Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photochromic properties of three 2D MOFs based on 1-carboxyethyl-4,4′-bipyridinine†
Jinjian Liu *,
Jing Li and
Wenbo Lu
*,
Jing Li and
Wenbo Lu
Key Laboratory of Magnetic Molecules & Magnetic Information Materials Ministry of Education, The School of Chemical and Material Science, Shanxi Normal University, Linfen 041004, China. E-mail: liujj@sxnu.edu.cn
First published on 17th October 2019
Abstract
Viologen units have been widely used to impart metal–organic frameworks (MOFs) with photochromic properties. However, construction of a stable photochromic system in viologen MOFs has not been fully explored. Herein, we report three examples of MOFs, namely, {[Cd(CEbpy)(m-BDC)(DMF)]·2H2O}n (1), {[Cd(CEbpy)(p-BDC)(H2O)]·H2O}n (2), and {[Zn(CEbpy)(p-HBDC)(p-BDC)0.5]·H2O}n (3) based on benzenedicarboxylic acids (m-H2BDC = 1,3-benzenedicarboxylic acid, p-H2BDC = 1,4-benzenedicarboxylic acid) and a viologen-derived ligand 1-carboxyethyl-4,4′-bipyridine (L = CEbpy). As expected, the incorporation of the viologen unit into the frameworks results in the predefined photochromism upon both sunlight and UV-light. Compounds 1–3 feature a two-dimensional (2D) layered structure and are all photochromic due to the formation of CEbpy radicals by photoinduced electron transfer (PET). The aggregates build an interesting stable crystalline framework that exhibits long-lived color constancy in the solid state.
1. Introduction
As a unique highly ordered and porous material, metal–organic frameworks (MOFs)1 have become increasingly popular due to their configurable structure, high surface area and adjustable porosity. Because of the adjustability and multifaceted modularity of MOFs, the functional organic moiety can be self-assembled, grafted or embedded to create a multifunctional MOF that has been used for gas storage/separation, catalysis, proton conduction and sensing.2 Photochromic materials allow for convenient visual monitoring due to photoinduced color changes, which may be accompanied by changes in other properties.3 Recently, as a kind of functional material, photochromic MOFs have attracted more and more attention because they can display attribute changes immediately by light irradiation without any cumbersome processing.4 It is feasible to synthesize such functional MOFs using a photoactive organic linker.It is well known that viologen/4,4′-bipyridinium derivatives have electron acceptability and redox activity.5 Because of the excellent electron acceptability and Lewis acidic sites, viologens are extensively studied in the field of chromic materials such as photochromism, thermochromism and electrochromism.6 The viologen cation (V2+) is capable of undergoing electron transfer (ET) to form a viologen radical (V˙+) and can generally be accompanied by an identifiable color change.7 The photoinduced electron transfer (PET) process occurs without bond cleavage/formation, thereby exerting little disturbance to the molecules and crystal structures, which facilitates reversible photochromism in the solid state.8 In addition, it is known that viologen derivatives are excellent photochromic organic ligands providing controllability of substituents on the N atom of the pyridinium ring, which can promote the production of photosensitive MOFs those have chemical stability and redox activity.9 Viologen derivatives are usually selected as the organic ligands to be introduced into MOFs to construct new photochromic materials due to their excellent characteristics,10 which have been reported as photochromic materials several times.11
Viologens are well known to be easily photo-reduced accompanied by obvious color changes.12 Furthermore, the photochromic behaviors can usually be observed under anaerobic conditions or a flash-photolytic scale, because the reduced radicals are highly sensitive to oxidation.13 However, the instability of organic radicals in the air remains a challenge for applications. To date, stable organic radicals obtained have been limited to a few systems with variable color behavior.14 The preparation of cross-linked viologen MOFs is reported to be a good way to perform PET and to generate stable free radicals in air, accompanied by color changes.15 By limiting air oxidation and reverse ET, photogenerated free radicals are stabilized by crystal packing, which undergoes diffusion control in the crystalline state. So far, to construct a stable photochromic system in viologen MOFs has not been fully explored.16
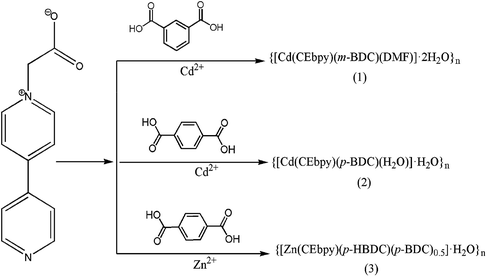
In this example, we propose a viologen-derived ligand, 1-carboxyethyl-4,4′-bipyridinine (L = CEbpy) with the terminal oxygen and nitrogen atoms serving as coordination sites, in which long-lived color species by embedding the viologen units into the condensed MOFs can be prepared. Three metal–viologen MOFs, formulated as {[Cd(CEbpy)(m-BDC)(DMF)]·2H2O}n (1), {[Cd(CEbpy)(p-BDC)(H2O)]·H2O}n (2), {[Zn(CEbpy)(p-HBDC)(p-BDC)0.5]·H2O}n (3) (m-H2BDC = 1,3-benzenedicarboxylic acid, p-H2BDC = 1,4-benzenedicarboxylic acid) are prepared. Furthermore, the photochromism of the three MOFs are investigated. As anticipated, under the irradiation of UV-light and sunlight, compounds 1–3 exhibit noticeable color changes due to the production of CEbpy radicals. It is notable that the photoinduced free radicals of the three MOFs can keep stable in air for months at room temperature.
