Open Access Article
Open Access ArticlePromoted dispersion and uniformity of active species on Fe–Ce–Al catalysts for efficient NO abatement†
Xiaobo Wang *ab,
Caojian Jianga,
Jia Wangc,
Keting Guid and
Hywel R. Thomasb
*ab,
Caojian Jianga,
Jia Wangc,
Keting Guid and
Hywel R. Thomasb
aSchool of Environmental Science, Nanjing Xiaozhuang University, Nanjing 211171, Jiangsu, China. E-mail: xb_wang88@126.com
bGeoenvironmental Research Centre, School of Engineering, Cardiff University, Cardiff, CF24 3AA, UK
cCollege of Chemical Engineering, Nanjing Forestry University, Nanjing 210037, Jiangsu, China
dSchool of Energy and Environment, Southeast University, Nanjing 210096, Jiangsu, China
First published on 4th November 2019
Abstract
Fe–Ce–Al catalysts were synthesized by the co-precipitation method (labeled as Fe–Ce–Al–P), co-impregnation method (Fe–Ce–Al–I), and direct mixing method (Fe–Ce–Al–M), respectively, and used for effective removal of NO. The synthesized catalysts were characterized by many methods including N2 physisorption, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), NH3-temperature programmed desorption (NH3-TPD), H2-temperature programmed reduction (H2-TPR), high-resolution transmission electron microscopy (HR-TEM), and energy dispersive spectroscopy (EDS) mapping. The results show that the synthesis methods greatly influence the catalytic performance of catalysts. The Fe–Ce–Al–P catalyst prepared by the co-precipitation method yields the highest catalytic performance, while the Fe–Ce–Al–I and Fe–Ce–Al–M catalysts exhibit relatively low catalytic activity. The co-precipitation method can promote the accumulation and dispersion of more surface active species on the catalyst surface, and provide smaller particle size of active species and generate more uniform particle size distribution, while these characteristics can't be obtained by the co-impregnation method and direct mixing method. Moreover, the co-precipitation method could produce the highest surface area and enhanced redox ability and surface acidity of the catalyst, which resulted from the high dispersion and uniform distribution of surface active species. These may be the key factors to the superior catalytic performance of the Fe–Ce–Al–P catalyst.
1. Introduction
Nitrogen oxides (NOx), as a typical pollutant from the combustion process, have caused severe problems in the environment and to human health.1 To date, several methods and technologies have been developed to remove NOx and selective catalytic reduction (SCR) is proven to be the most effective route for NO abatement.2 The key issue for SCR technology is the catalyst and the currently used catalysts for this process are V2O5/TiO2 or V2O5–WO3/TiO2.3 Although the V2O5–WO3–TiO2 (VWTi) catalyst system could yield high SCR catalytic activity, it also possesses inherent drawbacks, such as relatively narrow catalytic activity temperature window, the poisonous effect of VOx to the ecosystem and higher oxidation activity of SO2 to SO3.4 Due to this fact, many highly active and environmentally friendly catalysts without VOx have been developed and may be an alternative route to solve these problems mentioned above.According to this view, some transition-metal containing oxides, including FeOx, CuOx, MnOx, CeOx, and CrOx, have been designed for the replacement of the commercial SCR catalysts.5–9 Among them, the Fe-containing catalysts have attracted special attention due to its better catalytic activity, favorable N2 selectivity and nontoxicity.9,10 And especially the bimetallic Fe catalysts, including Fe–Mn,11,12 Fe–Ce,9,13 Fe–Cu14 and FexW1−xOδ,15 exhibited superior catalytic activity for the SCR reaction. For the Fe–Ce catalysts, many researchers have widely studied this system from different levels and angles. Jiang et al. doped Ce onto Fe/β by a simple impregnation step and found Fe–Ce/β catalyst showed high catalytic activity and better hydrothermal stability.9 Ma et al. supported Fe–Ce on carbon nanotube by an ethanol impregnation method and ascribed its excellent catalytic performance to the crystal CeO2 and enriched chemisorbed oxygen of catalyst surface.16 Zhang et al. synthesized Fe–S–Ce catalysts using the one-pot method and found the excellent SCR performance had a close relationship with the abundant chemisorbed oxygen and the enhanced surface acidity and redox property.17 Previous studies for the Fe–Ce catalysts mainly focused on the Fe–Ce mixed metal oxides prepared by the one-pot method and supported Fe–Ce on H-β zeolite and carbon nanotube, respectively, using the impregnation method. It is well known that the catalytic performance of catalysts greatly depends on the synthesis methods, support, precursors, oxidation state and crystallinity of metal oxides.18 However, for the Fe–Ce–Al catalysts, the effect of catalysts synthesis methods on the catalytic activity is still lack of study, and the research on promoting the uniformity of particle size distribution and dispersion characteristics of surface active species by different synthesis methods to achieve superior catalytic performance is not systematically reported in the literature so far, especially the research on the mechanism of optimizing the physicochemical properties of catalyst by promoting the uniformity and dispersion of surface active species. Therefore, based on the discussion above, it is of great importance to reveal the intrinsic relationship among the component, the structure and the catalytic performance of catalyst resulted from different synthesis methods in the angle of promoting active species dispersion and uniformity on Fe–Ce–Al catalysts.
