSC; (b) L. –Y. Xie, S. Peng, F. Liu, J.-Y. Yi, M. Wang, Z. Tang, X. Xu and W.-M. He, Adv. Synth. Catal., 2018, 360, 4259 CrossRef CAS; (c) C. Wu, L.-H. Lu, A. –Z. Peng, G.-K. Jia, C. Peng, Z. Cao, Z. Tang, W.-M. He and X. Xu, Green Chem., 2018, 20, 3683 RSC; (d) X. Gong, J. Chen, X. Li, W. Xie and J. Wu, Chem.–Asian J., 2018, 13, 2543 CrossRef CAS; (e) Y. Zhao, Y. Luo, Y. Zhu, H. Wang, H. Zhou, H. Tan and Z. Zhou, Synlett, 2018, 29, 773 CrossRef CAS; (f) Y.-H. Zhao, Y. Li, M. Luo, Z. Tang and K. Deng, Synlett, 2016, 27, 2597 CrossRef CAS; (g) Y. Zhao, Y. Li, T. Guo, Z. Tang, W. Xie and G. Zhao, Tetrahedron Lett., 2016, 57, 2257 CrossRef CAS; (h) T. Guo, Y. Liu, Y.-H. Zhao, P.-K. Zhang, S.-L. Han and H.-M. Liu, Tetrahedron Lett., 2016, 57, 4629 CrossRef CAS; (i) T. Guo, Y. Liu, Y.-H. Zhao, P.-K. Zhang, S.-L. Han and H.-M. Liu, Tetrahedron Lett., 2016, 57, 3920 CrossRef CAS; (j) L. Zhen, C. Fang, Y. Zheng, G. Qiu, X. Li and H. Zhou, Tetrahedron Lett., 2018, 59, 3934 CrossRef; (k) Y. Zong, Y. Lang, M. Yang, X. Li, X. Fan and J. Wu, Org. Lett., 2019, 21, 1935 CrossRef CAS.
- (a) G. Qiu, T. Liu and Q. Ding, Org. Chem. Front., 2016, 3, 510 RSC; (b) K. Moriyama, Y. Nkamura and H. Togo, Org. Lett., 2014, 16, 3812 CrossRef CAS; (c) U. Dutta, A. Deb, D. Lupton and D. Maiti, Chem. Commun., 2015, 51, 17744 RSC; (d) A. Podgorsek, S. Stavber, M. Zupan and J. Iskra, Tetrahedron Lett., 2006, 47, 7245 CrossRef CAS; (e) V. A. Schmidt, R. K. Quinn, A. T. Brusoe and E. J. Alexanian, J. Am. Chem. Soc., 2014, 136, 14389 CrossRef CAS; (f) T. Liu, M. C. Myers and Y.-Q. Yu, Angew. Chem., Int. Ed., 2017, 56, 306 CrossRef CAS; (g) X. Deng, H. Cao, C. Chen, H. Zhou and L. Yu, Sci. Bull., 2019, 64, 1280 CrossRef CAS; (h) L. Yu and X. Huang, Synlett, 2007, 1371 CAS; (i) L. R. Reddy, B. V. S. Reddy and E. J. Corey, Org. Lett., 2006, 8, 2819 CrossRef CAS; (j) X.-F. Wu, Chem.–Asian J., 2012, 7, 2502 CrossRef CAS; (k) X.-F. Wu and H. Neumann, Adv. Synth. Catal., 2012, 354, 3141 CrossRef CAS; (l) S. Enthaler, ACS Catal., 2013, 3, 150 CrossRef CAS.
- Regioselective radical reactions see: (a) X. Gong, X. Li, W. Xie, J. Wu and S. Ye, Org. Chem. Front., 2019, 6, 1863 RSC; (b) J. Zhang, W. Xie, S. Ye and J. Wu, Org. Chem. Front., 2019, 6, 2254 RSC; (c) S. Ye, T. Xiang, X. Li and J. Wu, Org. Chem. Front., 2019, 6, 2183 RSC; (d) S. Ye, X. Li, W. Xie and J. Wu, Asian J. Org. Chem., 2019, 8, 893 CrossRef CAS; (e) X. Wang, M. Yang, W. Xie, X. Fan and J. Wu, Chem. Commun., 2019, 55, 6010 RSC; (f) G. Liu, H. Liu, G. Qiu, S. Pu and J. Wu, Chem. Commun., 2012, 48, 7049 RSC; (g) G. Qiu, K. Zhou and J. Wu, Chem. Commun., 2018, 54, 12561 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra06646b |
| This journal is © The Royal Society of Chemistry 2019 |