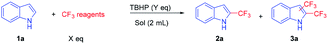Open Access Article
Open Access ArticleMetal-free oxidative trifluoromethylation of indoles with CF3SO2Na on the C2 position†
Jiao-Jiao Xie ,
Zhi-Qing Wang and
Guo-Fang Jiang
,
Zhi-Qing Wang and
Guo-Fang Jiang *
*
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, P. R. China. E-mail: gfjiang@hnu.edu.cn
First published on 30th October 2019
Abstract
An efficient method of synthesizing 2-trifluoromethylindoles from indoles with easy-to-handle, cheap and low-toxic CF3SO2Na under metal-free conditions is described, which selectively introduces trifluoromethyl to indoles on the C2 position. The desired product can be obtained in 0.7 g yield. A radical intermediate may be involved in this transformation.
Introduction
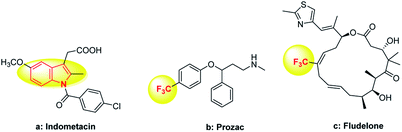
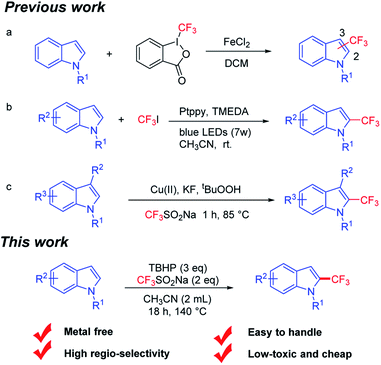
Indole compounds are widely found in nature and most of them are bioactive and diffusely used in medicine, as food additives and in other fields.1 For example, indometacin is one of the strongest prostaglandins and synthetic inhibitors (Fig. 1a).2 As a special functional group, trifluoromethyl is often applied to materials, pesticides and pharmaceuticals,3 which can enhance the polarity, stability and lipophilicity.4 For instance, Prozac is mainly used for the treatment of mental illness and fludelone can effectively inhibit cancer cells (Fig. 1b and c).5 Given the importance of indoles and trifluoromethyl groups, combining the two into a single entity would be very interesting. In this context, it is of profound significance to study new and potentially physiologically active indoles containing trifluoromethyl from the perspective of synthetic methodology and application prospects.The classical methods for the synthesis of trifluoromethyl compounds usually use Umemoto,6 Ruppert–Prakash,7 Langlois8 and Togni9 reagents or other reagents.10,12 For the past few decades, exploring effective ways to obtain 2-trifluoromethylindoles from indoles has gained increasing attention. Rey-Rodriguez et al.11 demonstrated the iron(II) catalyzed trifluoromethylation of indole under mild reaction conditions (Fig. 2a). Unfortunately, it showed low regioselectivity. Furthermore, Choi et al.12 described the platinum(II) complexes catalyzed trifluoromethylation of indole on the C2 position in the presence of CF3I and visible light; however, CF3I is difficult to preserve and toxic (Fig. 2b). In the recent years, CF3SO2Na has gradually become an environmentally friendly and cheap source of trifluoromethyl in the field of organic synthesis.13 Shi14 offered an efficient copper-catalyzed oxidation method for the trifluoromethylation of C3 position-blocked indoles (Fig. 2c). CF3SO2Na has been widely used as a CF3 source in organic synthesis; however, the metal-free and highly regioselective synthesis of 2-trifluoromethylindoles has rarely been reported. The disadvantages of expensive reagents, poor regioselectivity or difficult-to-handle reagents are often encountered. As part of our ongoing interest in the synthesis of indoles derivatives,15 we reported herein a tert-butyl hydroperoxide (TBHP)-promoted metal-free and highly regio-selective trifluoromethylation of indole on the C2 position using easy-to-handle and low-toxic CF3SO2Na as the trifluoromethyl source.