In the present work, the Fe–Ce/Al2O3 catalysts with the same Fe/Ce molar ratio were synthesized by three different synthetic strategies and used in the NH3-SCR reaction. The relationship between the physicochemical properties resulted from different synthesis methods and the excellent catalytic activity was explored via different characterization techniques, such as BET, XRD, H2-TPR, NH3-TPD, XPS, HR-TEM, and EDS mapping.
2. Materials and methods
2.1 Catalysts synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce being 1
Ce being 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. And then, 25 g Al2O3 power was added to the solution with magnetic stirring for 10 min at 50 °C. Later, the ammonia solution (wt 25%) was gradually added to with continuous stirring until the pH value reached 9–10. After stirring for 1.5 h, the resulting suspension was first dried at 120 °C for 12 h and then air-calcined at 450 °C for 5 h. And then, the calcined samples were compressed into tablet form and finally crushed and sieved to 30–60 mesh size for use. The sample was denoted as Fe–Ce–Al–P.
2. And then, 25 g Al2O3 power was added to the solution with magnetic stirring for 10 min at 50 °C. Later, the ammonia solution (wt 25%) was gradually added to with continuous stirring until the pH value reached 9–10. After stirring for 1.5 h, the resulting suspension was first dried at 120 °C for 12 h and then air-calcined at 450 °C for 5 h. And then, the calcined samples were compressed into tablet form and finally crushed and sieved to 30–60 mesh size for use. The sample was denoted as Fe–Ce–Al–P.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce = 1
Ce = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. And then the solution was stirred for 10 min at 50 °C. The following processes were the same as the co-precipitation method mentioned above without adding the ammonia solution (wt 25%) to the solution. The sample was named as Fe–Ce–Al–M.
2. And then the solution was stirred for 10 min at 50 °C. The following processes were the same as the co-precipitation method mentioned above without adding the ammonia solution (wt 25%) to the solution. The sample was named as Fe–Ce–Al–M.2.2 Catalyst characterization
N2 adsorption–desorption was carried out at −196 °C on a Quantachrome Quadrisorb SI instrument.X-ray diffraction (XRD) patterns were obtained on an X-ray diffractometer (Smartlab 9, Japan) to study the crystal structure of the sample with CuKα radiation in the 2θ range from 10 to 90°.
X-ray photoelectron spectroscopy (XPS) analysis was performed to investigate the surface elements and chemical states of the samples on Thermo Fisher Scientific XPS (EXCALAB 250Xi) spectroscopy.