2. Experimental section
2.1 Materials and instruments
All reagents and solvents were commercially purchased and used without further purification. 1-Carboxyethyl-4,4′-bipyridine (L = CEbpy) was synthesized according to the literature;17 Elemental analyses (C, H, and N) were conducted on a Vario EL III CHNOS elemental analyzer; X-ray powder diffraction (PXRD) data were obtained with a Rigaku Ultima IV-185 diffractometer; UV-vis diffuse reflectance spectra were performed at room temperature with a Varian Cary 5000 UV-visible spectrophotometer; electron paramagnetic resonance (EPR) spectroscopy were recorded at room temperature on a Bruker A300-10/12 electron resonance spectrometer; a ThermoFisher ESCALAB 250 X-ray photoelectron spectrometer (powered at 150 W) by Al Kα radiation (λ = 8.357 Å; spot size, 500 m) was used to perform X-ray photoelectron spectroscopy (XPS).The powder X-ray diffraction experimental spectra of the synthesized three MOFs synthesized are in good agreement with the simulated samples, confirming the solid phase purity (Fig. S1†) (Scheme 1).
2.2 X-ray crystallography
Crystallographic data were recorded at 293 K using graphite monochrome Mo-Kα (λ = 0.71073) on an Oxford Gemini diffractometer for X-ray diffraction data of compounds 1–3. Empirical absorption correction of spherical harmonics was implemented in the SCALE3 ABSPACK scaling algorithm.18 The SHELXTL-97 crystallographic software package was used to solve and refine the structures solved by direct method and refined on F2 by full matrix least squares techniques.19 All non-hydrogen atoms were refined anisotropically. The crystallographic data of 1–3 is listed in Table 1, and the selected bond lengths and bond angles are listed in Table S1.†| a R1 = ∑‖Fo| − Fc‖/∑Fo|b wR = [∑w(Fo2 − Fc2)]/∑w(Fo2)2]1/2 | ||||
|---|---|---|---|---|
| Compound | 1 | 1UP | 2 | 3 |
| CCDC code | 1956829 | 1956570 | 1902518 | 1918048 |
| Empirical formula | C23H25CdN3O9 | C23H25CdN3O9 | C20H18CdN2O8 | C24H19ZnN2O9 |
| Formula weight | 599.87 | 599.87 | 526.76 | 544.80 |
| Crystal size (mm) | 0.4 × 0.4 × 0.3 | 0.3 × 0.3 × 0.3 | 0.34 × 0.33 × 0.3 | 04 × 0.3 × 0.3 |
| Crystal system, | Monoclinic | Monoclinic | Triclinic | Monoclinic |
| Space group | P21/c | P21/c | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/c |
| a (Å) | 10.624(9) | 10.5787(6) | 9.6740(6) | 7.4557(5) |
| b (Å) | 16.457(13) | 16.3960(16) | 10.0787(6) | 19.1019(13) |
| c (Å) | 14.415(11) | 14.3239(13) | 11.7148(7) | 15.6785(10) |
| α (°) | 90 | 90 | 96.8800(10) | 90 |
| β (°) | 110.510(19) | 110.304(2) | 92.3220(10) | 93.186(2) |
| γ (°) | 90 | 90 | 114.9100(10) | 90 |
| Volume (Å3) | 2361(3) | 2330.1(4) | 1023.23(11) | 2229.4(3) |
| Z | 4 | 4 | 2 | 4 |
| Dc (g cm−3) | 1.679 | 1.701 | 1.710 | 1.566 |
| F(000) | 1204 | 1204 | 1056 | 1072 |
| m (mm−1) | 0.984 | 1.997 | 1.118 | 1.156 |
| R1/wR2, [I ≥ 2σ(I)]a,b | 0.0552, 0.1662 | 0.0515, 0.1589 | 0.0336, 0.0927 | 0.0525, 0.1735 |
| R1/wR2, (all data) | 0.0597, 0.1755 | 0.0546, 0.1644 | 0.0374, 0.1089 | 0.0578, 0.1797 |
3. Results and discussion
3.1 Structural description
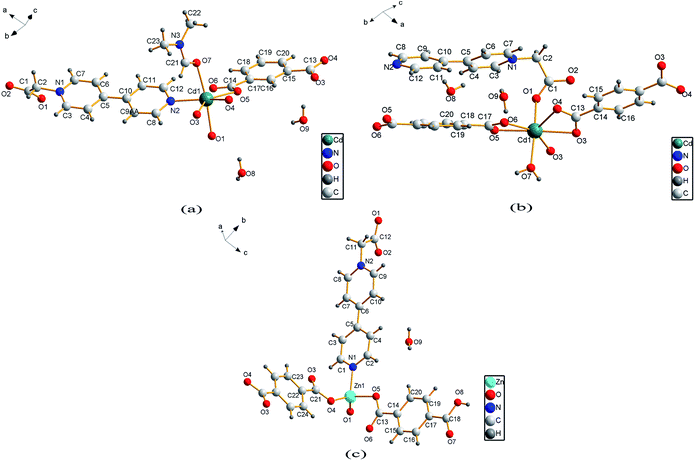