Initially, indole (1a) and CF3SO2Na were selected as model substrates for the optimization of the reaction conditions (Table 1). When 1a, 1 equiv. of CF3SO2Na, 2 equiv. of TBHP and 2 mL CH3CN were added to a sealed Pyrex test tube under room temperature, 2a was obtained in the yield of 15% (entry 1). Subsequently, higher temperature (80 °C and 140 °C) gave the desired product 2a in higher yields (36% and 45%, respectively) (entries 2–3). Next, we switched the ratio of raw materials. It was found that the amount of CF3SO2Na would greatly affect the yield of 2a, which showed that a small loading of CF3SO2Na resulted in less desired product and a higher loading of CF3SO2Na would result in byproduct 3a (entries 5–6). A higher yield of 2a was obtained in the presence of 3 equiv. of TBHP (entries 3–5). A reaction time of 18 hours was found to be enough for this transformation, which gave the desired product 2a in 66% yield (entry 5). Shortening the reaction time to 16 hours or prolonging the reaction time to 20 hours (even 24 hours) afforded 2a in the yield of 53% and 65% (66%), respectively (entries 5–9). When toluene, DMF and H2O were used as solvents, 2a was obtained in the yield of 49%, 41% and 30%, respectively (entries 10–11), while no targeting product 2a was obtained with DMSO or 1,4-dioxane as solvents. Besides, we also investigated other trifluoromethylation reagents such as TMSCF3 (trifluoromethyltrimethylsilane), using which only trace 2a was observed. In brief, CF3SO2Na (2.0 equiv.), TBHP (3.0 equiv.), CH3CN (2 mL), 140 °C and 18 h are the optimized reaction conditions, as shown in entry 5. The two-dimensional NMR (H–H COSEY) plot also supported that trifluoromethylation was on the C2 position of indoles (ESI 5†).
| Entry | CF3 reagent(X eq.) | Y eq. | Sol (2 mL) | Temp/°C | Time/h | Yieldb 2a/(3a) % | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Unless otherwise noted, the reaction was carried out with 1a (0.3 mmol) and solvent CH3CN (2 mL), 140 °C, stirred for 18 h in air.b Isolated yields. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | CF3SO2Na(2 eq.) | 1 | CH3CN | 25 | 18 | 15 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | CF3SO2Na(2 eq.) | 1 | CH3CN | 80 | 18 | 36 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | CF3SO2Na(2 eq.) | 1 | CH3CN | 140 | 18 | 45 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | CF3SO2Na(2 eq.) | 2 | CH3CN | 140 | 18 | 50 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | CF3SO2Na(2 eq.) | 3 | CH3CN | 140 | 18 | 66 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | CF3SO2Na(3 eq.) | 3 | CH3CN | 140 | 18 | 56(16) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | CF3SO2Na(2 eq.) | 3 | CH3CN | 140 | 16 | 53 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | CF3SO2Na(2 eq.) | 3 | CH3CN | 140 | 20 | 65 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | CF3SO2Na(2 eq.) | 3 | CH3CN | 140 | 24 | 66 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | CF3SO2Na(2 eq.) | 3 | Toluene | 140 | 18 | 49 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 | CF3SO2Na(2 eq.) | 3 | DMF | 140 | 18 | 41 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 | CF3SO2Na(2 eq.) | 3 | H2O | 140 | 18 | 30 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 | CF3SO2Na(2 eq.) | 3 | DMSO | 140 | 18 | nr | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 | CF3SO2Na(2 eq.) | 3 | 1,4-Dioxane | 140 | 18 | nr | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 | TMSCF3(2 eq.) | 3 | CH3CN | 140 | 18 | Trace | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
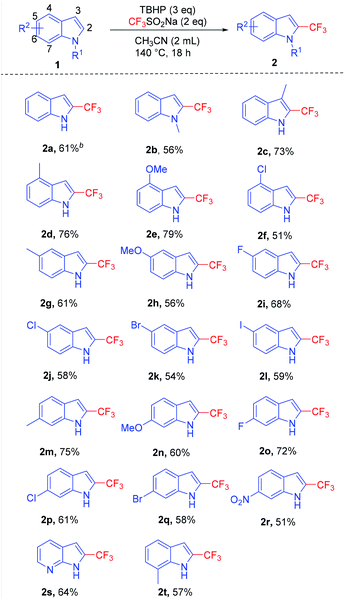