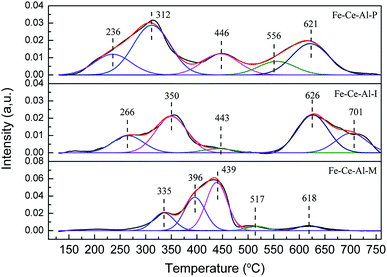
NH3-TPD and H2-TPR experiments were carried out on a Micromeritics Autochem 2920 II instrument to investigate the acidity and redox property of each studied sample. For the H2-TPR experiments, about 110 mg sample was used and pretreated in N2 at 200 °C for 1 h. And the data was recorded from 100 to 760 °C with a heating rate of 10 °C min−1. For the NH3-TPD experiments, about 200 mg sample was used and pretreated with He at 450 °C for 1 h. And the TPD experiment was conducted from 100 to 800 °C with a heating rate of 10 °C min−1.
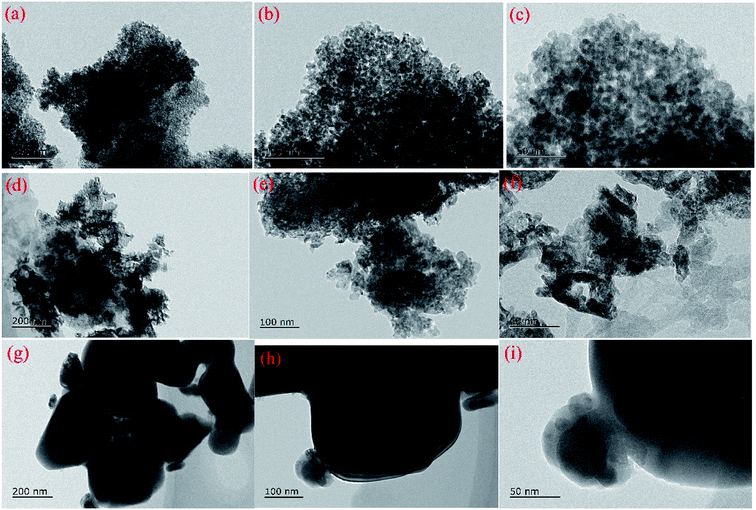
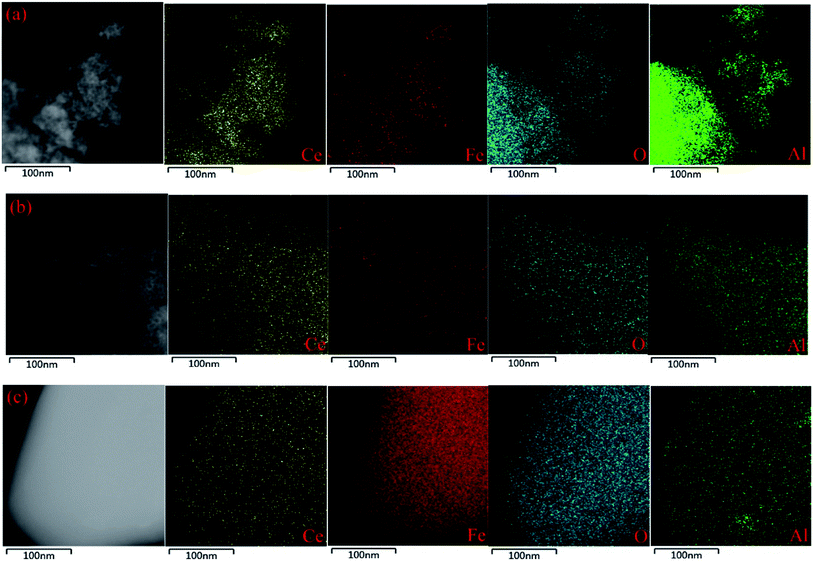
HR-TEM images and EDS mappings were obtained using a JOEL JEM-2100 electron microscope operated at 200 kv to investigate the microstructure of the samples and the elements dispersion.