Single crystal X-ray analysis reveals that compound 1 is a two-dimensional (2D) MOF crystallizing in the monoclinic space group P21/c. As shown in Fig. 1a, the asymmetric unit of 1 contains one Cd2+ cation, one CEbpy ligand, one m-BDC2− ligand, one DMF ligand and two lattice water molecules. Every Cd2+ cation is six-coordinated in a distorted octahedral geometry by five O atoms (dCd–O = 2.221 − 2.475 Å) from one CEbpy ligand, two m-BDC2− ligands, one DMF ligand and one N atom (dCd–N = 2.304 Å) from another CEbpy ligand. There are two different coordination modes in the two carboxylate groups of the m-BDC2− ligand, one is monodentate, while the other is bidentate. The carboxylate group of the CEbpy ligand is monodentate. The coordinated DMF molecule acts as a terminal ligand to complete the octahedral coordination environment of the Cd2+ ion. As illustrated in Fig. 3a, the coordination interaction among the two bis-CEbpy groups (Fig. 2a), four m-BDC2− ligands and six coordinated Cd2+ ions makes compound 1 a 2D network with (6, 3) topology. Strong π–π stacking interactions between the pyridine rings of CEbpy ligands in the layer exist with the center–center distance of 3.737 Å (Fig. S2a†), and C–H⋯O, O–H⋯O hydrogen bonds are observed (Table S2†), which will well stabilize the crystal structure.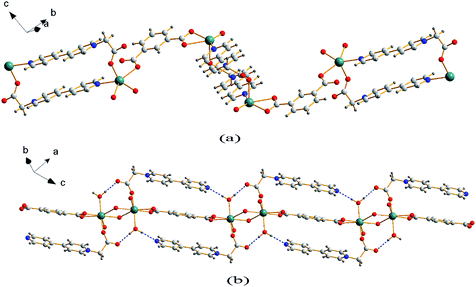 | ||
| Fig. 2 1D coordination chain of 1 (a) and 2 (b) based on the CEbpy ligand, benzenedicarboxylic acids and Cd2+ ion. | ||
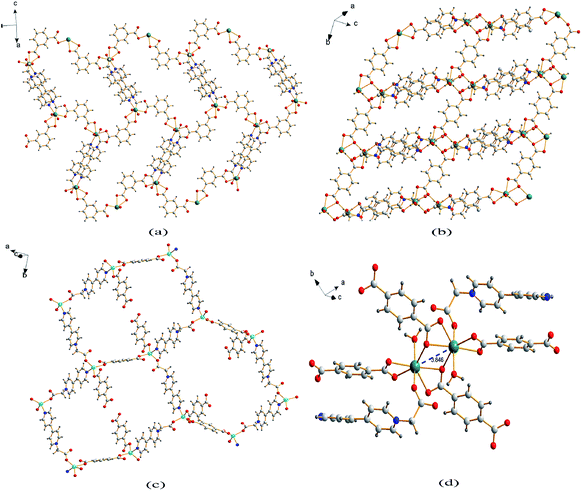 | ||
| Fig. 3 2D coordination layer of 1 (a), 2 (b) and 3 (c); (d) the Cd2 molecular building block with a Cd⋯Cd separation for 2. | ||
Compound 2 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and also features a 2D framework structure. The asymmetric unit of 2 consists of one Cd2+ ion, one CEbpy ligand, two half p-BDC2− ligands, one coordinated water and two half lattice water molecules (Fig. 1b). Each Cd ion shows a slightly distorted pentagonal bipyramid structure, coordinated by five O atoms from three different p-BDC2− ligands, one O atom from the CEbpy ligand and one O atom from the coordinated water. The Cd–O distances vary from 2.273 Å to 2.414 Å. Unlike the 2-connected node of CEbpy in compound 1, it acts as a 1-connected node in 2, the N atom does not participate in coordination, but forms a stronger hydrogen bond O(7)–H⋯N(2) (2.040 Å) with the coordinated water molecule, which also forms another strong hydrogen bond O(7)–H⋯O(2) (1.887 Å) with the carboxylate O atom not involved in the coordination in the CEbpy ligand on the other side (Fig. 2b). One prominent structural feature of 2 is the presence of a binuclear Cd2 unit Cd2(CO2)6(H2O)2. The p-BDC2− linker with four carboxylate O atoms in the μ-4 mode links with four Cd2+ ions to form two Cd2 clusters with a Cd⋯Cd separation of 3.846 Å (Fig. 3d). Each Cd2 cluster can be viewed as a second building unit (SBU) surrounded by four p-BDC2− ligands with bidentate chelating modes. The binuclear unit is further connected to adjacent ones by a p-BDC2− ligand and two CEbpy ligands to form a 1D three rows of horizontal chain network (Fig. 2b). The adjacent chains are further linked by p-BDC2− ligands to form a 2D layer (Fig. 3b). The 2D layers are stacked by face-to-face π⋯π stacking interactions (the center–center distance is 3.764 Å, Fig. S2b†) and hydrogen bonds to form the supramolecular array.