With the optimized reaction conditions in hand, we investigated the substrate scopes with respect to different substituents on indole scaffolds shown in Table 2. To our delight, most of the transformations went smoothly and provided the corresponding products in moderate to good yields. When the hydrogen of NH was replaced by methyl, trifluoromethylation took place and gave 2b in the yield of 56%. It should be noted that when the position of the methyl group on the scaffold of indole was changed from C3 to C7, the corresponding products were obtained with the yield of 73% to 57% (2c, 2d, 2g, 2m, 2t). Indoles with the methyl group substituted at C4 or C6 position successfully converted into corresponding products with the yields of 76% (2d) and 75% (2m), respectively, showing a higher activity. Importantly, this approach was compatible with halogen groups such as bromine (2k) and iodine (2l), which presented corresponding products with the yield of 54% and 59%, respectively. Except for the fluorine-containing substituents, it was found that the yield of electron-donating groups was slightly higher than that of the electron-withdrawing groups (2h, 2n, 2r). For example, 7-azaindole was successfully converted into its corresponding trifluoromethylation product 2s with the yield of 64%.
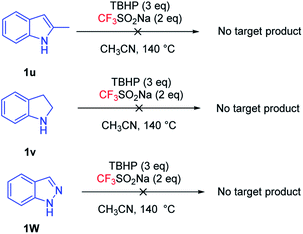
Subsequently, we studied other indole derivatives under optimized conditions (Fig. 3), such as 2-methylindole (1u), indoline (1v) and indazole (1w). The desired product was not obtained, which may indicate that trifluoromethylation occurred on the C2 position and indoline could not be compatible in the present system.
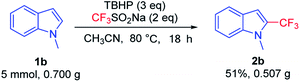
Investigations established that a large-scale amplification experiment of 1b (5 mmol) under optimized conditions at 80 °C smoothly gave 2b. We were pleased to observe that 0.507 g of the corresponding product was isolated with the yield being 51% (Fig. 4).
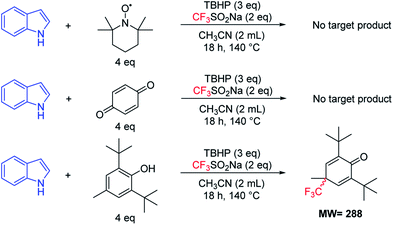
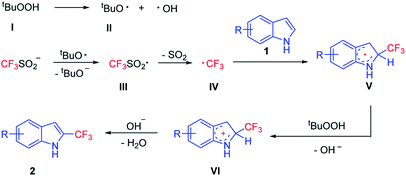
In order to get insights into the reaction mechanism, a series of control experiments were conducted. When 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) (4 equiv.), butylatedhydroxytoluene (BHT) (4 equiv.) or 1,4-benzoquinone (4 equiv.) was respectively added under the standard reaction conditions (Table 1, entry 6), almost no desired products were detected (Fig. 5). In addition, a radical intermediate (molecular weight: 288) was detected by GC-MS when a radical scavenger (BHT) was used. Based on these results, we proposed herein a plausible reaction pathway (Fig. 6).8a,14 At first, a free radical, tBuO˙ (II) generated from TBHP (I) by heating reacts with a trifluorosulfinate anion to provide the free radical 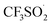 (III). Subsequently, a free radical
(III). Subsequently, a free radical 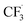 was formed by releasing SO2 from the radical
was formed by releasing SO2 from the radical 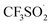 (III). The intermediate (V) was generated from the radical
(III). The intermediate (V) was generated from the radical 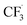 with substrate 1. TBHP accepted one electron from the intermediate (V) to give the intermediate cation (VI) and release OH−. Finally, the product 2 was obtained by releasing a proton from the intermediate cation (VI).
with substrate 1. TBHP accepted one electron from the intermediate (V) to give the intermediate cation (VI) and release OH−. Finally, the product 2 was obtained by releasing a proton from the intermediate cation (VI).