2.3 Catalytic activity measurement
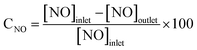
SCR activity measurement was accomplished in a fixed bed reactor using a 5 mL sample (30–60 mesh). The simulated flue gas was composed of NH3 (500 ppm), NO (500 ppm), 3% O2 and balance N2. The reactions were executed with a total gas flow rate of 1.5 L min−1. All the data were measured online by a flue gas analyzer (rbr ECOM–J2KN, Germany) under steady state. The NOx conversion was calculated via:
 | (1) |
3. Results and discussions
3.1 Catalytic performance
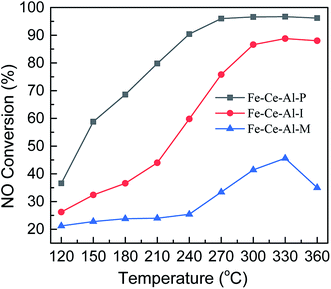
The NO conversion of Fe–Ce–Al catalysts prepared by different methods is shown in Fig. 1. It can be seen that the Fe–Ce–Al–M catalyst exhibits the lowest NO conversion, which is less than 45.6% at 120–360 °C. And the SCR catalytic activity of the Fe–Ce–Al–I catalyst is greatly improved compared with that of the Fe–Ce–Al–M catalyst. With the increase of reaction temperature, the NO conversion of Fe–Ce/Al2O3–I catalyst gradually increases from 26.2% to 88.8% at 120–330 °C and then decreases to 88% at 360 °C. For the Fe–Ce–Al–P catalyst, it yields the highest SCR activity in the whole temperature range. More than 96% NO conversion can be obtained in 270–360 °C. These three catalysts prepared by different methods have the same chemical compositions but show a significant difference in catalytic activity. The results demonstrate that the preparation methods have a great effect on its catalytic activity. Furthermore, as shown in Fig. S1,† after a 6 h long reaction at 330 °C, the Fe–Ce–Al–P catalyst still exhibits the best catalytic activity, suggesting the superior activity of the Fe–Ce–Al–P catalyst. The effect of different physicochemical properties of catalysts resulted from the preparation methods on the catalytic performance will be systematically investigated using various characterization technique in the following sections.3.2 Catalyst characterization
| Catalysts | SBET (m2 g−1) | Pore volume (mm3 g−1) | Pore diameter (nm) |
|---|---|---|---|
| Fe–Ce–Al–P | 122.30 | 0.23 | 3.72 |
| Fe–Ce–Al–I | 94.88 | 0.20 | 3.51 |
| Fe–Ce–Al–M | 94.09 | 0.17 | 3.94 |
| Al2O3 | 168.30 | 0.27 | 3.51 |
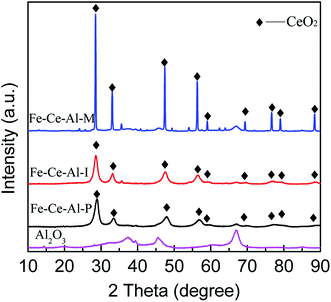
| Catalysts | 2 Theta (°) | Area | FMWH (rad) | D (nm) |
|---|---|---|---|---|
| Fe–Ce–Al–P | 28.68 | 181![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128 128 |
1.22 | 6.8 |
| Fe–Ce–Al–I | 28.56 | 173![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 729 729 |
1.11 | 7.4 |
| Fe–Ce–Al–M | 28.47 | 269![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135 135 |
0.17 | 59.6 |
| Catalysts | Binding energy (eV) | Surface atomic ratio (%) | ||||
|---|---|---|---|---|---|---|
| Fe 2p | O 1s | Fe3+/(Fe3+ + Fe2+) | Ce3+/(Ce3+ + Ce4+) | Oα/(Oα + Oβ) | ||
