Open Access Article
Open Access ArticleBio-based cyclized Eucommia ulmoides gum elastomer for promising damping applications†
Xin Qi a,
Fei Xie
a,
Fei Xie a,
Jichuan Zhangab,
Liqun Zhang
a,
Jichuan Zhangab,
Liqun Zhang ab and
Dongmei Yue
ab and
Dongmei Yue *ab
*ab
aState Key Laboratory of Organic–Inorganic Composites, Beijing University of Chemical Technology, Beijing 100029, PR China. E-mail: yuedm@mail.buct.edu.cn
bKey Laboratory of Beijing City on Preparation and Processing of Novel Polymer Materials, Beijing 100029, PR China
First published on 20th December 2019
Abstract
Eucommia ulmoides gum (EUG) is an important bio-based material with a structure similar to that of natural rubber. However, EUG is a hard plastic at room temperature due to crystallization, which limits its wide application. In this paper, a bio-based cyclized Eucommia ulmoides gum (CEUG) elastomer with various degrees of cyclization was prepared using TiCl4/CH3COOH as catalysts. 1H-NMR and FT-IR techniques were used to obtain structure information. It was found that di-, tri-, and tetra-substituted olefins in the cyclized sequence were formed during cyclization. DSC and XRD results indicated that the cyclized structure could inhibit crystallization. When the degree of cyclization reached 8.2%, crystallization disappeared and the material transformed from a plastic into an elastomer. With increasing of the degree of cyclization, the glass transition temperature (Tg) of CEUG increased and the thermal stability was enhanced, but the molecular weight decreased significantly. Above all, DMA results showed that the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δmax could reach 1.2 when the degree of cyclization was 20.0%, and the damping temperature range could be adjusted by controlling the degree of cyclization. This new elastomer is expected to contribute to the development of damping materials.
δmax could reach 1.2 when the degree of cyclization was 20.0%, and the damping temperature range could be adjusted by controlling the degree of cyclization. This new elastomer is expected to contribute to the development of damping materials.
1. Introduction
In recent years, it has become a new trend to replace non-renewable fossil resources with renewable biomass.1 The promotion of sustainable bio-based materials could reduce carbon emissions and mitigate the adverse environmental effects.2 Eucommia ulmoides gum (EUG), also called gutta percha or balata, is one of the most important bio-based polymer materials that are mainly native to central and Southern China.3 Because EUG is rich in resources and renewable, more and more researchers are beginning to study the application of it.EUG exists in many tissues of Eucommia ulmoides Oliv, such as leaves, barks, roots, fruit coatings and cotyledon.3,4 Its chemical structure is trans-1, 4-polyisoprene (TPI), which is the isomer of nature rubber (NR). Owing to the transconfiguration, it crystallizes easily. It is a hard plastic at room temperature with highly anti-impact strength and hot melt binding properties.5,6 So, EUG could blend with some materials like polypropylene (PP),7 polylactide (PLA)8 to improve impact strength, or blend with high-density polyethylene (HDPE)9 to prepare shape memory materials. However, due to lack of elasticity at room temperature, EUG was not widely used like NR. Hence, it is of great significance to transform it into elastomer through chemical modifications. Yan firstly turned it into elastomer by curing reaction of the double bond in 1984.10 After that, other researchers have tried to modify it by some ways, such as hydrogenation and epoxidation,10,11 but little paper about its application in the field of function elastomers, such as damping elastomers, has been seen.
Invalid vibration and noise have become important issues in modern industry.12 Because of their unique viscoelastic properties, elastomer has been extensively utilized as damping materials.13 The loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) defined by the ratio of loss modulus (E′′) to storage modulus (E′), is used as an assessment of the ability to dissipate energy. High-performance damping materials should meet the requirement of tan
δ) defined by the ratio of loss modulus (E′′) to storage modulus (E′), is used as an assessment of the ability to dissipate energy. High-performance damping materials should meet the requirement of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ > 0.3 over a broad temperature range.14 The damping properties of elastomer dominated by its glass transition, and it always has narrow damping temperature range around the glass transition temperature (Tg), which can't satisfy the actual requirements.15 One way to solve this problem is to optimize the Tg to the desired temperatures by blending materials with low or high Tg,16 usually different materials have poor compatibility. So, it is a meaningful study to regulate the damping temperature range by chemical methods.