Conclusions
In summary, we have developed an original method of synthesizing 2-trifluoromethylindoles from indoles with easy-to-handle, cheap and low-toxic CF3SO2Na under metal-free conditions, which selectively proceeded on the C2 position of indoles. High functional group tolerance and moderate to good yield were presented in this transformation. The control experiments provided evidences that the reaction may undergo a free radical pathway. Further applications of 2-trifluoromethylindoles are currently being carried out in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the financial support from the National Natural Science Foundation of China (51578224).Notes and references
- (a) B. Zhang and A. Studer, Org. Lett., 2014, 16, 1216 CrossRef CAS; (b) J. P. Brand, J. Charpentier and J. Waser, Angew. Chem., Int. Ed., 2009, 48, 9346 CrossRef CAS; (c) W. Wang, X. Lian, D. Chen, X. Liu, L. Lin and X. Feng, Chem. Commun., 2011, 47, 7821 RSC; (d) L. Han, X. Ma, Y. Liu, Z. Yu and T. Liu, Org. Chem. Front., 2018, 5, 725 RSC; (e) Y. Yamamoto, E. Ohkubo and M. Shibuya, Adv. Synth. Catal., 2017, 359, 1747 CrossRef CAS; (f) E. Xie, A. Rahman and X. Lin, Org. Chem. Front., 2017, 4, 1407 RSC; (g) B. Ernst, A. Ruehling, B. Wibbeling and F. Glorius, Chem.–Eur. J., 2016, 22, 4400 CrossRef.
- (a) D. Tu, J. Luo and C. Jiang, Chem. Commun., 2018, 54, 2514 RSC; (b) S. Jana, A. Verma, R. Kadu and S. Kumar, Chem. Sci., 2017, 8, 6633 RSC.
- (a) G. L. Gao, C. Yang and W. Xia, Chem. Commun., 2017, 53, 1041 RSC; (b) Y. Ye, K. P. S. Cheung, L. He and G. C. Tsui, Org. Chem. Front., 2018, 5, 1511 RSC; (c) G. Blay, I. Fernandez, M. Carmen Munoz, J. R. Pedro and C. Vila, Chem.–Eur. J., 2010, 16, 9117 CrossRef CAS; (d) L. Jiang, W. Yi and Q. Liu, Adv. Synth. Catal., 2016, 358, 3700 CrossRef CAS.
- (a) B. Chaudhary, M. Diwaker and S. Sharma, Org. Chem. Front., 2018, 5, 3133 RSC; (b) D. A. Nagib and D. W. C. MacMillan, Nature, 2011, 480, 224 CrossRef CAS; (c) D. A. Borkin, S. M. Landge and B. Toeroek, Chirality, 2011, 23, 612 CrossRef CAS; (d) Q. Lou, Y. Ding, D. Xu, G. Liu and J. Zhao, Adv. Synth. Catal., 2017, 359, 2557 CrossRef CAS.
- (a) C. P. Zhang, Z. L. Wang, Q. Y. Chen, C. T. Zhang, Y. C. Gu and J. C. Xiao, Angew. Chem., Int. Ed., 2011, 50, 1896 CrossRef CAS; (b) M. S. Wiehn, E. V. Vinogradova and A. Togni, J. Fluorine Chem., 2010, 131, 951 CrossRef CAS; (c) Y. Huang, S. Suzuki, G. Liu, E. Tokunaga, M. Shiro and N. Shibata, New J. Chem., 2011, 35, 2614 RSC.
- C. Xu, J. Liu, W. Ming, Y. Liu, J. Liu and M. Wang, Chem.–Eur. J., 2013, 19, 9104 CrossRef CAS.
- (a) S. Seo, J. B. Taylor and M. F. Greaney, Chem. Commun., 2013, 49, 6385 RSC; (b) X. Mu, S. Chen, X. Zhen and G. Liu, Chem.–Eur. J., 2011, 17, 6039 CrossRef CAS.