| Fe3+ | Fe2+ | Oα | ||||
| Fe–Ce–Al–P | 711.5 | 710.5 | 531.8 | 68.62 | 37.91 | 42.72 |
| Fe–Ce–Al–I | 712.5 | 710.7 | 531.8 | 38.47 | 19.16 | 27.99 |
| Fe–Ce–Al–M | 713.5 | 710.8 | 532.6 | 18.24 | 17.29 | 20.28 |
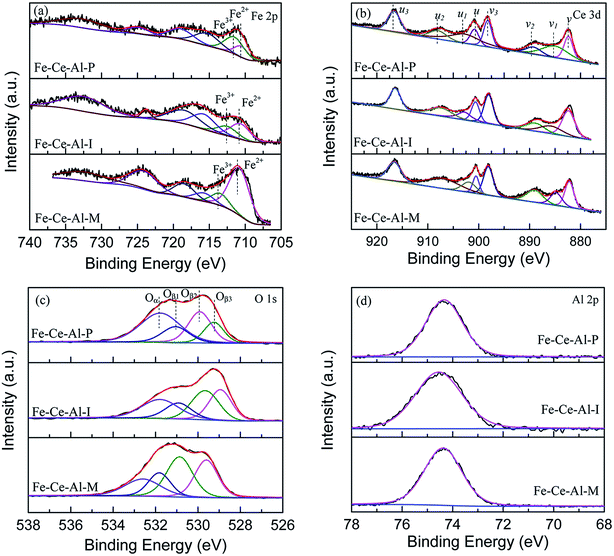
The Fe 2p spectra for the investigated catalysts are shown in Fig. 3(a). Two characteristic peaks located at about 710.8–711.3 eV and 723.8–725 eV could be ascribed to Fe 2p3/2 and Fe 2p1/2, respectively. And the spectra of Fe 2p3/2 can be fitted into two sub-bands. The peak locates at about 710.5 eV is attributed to Fe2+, while the peak at about 712.5 eV is ascribed to the Fe3+, indicating the coexistence of FeO and Fe2O3 on the surface of studied samples.22 Moreover, compared with that of the Fe–Ce–Al–I and Fe–Ce–Al–M catalysts, the binding energies for Fe2+ and Fe3+ over Fe–Ce–Al–P catalyst are shifted to lower values, indicating the Fe species on the surface of Fe–Ce–Al–P catalyst prepared by co-precipitation method are more active, which will help to promote the catalytic performance.7 The relative proportions of Fe3+ and Fe2+ were calculated by the peak area of Fe 2p3/2 for Fe3+ and Fe2+ (listed in Table 3). The Fe3+/(Fe3++Fe2+) over the Fe–Ce–Al–P catalyst is 68.62%, while it is only 38.47% and 18.24% for the Fe–Ce–Al–I and Fe–Ce–Al–M catalysts, respectively. It indicates that the Fe–Ce–Al–P catalyst prepared by the co-precipitation method can generate more Fe3+ species on the catalyst surface. And more Fe3+ sites could promote the conversion of NO to NO2 during the SCR reaction and thus enhance the catalytic activity through the “Fast SCR” reaction.22,23
The XPS spectra of the Ce 3d for the three catalysts are displayed in Fig. 3(b), which all can be fitted into peaks labeled as u, u1, u2, u3, v, v1, v2, and v3, respectively. The peaks denoted as u1 and v1 are attributed to the Ce3+, while others are ascribed to Ce4+, suggesting the coexistence of the Ce4+ and Ce3+ on the catalyst surface. In addition, the Ce3+/(Ce4+ + Ce3+) ratio over the three samples is decreased as follows: Fe–Ce–Al–P (37.91%) > Fe–Ce–Al–I (19.16%) > Fe–Ce–Al–M (17.29%). It fully indicates that the contents of Ce3+ species are closely related to the preparation methods of the catalysts and more Ce3+ species can be generated on the catalyst surface by the co-precipitation method. On one hand, more Ce3+ species can generate more charge imbalance and oxygen vacancies on the catalyst surface, leading to the enhancement of surface chemisorbed oxygen and therefore facilitation of NO oxidation;21,24,25 on the other hand, more surface Ce3+ species can promote the adsorption of NO and NH3, and thus contribute to the enhancement of catalytic activity.26,27 These may be the reasons for the superior catalytic activity of the Fe–Ce–Al–P catalyst.