and also features a 2D framework structure. The asymmetric unit of 2 consists of one Cd2+ ion, one CEbpy ligand, two half p-BDC2− ligands, one coordinated water and two half lattice water molecules (Fig. 1b). Each Cd ion shows a slightly distorted pentagonal bipyramid structure, coordinated by five O atoms from three different p-BDC2− ligands, one O atom from the CEbpy ligand and one O atom from the coordinated water. The Cd–O distances vary from 2.273 Å to 2.414 Å. Unlike the 2-connected node of CEbpy in compound 1, it acts as a 1-connected node in 2, the N atom does not participate in coordination, but forms a stronger hydrogen bond O(7)–H⋯N(2) (2.040 Å) with the coordinated water molecule, which also forms another strong hydrogen bond O(7)–H⋯O(2) (1.887 Å) with the carboxylate O atom not involved in the coordination in the CEbpy ligand on the other side (Fig. 2b). One prominent structural feature of 2 is the presence of a binuclear Cd2 unit Cd2(CO2)6(H2O)2. The p-BDC2− linker with four carboxylate O atoms in the μ-4 mode links with four Cd2+ ions to form two Cd2 clusters with a Cd⋯Cd separation of 3.846 Å (Fig. 3d). Each Cd2 cluster can be viewed as a second building unit (SBU) surrounded by four p-BDC2− ligands with bidentate chelating modes. The binuclear unit is further connected to adjacent ones by a p-BDC2− ligand and two CEbpy ligands to form a 1D three rows of horizontal chain network (Fig. 2b). The adjacent chains are further linked by p-BDC2− ligands to form a 2D layer (Fig. 3b). The 2D layers are stacked by face-to-face π⋯π stacking interactions (the center–center distance is 3.764 Å, Fig. S2b†) and hydrogen bonds to form the supramolecular array.
Compound 3 crystallizes in the monoclinic space group P21/c, and also possesses a 2D framework structure with (6, 3) topology like compound 1. The asymmetric unit of 3 consists of one Zn2+ cation, one CEbpy ligand, one coordinated p-HBDC− ligand, a half coordinated p-BDC2− ligand and one lattice water molecule (Fig. 1c). Each Zn ion is bound by one O atom from the p-HBDC− ligand, one O atom from the p-BDC2− ligand, one O atom from the CEbpy ligand and one N atom from another CEbpy ligand, showing a distorted tetrahedron coordination mode. Each Zn ion connects CEbpy ligands and p-BDC2− ligands to form a 2D grid-like layered motif (Fig. 3c). The bond lengths of Zn–O vary from 1.953 Å to 1.980 Å, and the bond length of Zn–N is 2.078 Å in the structure. The two carboxyl groups in the p-BDC2− ligand both participate in the coordination. However, only one carboxyl group in the p-HBDC− ligand participates in the coordination. Every CEbpy ligand adopts a bidentate coordination mode bridging two Zn2+ cations, which is the same as 1, but different from 2.
3.2 Photochromism
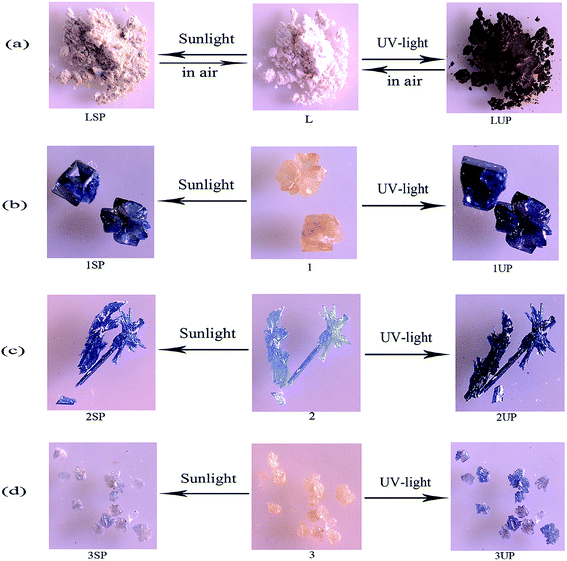
Because of the presence of the electron-deficient viologen moiety, the photochromic behaviors of the three MOFs are investigated.20 Compounds 1–3 are found sensitive to both sunlight and UV-light and undergo photochromic transformations upon irradiation at room temperature in air.Compound 1 is photosensitive, and exhibits a visible color change from yellow to dark blue (1SP) when exposed to sunlight for 5 min in air at room temperature, and after 20 min of irradiation, the coloration is completely saturated. Compound 1 also exhibits a visually detectable change from yellow to darker blue (1UP) within 2 min (Fig. 4b) upon UV-light irradiation (Hg lamp, 365 nm, 175 W). The similar photochromic behaviors can also be found in 2 and 3. As shown in Fig. 4c, for 2, a color change from light blue to blue (2SP) upon sunlight irradiation after 10 min and to dark blue (2UP) upon UV-light irradiation after 1 min. Compound 3 undergoes a slower color change from yellow to brown (3SP) upon sunlight irradiation after 40 min, and turns to