- (a) B. R. Langlois, E. Laurent and N. Roidot, Tetrahedron Lett., 1991, 32, 7525 CrossRef CAS; (b) S. A. Miller, B. van Beek, T. A. Hamlin, F. M. Bickelhaupt and N. E. Leadbeater, J. Fluorine Chem., 2018, 214, 94 CrossRef CAS; (c) Y. Ji, T. Brueckl, R. D. Baxter, Y. Fujiwara, I. B. Seiple, S. Su, D. G. Blackmond and P. S. Baran, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 14411 CrossRef CAS.
- (a) E. Mejía and A. Togni, ACS Catal., 2012, 2, 521 CrossRef; (b) R. Shimizu, H. Egami, T. Nagi, J. Chae, Y. Hamashima and M. Sodeoka, Tetrahedron Lett., 2010, 51, 5947 CrossRef CAS; (c) L. Hu, X. Chen and G. Zhu, Chem. Commun., 2016, 52, 6845 RSC; (d) S. P. Pitre, C. D. McTiernan, H. Ismaili and J. C. Scaiano, ACS Catal., 2014, 4, 2530 CrossRef CAS; (e) J. Liu, L. Li, L. Yu, L. Tang, Q. Chen and M. Shi, Adv. Synth. Catal., 2018, 360, 2959 CrossRef CAS; (f) Y.-T. He, Q. Wang, J. Zhao, X.-Z. Wang, Y.-F. Qiu, Y.-C. Yang, J.-Y. Hu, X.-Y. Liu and Y.-M. Liang, Adv. Synth. Catal., 2015, 357, 3069 CrossRef CAS.
- (a) Z. Wang, F. Ge, W. Wan, H. Jiang and J. Hao, J. Fluorine Chem., 2007, 128, 1143 CrossRef CAS; (b) N. Iqbal, S. Choi, E. Ko and E. J. Cho, Tetrahedron Lett., 2012, 53, 2005 CrossRef CAS; (c) M. Chen, Z.-T. Huang and Q.-Y. Zheng, Chem. Commun., 2012, 48, 11686 RSC; (d) B. Torok, M. Abid, G. London, J. Esquibel, M. Torok, S. C. Mhadgut, P. Yan and G. K. S. Prakash, Angew. Chem., Int. Ed., 2005, 44, 3086 CrossRef; (e) P. Li, G. Zhao and S. Zhu, Chin. J. Chem., 2011, 29, 2749 CrossRef CAS; (f) Y. Hui, W. Chen, W. Wang, J. Jiang, Y. Cai, L. Lin, X. Liu and X. Feng, Adv. Synth. Catal., 2010, 352, 3174 CrossRef CAS.
- R. Rey-Rodriguez, P. Retailleau and P. Bonnet andI.Gillaizeau, Chem.–Eur. J., 2015, 21, 3572 CrossRef CAS PubMed.
- W. J. Choi, S. Choi, K. Ohkubo, S. Fukuzumi, E. J. Cho and Y. You, Chem. Sci., 2015, 6, 145 Search PubMed.
- (a) B. Chang, H. Shao, P. Yan, W. Qiu, Z. Weng and R. Yuan, ACS Sustainable Chem. Eng., 2016, 5, 334 CrossRef; (b) Y. Zhang, X. Han, J. Zhao, Z. Qian, T. Li and Y. Tang, Adv. Synth. Catal., 2018, 360, 2659 CrossRef CAS; (c) L. Jiang, J. Qian, W. Yi, G. Lu, C. Cai and W. Zhang, Angew. Chem., Int. Ed., 2015, 54, 14965 CrossRef CAS.
- X. Shi, X. Li, L. Ma and D. Shi, Catalysts, 2019, 9, 278 CrossRef.
- C. P. Wang and G. F. Jiang, Tetrahedron Lett., 2017, 58, 1747 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra07785e |
| This journal is © The Royal Society of Chemistry 2019 |