The O 1s spectra of the studied samples after peak fitting is shown in Fig. 3(c) can be separated into two groups. The peak Oα located at about 532 eV is assigned to the surface adsorbed oxygen, such as O22−, OH, and O− species;1,28 while the peak Oβ centered at about 530 eV is ascribed to the lattice oxygen.29 After further peak fitting, the peak Oβ can be divided into three peaks labeled as Oβ1, Oβ2 and Oβ3, respectively. The Oβ1 recorded at about 529.3 ± 0.3 eV is originated from the CeO2,30–32 and the Oβ2 located at 529.9 ± 0.3 eV can be attributed to oxygen species corresponding to the Fe2O3;33–36 while the Oβ3 at about 531 eV can be assigned to the Al–O bonds of Al2O3.37–42 Additionally, the sequence of the relative amount of Oα is ranked by Fe–Ce–Al–P > Fe–Ce–Al–I > Fe–Ce–Al–M catalyst. As the most active catalyst, the Fe–Ce–Al–P catalyst is provided with the highest content of surface adsorbed oxygen with the lowest binding energy, implying the improved oxygen mobility, which is beneficial to promote SCR performance.
The XPS spectra of Al 2p for different samples are detailed in Fig. 3(d). The binding energies of the Al 2p are all centered at about 74.4 ± 0.1 eV. The basically unchanged binding energies of Al 2p demonstrates that the synergistic effect between Fe and Ce species is dominant over the catalysts.
Based on the XPS discussion above, the Fe–Ce–Al–P catalyst possesses the highest content of Fe3+ and Ce3+, the redox couples between Fe3+/Fe2+ and Ce4+/Ce3+ may enhance the redox cycle, which would accelerate the electron transfer and promote the generation of oxygen vacancy, and therefore facilitate the catalytic performance.29,43
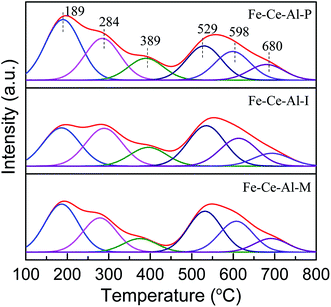
| Catalysts | Acidity (mmol g−1) | |||
|---|---|---|---|---|
| Weak | Moderate | Strong | Total | |
| Fe–Ce–Al–P | 0.218 | 0.046 | 0.167 | 0.431 |
| Fe–Ce–Al–I | 0.167 | 0.041 | 0.178 | 0.386 |
| Fe–Ce–Al–M | 0.161 | 0.026 | 0.167 | 0.354 |
4. Conclusion
In this work, Fe–Ce–Al catalysts were prepared by three different methods and the synthesis methods have a direct impact on the catalytic performance of catalysts. The Fe–Ce–Al–P catalyst prepared by the co-precipitation method yields the highest catalytic performance, while the Fe–Ce–Al–I and Fe–Ce–Al–M catalyst exhibit relatively low denitration efficiency. Compared with that of Fe–Ce–Al–I and Fe–Ce–Al–M catalyst, the Fe–Ce–Al–P catalysts before and after the reaction possess the best dispersion of active species and more surface active species are accumulated on the surface of Fe–Ce–Al–P catalyst. And the Fe–Ce–Al–P catalyst could provide smaller particle sizes of active species and generate more uniform particle sizes distribution, all mentioned above may be the key factors to the superior catalytic performance. Moreover, the co-precipitation method could produce the highest surface area and the enhanced redox ability and surface acidity of the catalyst due to the high dispersion and uniform distribution of surface active pieces, and these characteristics will also directly contribute to the superior catalytic performance of Fe–Ce–Al–P catalyst.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51276039), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (17KJB610005), the Jiangsu Government Scholarship for Overseas Studies (JS-2018), and a project funded by Nanjing Xiaozhuang University (2016NXY41).References
- J. Liu, R. T. Guo, M. Y. Li, P. Sun, S. M. Liu, W. G. Pan, S. W. Liu and X. Sun, Fuel, 2018, 223, 385–393 CrossRef CAS.
- L. J. France, Q. Yang, W. Li, Z. Chen, J. Guang, D. Guo, L. Wang and X. Li, Appl. Catal., B, 2017, 206, 203–215 CrossRef CAS.
- R. T. Guo, X. Sun, J. Liu, W. G. Pan, M. Y. Li, S. M. Liu, P. Sun and S. W. Liu, Appl. Catal., A, 2018, 558, 1–8 CrossRef CAS.