blue (3UP) upon UV-light irradiation after 5 min (Fig. 4d). It should be noted that, there is also a color change for the ligand CEbpy during irradiation, indicating that the ligand CEbpy alone exhibits photochromic behaviors. As shown in Fig. 4a, white crystals of CEbpy turn to khaki (LSP) after being irradiated with sunlight for 1 h, and turn to dark brown (LUP) upon UV-light irradiation after 15 min. After exposure to sunlight, the photoproduct of CEbpy is fully decolored in the dark after one day, and the photoproduct upon UV-light irradiation takes two days to decolorize. However, all the three MOFs based on the CEbpy ligand are more stable in air. Unlike the ligand CEbpy, the photoproducts of the three MOFs can keep stable in the dark for a long period of time before they return to the original states. (compound 1, 25 days after sunlight irradiation to decolorize, 76 days after UV-light irradiation; compound 2, 94 days after sunlight irradiation, over six months after UV-light irradiation; compound 3, 15 days after sunlight irradiation, 62 days after UV-light irradiation). It shows that the preparation of cross-linked viologen MOFs is a good method to undergo PET and generate stable radicals in the solid state in air at the room temperature. The decolorization processes for the photoproducts of the three MOFs can be also accomplished by heating the samples at 140 °C for 4–6 hours. These colorization–decolorization processes can be repeated at least for six cycles by alternating light irradiation and heating treatment, showing good reversibility.
In order to clarify the photochromic processes, the solid UV-vis reflectance and EPR spectra of the three MOFs before and after irradiation are investigated. As shown in Fig. 5a and c, the UV-vis spectra of photoproducts (1SP, 1UP, 3SP, 3UP) show two new bands at about 410 nm and 610 nm compared to origin samples 1 and 3 after sunlight and UV-light irradiation. However, it is found that the UV-vis reflectance spectrum of the original sample 2 has the absorption bands similar to the photoproducts of 1 and 3. The absorption bands get enhanced after irradiation with sunlight and UV-light, (Fig. 5b). The difference in the UV-vis reflectance spectra for the three MOFs should be due to the fact that 2 is more sensitivity upon indoor-light than compounds 1 and 3. The viologen moiety is known to be redox-active and can generate radicals under light irradiation. Based on the previous works on the viologen derivatives,21 we can conclude that the color change of the three MOFs should be due to the production of CEbpy radicals. EPR spectra confirm this radical generation. For compounds 1 and 3, there is no EPR signal observed before irradiation, and strong radical signals of g = 2.0037 occur after irradiation (Fig. 5d and f). For compound 2, a strong EPR signal at g = 2.0037 has already been observed due to the sensitivity to indoor-light, and the EPR signals became stronger after sunlight and UV-light irradiation (Fig. 5e). At the same time, the single crystal XRD data and PXRD patterns indicate that the photochromism is unrelated to photolysis or photoinduced isomerisation (Table 1 and Fig. S1†).22 The XPS test of 1 before and after UV-light irradiation is used to identify the PET process. As shown in Fig. S3a and b,† the core energy level spectra of Cd 3d and C 1s are almost the same before and after irradiation. However, the variation of those of O 1s and N 1s are discernible. The O 1s core level spectrum has a transition to higher binding energy from 530.58 eV to 530.88 eV (Fig. S3c†), suggesting that the O atoms lose electrons. Different from the case of O 1s, the N 1s peaks shift to lower energy (Fig. S3d†). This result indicates that photochromism of 1 should originate from ET from the carboxylate O to the N atom of the pyridinium ring.