- S. Han, Q. Ye, S. Cheng, T. Kang and H. Dai, Catal. Sci. Technol., 2017, 7, 703–717 RSC.
- F. Cao, S. Su, J. Xiang, P. Wang, S. Hu, L. Sun and A. Zhang, Fuel, 2015, 139, 232–239 CrossRef CAS.
- L. Zhu, L. Zhang, H. Qu and Q. Zhong, J. Mol. Catal. A: Chem., 2015, 409, 207–215 CrossRef CAS.
- B. Shen, F. Wang and T. Liu, Powder Technol., 2014, 253, 152–157 CrossRef CAS.
- R. Yang, H. Huang, Y. Chen, X. Zhang and H. Lu, Chin. J. Catal., 2015, 36, 1256–1262 CrossRef CAS.
- S.-Y. Jiang and R.-X. Zhou, Fuel Process. Technol., 2015, 133, 220–226 CrossRef CAS.
- B. Feng, Z. Wang, Y. Sun, C. Zhang, S. Tang, X. Li and X. Huang, Catal. Commun., 2016, 80, 20–23 CrossRef CAS.
- S. Yang, F. Qi, S. Xiong, H. Dang, Y. Liao, P. K. Wong and J. Li, Appl. Catal., B, 2016, 181, 570–580 CrossRef CAS.
- W. Mu, J. Zhu, S. Zhang, Y. Guo, L. Su, X. Li and Z. Li, Catal. Sci. Technol., 2016, 6, 7532–7548 RSC.
- Y. Shu, T. Aikebaier, X. Quan, S. Chen and H. Yu, Appl. Catal., B, 2014, 150–151, 630–635 CrossRef CAS.
- T. Zhang, J. Liu, D. Wang, Z. Zhao, Y. Wei, K. Cheng, G. Jiang and A. Duan, Appl. Catal., B, 2014, 148–149, 520–531 CrossRef CAS.
- H. Wang, Z. Qu, S. Dong, H. Xie and C. Tang, Environ. Sci. Technol., 2016, 50, 13511–13519 CrossRef CAS.
- Y. Ma, D. Zhang, H. Sun, J. Wu, P. Liang and H. Zhang, Ind. Eng. Chem. Res., 2018, 57, 3187–3194 CrossRef CAS.
- X. Zhang, J. Wang, Z. Song, H. Zhao, Y. Xing, M. Zhao, J. Zhao, Z. a. Ma, P. Zhang and N. Tsubaki, Mol. Catal., 2019, 463, 1–7 CrossRef CAS.
- S. S. R. Putluru, L. Schill, A. D. Jensen, B. Siret, F. Tabaries and R. Fehrmann, Appl. Catal., B, 2015, 165, 628–635 CrossRef CAS.
- W. Zhao, Q. Zhong, Y. Pan and R. Zhang, Chem. Eng. J., 2013, 228, 815–823 CrossRef CAS.
- S. Andreoli, F. A. Deorsola, C. Galletti and R. Pirone, Chem. Eng. J., 2015, 278, 174–182 CrossRef CAS.
- K. Chen, R. Chen, H. Cang, A. Mao, Z. Tang and Q. Xu, Environ. Technol., 2018, 39, 1753–1764 CrossRef CAS.
- H. Xu, Y. Li, B. Xu, Y. Cao, X. Feng, M. Sun, M. Gong and Y. Chen, J. Ind. Eng. Chem., 2016, 36, 334–345 CrossRef CAS.
- G. Delahay, D. Valade, A. Guzmanvargas and B. Coq, Appl. Catal., B, 2005, 55, 149–155 CrossRef CAS.
- T. Wang, H. Liu, X. Zhang, Y. Guo, Y. Zhang, Y. Wang and B. Sun, Fuel Process. Technol., 2017, 158, 199–205 CrossRef CAS.
- H. Li, C.-Y. Wu, Y. Li and J. Zhang, Appl. Catal., B, 2012, 111–112, 381–388 CrossRef CAS.
- Z. Song, Q. Zhang, P. Ning, J. Fan, Y. Duan, X. Liu and Z. Huang, J. Taiwan Inst. Chem. Eng., 2016, 65, 149–161 CrossRef CAS.
- C. Liu, L. Chen, H. Chang, L. Ma, Y. Peng, H. Arandiyan and J. Li, Catal. Commun., 2013, 40, 145–148 CrossRef CAS.
- P. Sun, S. X. Huang, R. T. Guo, M. Y. Li, S. M. Liu, W. G. Pan, Z. G. Fu, S. W. Liu, X. Sun and J. Liu, Appl. Surf. Sci., 2018, 447, 479–488 CrossRef CAS.
- T. Wang, Z. Wan, X. Yang, X. Zhang, X. Niu and B. Sun, Fuel Process. Technol., 2018, 169, 112–121 CrossRef CAS.
- R. Eloirdi, P. Cakir, F. Huber, A. Seibert, R. Konings and T. Gouder, Appl. Surf. Sci., 2018, 457, 566–571 CrossRef CAS.
- G. Praline, B. E. Koel, R. L. Hance, H. I. Lee and J. M. White, J. Electron Spectrosc. Relat. Phenom., 1980, 21, 17–30 CrossRef CAS.
- H. L. Dauscher, F. L. Normand, W. Müller, G. Maire and A. Vasquez, et al., Surf. Interface Anal., 1990, 16, 341–346 CrossRef.
- W. Liu, M. Zhu, J. Liu, X. Li and J. Liu, Chin. Chem. Lett., 2019, 30, 750–756 CrossRef CAS.
- K. Park, H. Y. Hong, G. W. Jung, D. H. Kim, D. A. Hakeem and A. Iqbal, Ceram. Int., 2018, 44, 15024–15034 CrossRef CAS.
- H. Liu, Z. G. Zhang, X. X. Wang, G. D. Nie, J. Zhang, S. X. Zhang, N. Cao, S. Y. Yan and Y. Z. Long, J. Phys. Chem. Solids, 2018, 121, 236–246 CrossRef CAS.
- X. Zhang, Y. Yang, L. Song, Y. Wang, C. He, Z. Wang and L. Cui, Mol. Catal., 2018, 447, 80–89 CrossRef CAS.
- H. Y. Yu, M. F. Li, B. J. Cho, C. C. Yeo, M. S. Joo, D. L. Kwong, J. S. Pan, C. H. Ang, J. Z. Zheng and S. Ramanathan, Appl. Phys. Lett., 2002, 81, 376–378 CrossRef CAS.
- N. M. Figueiredo, N. J. M. Carvalho and A. Cavaleiro, Appl. Surf. Sci., 2011, 257, 5793–5798 CrossRef CAS.
- J. Ding, C. Yu, J. Liu, C. Deng and H. Zhu, Mater. Chem. Phys., 2018, 206, 193–203 CrossRef CAS.
- C. K. Gao, J. Y. Yan, L. Dong and D. J. Li, Appl. Surf. Sci., 2013, 285, 287–292 CrossRef CAS.
- I. Iatsunskyi, M. Kempiński, M. Jancelewicz, K. Załęski, S. Jurga and V. Smyntyna, Vacuum, 2015, 113, 52–58 CrossRef CAS.
- X. Zhu, J. Yu, C. Jiang and B. Cheng, Phys. Chem. Chem. Phys., 2017, 19, 6957–6963 RSC.
- Q. Xu, R. Su, L. Cao, Y. Li, C. Yang, Y. Luo, J. Street, P. Jiao and L. Cai, RSC Adv., 2017, 7, 48785–48792 RSC.
- K. Zhuang, Y. P. Zhang, T. J. Huang, B. Lu and K. Shen, J. Fuel Chem. Technol., 2017, 45, 1356–1364 CrossRef CAS.
- F. Xia, Z. Song, X. Liu, X. Liu, Y. Yang, Q. Zhang and J. Peng, Res. Chem. Intermed., 2018, 44, 2703–2717 CrossRef CAS.
- A. Phuruangrat, S. Thongtem and T. Thongtem, Mater. Lett., 2017, 196, 61–63 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra06875a |
| This journal is © The Royal Society of Chemistry 2019 |