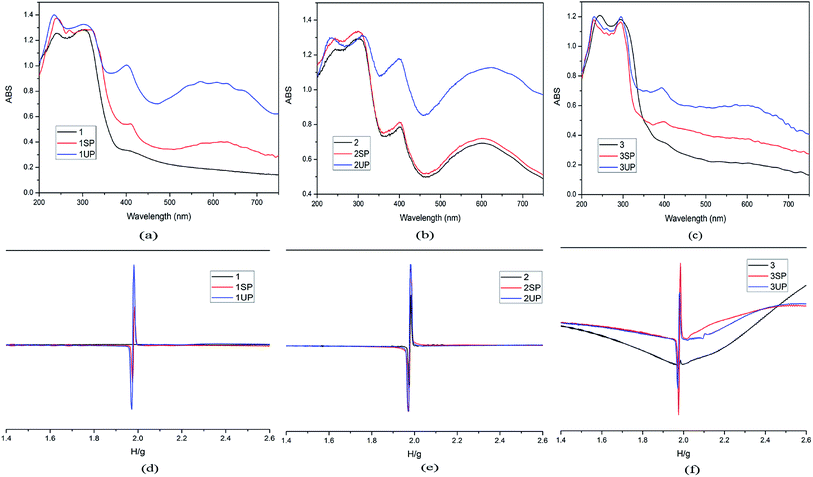 | ||
| Fig. 5 UV-vis spectra (a for 1, b for 2, c for 3) and EPR spectra (d for 1, e for2, f for 3) before and after irradiation for the three MOFs. | ||
It is well known that there are many factors affecting the photochromic behavior of the viologens, the main factor is the electron transfer distance between the electron-rich group and the electron-deficient bipyridinium unit.23 In compounds 1–3, the electron donors are the carboxyl O atoms of CEbpy ligands and benzenedicarboxylic acids to provide electrons to the pyridinium N atoms. Structural analysis of the shortest ET distances for the three MOFs reveals that the O1⋯N1 distance is 2.767 Å and the O1⋯N1⋯C2 angle is 60.39° in compound 1, the O1⋯N1 distance is 2.709 Å and the O1⋯N1⋯C2 angle is 60.78° in compound 2, the O2⋯N2 distance is 2.717 Å and the O1⋯N1⋯C2 angle is 60.31° in compound 3. These values favor the interaction between the carboxylate group donor and the viologen acceptor unit.24 The tight condensed packing mode provides an opportunity for intramolecular ET and creates a viologen radical with color changes. The ET distances of the three compounds are much shorter than those commonly reported (>3.0 Å),25 thus it may be the reason that why the three compounds are not only sensitive to UV-light but also sensitive to sunlight. Compound 2 is also found sensitive to indoor-light, rather than compounds 1 and 3. By analyzing and comparing the interactions around the pyridine rings of the three compounds, this phenomenon is mainly caused by the following three reasons: firstly, the distance (2.709 Å) between the carboxylate O and the pyridinium N in 2 is shorter than 1 (2.767 Å), and similar to 3 (2.717 Å); secondly, comparing the structures of 2 and 3, compound 2 forms a strong π–π stacking interaction between the pyridine rings of CEbpy ligands, promoting the ET progress, while in 3, there is no π–π stacking interaction; at last, compared to 3, the interactions of the O(7)–H⋯N(2) hydrogen bonds between the CEbpy ligand and the coordinated water molecules in 2 may also improve the ET.
4. Conclusions
In summary, three MOFs {[Cd(CEbpy)(m-BDC)(DMF)]·2H2O}n (1), {[Cd(CEbpy)(p-BDC)·(H2O)]·H2O}n (2), {[Zn(CEbpy)(p-HBDC)(p-BDC)0.5]·H2O}n (3) based on a viologen-derived ligand 1-carboxyethyl-4,4′-bipyridine (CEbpy) and benzenedicarboxylic acids (m-H2BDC = 1,3-benzenedicarboxylic acid, p-H2BDC = 1,4-benzenedicarboxylic acid) have been synthesized and characterized. The introduction of the carboxylate-viologen ligand into the closely packed MOFs facilitates long-lived color constancy achieved by the condensed packing mode. Given the existence of many known stable MOFs, structural designs can provide an opportunity to create novel stable crystalline materials with various photochromic components as building blocks.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the 1331 Engineering of Shanxi Province.References
- (a) A.-Y. Robin and K.-M. Fromm, Coord. Chem. Rev., 2006, 250, 2127 CrossRef CAS; (b) X.-X. Li, H.-G. Xu, F.-Z. Kong and R.-H. Wang, Angew. Chem., Int. Ed., 2013, 51, 14014 CrossRef; (c) J.-K. Sun and J. Zhang, Dalton Trans., 2015, 44, 19041 RSC.
- (a) S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS; (b) M.-H. Li and P. Keller, Soft Matter, 2009, 5, 927 RSC; (c) G. Rogez, N. Viart and M. Drillon, Angew. Chem., Int. Ed., 2010, 49, 1921 CrossRef CAS.
- (a) Y.-X. Xie, W.-N. Zhao, G.-C. Li, P.-F. Liu and L. Han, Inorg. Chem., 2016, 55, 549 CrossRef CAS; (b) W. Yu, X.-Y. Wang, J. Li, Z.-T. Li, Y.-K. Yan, W. Wang and J. Pei, Chem. Commun., 2013, 49, 54 RSC; (c) P.-F. Hao, H.-H. Li, T.-L. Yu and Y.-L. Fu, Dyes Pigm., 2017, 136, 825 CrossRef CAS; (d) Y.-J. Ma, J.-X. Hu, S.-D. Han, J. Pan, J.-H. Li and G.-M. Wang, Chem. Commun., 2019, 55, 5631 RSC.
- (a) Q.-W. Zhang, H.-Q. Sun, X.-S. Wang, X.-H. Hao and S.-L. An, ACS Appl. Mater. Interfaces, 2015, 7, 25289 CrossRef CAS PubMed; (b) H. Zhou, L. Qin, M.-K. Wu and L. Han, Cryst. Growth Des., 2018, 18, 5738 CrossRef; (c) H.-Y. Wang, S. Liu, C. Fu and H. Zhang, CrystEngComm, 2019, 21, 1635 RSC.
- (a) J.-K. Sun, P. Wang, Q.-X. Yao, Y.-J. Chen, Z.-H. Li, Y.-F. Zhang, L.-M. Wu and J. Zhang, J. Mater. Chem., 2012, 22, 12212 RSC; (b) Q. Sui, Y. Yuan, N.-N. Yang, X. Li, T. Gong and E.-Q. Gao, J. Mater. Chem. C, 2017, 5, 12400 RSC; (c) T.-M. Bockman and J.-K. Kochi, J. Org. Chem., 1990, 55, 4127–4135 CrossRef CAS.
- (a) Y. Alesanco, A. Viñuales, J. Palenzuela, I. Odriozola, G. Cabañero, J. Rodriguez and T.-Z. Ramón, ACS Appl. Mater. Interfaces, 2016, 8, 14795 CrossRef CAS; (b) H.-G. Chen, M. Li, G.-M. Zheng, Y.-F. Wang, Y. Song, C.-H. Han, Z.-Y. Fu, S.-J. Liao and J.-C. Dai, RSC Adv., 2014, 4, 42983 RSC.
- (a) Q. Sui, P. Li, N.-N. Yang, T. Gong, R. Bu and E.-Q. Gao, ACS Appl. Mater. Interfaces, 2018, 10, 11056 CrossRef CAS; (b) A.-S. Abouelwafa, V. Mereacre, T.-S. Balaban, C.-E. Anson and A.-K. Powell, CrystEngComm, 2010, 12, 94 RSC; (c) K. Fu, C.-X. Ren, C. Chen, L.-X. Cai, B. Tan and J. Zhang, CrystEngComm, 2014, 16, 5134 RSC.
- (a) X.-D. Yang, C. Chen, Y.-J. Zhang, L.-X. Cai and J. Zhang, Inorg. Chem. Commun., 2015, 60, 2122 Search PubMed; (b) S.-Z. Hu, M.-L. You, S.-H. Chen and Z.-Y. Fu, CrystEngComm, 2016, 18, 7221 RSC.
- (a) C.-C. Ko and V.-W.-W. Yam, Acc. Chem. Res., 2018, 51, 149 CrossRef CAS; (b) J. Chai, Y.-B. Wu, B.-S. Yang and B. Liu, J. Mater. Chem. C, 2018, 6, 4057 RSC; (c) J.-Z. Liao, S.-S. Wang, X.-Y. Wu, R.-M. Yu, C.-Z. Lu and X.-L. Chen, Dalton Trans., 2018, 47, 1027 RSC.
- (a) C. Tao, J. Wu, Y. Yan, C. Shi and J. Li, Inorg. Chem. Front., 2016, 3, 541 RSC; (b) X.-H. Jin, J.-K. Sun, X.-M. Xu, Z.-H. Li and J. Zhang, Chem. Commun., 2010, 46, 4695 RSC.
- (a) J.-J. Liu and J. Li, Dyes Pigm., 2019, 170, 107552 CrossRef CAS; (b) J.-J. Liu, Dyes Pigm., 2019, 160, 476 CrossRef CAS; (c) X.-D. Yang, R. Zhu, J.-P. Yin, L. Sun, R.-Y. Guo and J. Zhang, Cryst. Growth Des., 2018, 18, 3236 CrossRef CAS.
- (a) G. Xu, G.-C. Guo, M.-S. Wang, Z.-J. Zhang, W.-T. Chen and J.-S. Huang, Angew. Chem., Int. Ed., 2007, 46, 3249 CrossRef CAS; (b) Z.-J. Zhang, S.-C. Xiang, G.-C. Guo, G. Xu, M.-S. Wang, J.-P. Zou, S.-P. Guo and J.-S. Huang, Angew. Chem., Int. Ed., 2008, 47, 4149 CrossRef CAS; (c) N. Leblanc, M. Allain, N. Mercier and L. Sanguinet, Cryst. Growth Des., 2011, 11, 2064 CrossRef CAS.
- (a) R.-G. Lin, G. Xu, M.-S. Wang, G. Lu, P.-X. Li and G.-C. Guo, Inorg. Chem., 2013, 52, 1199 CrossRef CAS; (b) N. Leblanc, W. Bi, N. Mercier, P. Auban-Senzier and C. Pasquier, Inorg. Chem., 2010, 49, 5824 CrossRef CAS.
- (a) Y. Zeng, Z.-Y. Fu, H.-J. Chen, C.-C. Liu, S.-J. Liao and J.-C. Dai, Chem. Commun., 2012, 48, 8114 RSC; (b) M. Antonietta Loi, P. Denk, H. Hoppe, H. Neugebauer, C.-D. Meissner, C. Brabec, N. S. Sariciftci, A. Gouloumis, P. Vázquez and T. Torres, J. Mater. Chem., 2003, 13, 700 RSC.
- (a) J.-H. Li, S.-D. Han, J. Pan, Z.-Z. Xue, G.-M. Wang, Z.-H. Wang and Z.-Z. Bao, CrystEngComm, 2017, 19, 1160 RSC; (b) F.-P. Huang, J.-B. Lei, Q. Yu, H.-D. Bian and S.-P. Yan, Polyhedron, 2012, 34, 129 CrossRef CAS; (c) X.-D. Yang, C. Chen, Y.-J. Zhang, L.-X. Cai, B. Tan and J. Zhang, Dalton Trans., 2016, 45, 4522 RSC.
- (a) F. Wan, L.-X. Qiu, L.-L. Zhou, Y.-Q. Sun and Y. You, Dalton Trans., 2015, 44, 18320 RSC; (b) J.-J. Liu, Y. Chen, M.-J. Lin, C.-C. Huang and W.-X. Dai, Dalton Trans., 2016, 45, 6339 RSC; (c) X.-D. Yang, L. Sun, C. Chen, Y.-J. Zhang and J. Zhang, Dalton Trans., 2017, 46, 4366 RSC.
- A. Cousson, B. Bachet, B. Koket and M. Hubert-Habart, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1993, 49, 942 CrossRef.
- CrysAlisPro, version 1.171.33.56, Oxfordshire, UK, Oxford diffraction Ltd, 2010 Search PubMed.
- (a) G.-M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr, 2008, A64, 112 CrossRef; (b) G.-M. Sheldrick, SHELXS-97, Göttingen University, Germany, 1997 Search PubMed.
- (a) P.-X. Li, M.-S. Wang, L.-Z. Cai, G.-E. Wang and G.-C. Guo, J. Mater. Chem. C, 2015, 3, 253 RSC; (b) q.-x. Yao, L. Pan, X.-H. Jin, J. Li, Z.-F. Ju and J. Zhang, Chem.–Eur. J., 2009, 15, 11890 CrossRef CAS; (c) A.-Y. Ni, Y. Mu, J. Pan, S.-D. Han, M.-M. Shang and G.-M. Wang, Chem. Commun., 2018, 54, 3712 RSC.
- (a) M.-S. Wang, G. Xu, Z.-J. Zhang and G.-C. Guo, Chem. Commun., 2010, 46, 361 RSC; (b) N. Mercier, Eur. J. Inorg. Chem., 2013, 2013, 19 CrossRef; (c) W.-B. Li, Q.-W. Yao, L. Sun, X.-D. Yang, R.-Y. Guo and J. Zhang, CrystEngComm, 2017, 19, 722 RSC.
- (a) T. Gong, X. Yang, J.-J. Fang, Q. Sui, F.-G. Xi and E.-Q. Gao, ACS Appl. Mater. Interfaces, 2017, 9, 5503 CrossRef CAS; (b) C.-H. Zhang, L.-B. Sun, Y. Yan, H.-Z. Shi, B.-L. Wang and Z.-Q. Liang, J. Mater. Chem. C, 2017, 5, 8999 RSC.
- (a) T. Gong, P. Li, Q. Sui, L.-J. Zhou, N.-N. Yang and E.-Q. Gao, Inorg. Chem., 2018, 57, 6791 CrossRef CAS; (b) T. Gong, Q. Sui, P. Li, X. F. Meng, L.-J. Zhou, J.-Q. Chen, J.-H. Xu, L. Wang and E.-Q. Gao, Small, 2019, 15, 1803468 CrossRef; (c) Q. Sui, N.-N. Yang, T. Gong, P. Li, Y. Yuan, E.-Q. Gao and L. Wang, J. Phys. Chem. Lett., 2017, 8, 5450 CrossRef CAS.
- (a) J.-J. Liu, Y.-F. Guan, M.-J. Lin, C.-C. Huang and W.-X. Dai, Cryst. Growth Des., 2016, 16, 2836 CrossRef CAS; (b) S.-L. Li, M. Han, B. Wu, J. Wang and X.-M. Zhang, Cryst. Growth Des., 2018, 18, 3883 CrossRef CAS.
- (a) W. Yao, Y.-L. Yan, L. Xue, C. Zhang, G.-P. Li, Q.-D. Zheng, Y.-S. Zhao, H. Jiang and J.-N. Yao, Angew. Chem., Int. Ed., 2013, 52, 8713 CrossRef CAS; (b) H.-J. Chen, G.-M. Zheng, M. Li, Y.-F. Wang, Y. Song, C.-H. Han, J.-C. Dai and Z.-Y. Fu, Chem. Commun., 2014, 50, 13544 RSC; (c) J.-J. Liu, S.-B. Xia, Y.-L. Duan, T. Liu, F.-X. Cheng and C.-K. Sun, Polymers, 2018, 10, 165 CrossRef; (d) Y.-X. Hu, Y.-B. Dong, X.-N. Sun, G.-R. Zuo, J. Yin and S.-H. Liu, Dyes Pigm., 2018, 156, 260 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1956829, 1922958, 1956570, 1902518 and 1918048. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra06703e |
| This journal is © The Royal Society of Chemistry 2019 |