δ > 0.3 over a broad temperature range.14 The damping properties of elastomer dominated by its glass transition, and it always has narrow damping temperature range around the glass transition temperature (Tg), which can't satisfy the actual requirements.15 One way to solve this problem is to optimize the Tg to the desired temperatures by blending materials with low or high Tg,16 usually different materials have poor compatibility. So, it is a meaningful study to regulate the damping temperature range by chemical methods.
The cyclized rubbers can be made by the cyclization of unsaturated rubber or the cyclopolymerization of conjugated dienes,17 which are used in the formulation of adhesives, paintings, inks, thermoplastic elastomers and reinforcing filler.18 Cyclized rubber can be prepared in solution, solid or emulsion.19 Different from the other two approaches, molecule chains in solution state can move freely, so the catalyst can contact them more easily, leading to higher efficiency of cyclization. Many catalysts can be used in rubber cyclization, such as H2SO4, Lewis acid,20–22 trimethylsilyl triflate,19 CF3SO3H.23 Above all, the Tg will greatly increase after cyclization, which might be used to adjust damping temperature range.
In this paper, EUG was cyclized in xylene by using TiCl4/CH3COOH as catalysts for the first time to prepare damping elastomer (shown in Scheme 1).24,25 The cyclization reaction conditions were discussed, the structure and properties of CEUG and its vulcanizates were also evaluated. After cyclization, its tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ increased greatly. No need to blend with other materials, its damping temperature could be tailored by controlling the degree of cyclization, which would have potential applications in the field of damping materials.
δ increased greatly. No need to blend with other materials, its damping temperature could be tailored by controlling the degree of cyclization, which would have potential applications in the field of damping materials.
2. Experimental
2.1 Materials
Eucommia ulmoides gum (EUG) was provided by Beijing University of Chemical Technology, and was purified as follows: dissolved it in toluene and removed the insoluble materials by centrifugation, followed by precipitated in alcohol to get pure EUG (Mn = 1.2 × 106, PDI = 1.2). Titanium tetrachloride (AR, 99%) was purchased from Sinopharm Chemical Reagent Co., Ltd, acetic acid (AR, 98%) and other solvents were purchased from Beijing Chemical Works. The processing agents, such as dicumyl peroxide (DCP) and carbon black were commercially available and were used as received.2.2 Preparation of CEUG
The following procedure was typically used to prepare CEUG. 1 g of EUG was dissolved in 50 ml of xylene, then 1 ml of CH3COOH and 0.3 g of TiCl4 were added into solution to react for 30 min at 60 °C. The solution sample coagulated with ethanol, then washed with deionized water, and finally dried at 50 °C.2.3 Preparation of CEUG vulcanizates
100 g of CEUG was mixing on a two-roll mill for plasticization. Then, 6 g of DCP and 50 g of carbon black were added and mixed fully at room temperature. Finally, the blend was cured at 150 °C under 15 MPa pressure.2.4 Measurements
Fourier transform infrared (FT-IR) spectroscopy was performed with infrared spectrometer (BRUKER Tensor II, USA). The sample was dissolved in trichloromethane first and then coated on KBr.1H NMR spectra were recorded using UNITY300 NMR (VARIAN Company, USA). The measurements at 500 MHz were carried out at room temperature by using CDCl3 as solvent with TMS as internal standard. The degree of cyclization was calculated according to eqn (1):19
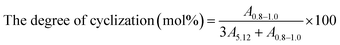 | (1) |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C consumption (mol%) was calculated according to eqn (2):
C consumption (mol%) was calculated according to eqn (2):
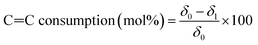 | (2) |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH protons (TPI) compared to all the polymer peaks of EUG and CEUG, respectively.
CH protons (TPI) compared to all the polymer peaks of EUG and CEUG, respectively.
The number-average molecular weight (Mn) and polymer dispersity index (PDI) were determined by GPC in toluene at 40 °C (Agilent Technologies, USA).
The crystalline structures were determined by XRD (max2500, Rigaku), Cu(Kα1), λ = 1.54056 Å, 5° < 2θ < 30°, the scan rate = 3° min−1.
Melting-crystallizing behaviour and glass transition temperature (Tg) were measured by DSC analyser (PerkinElmer, USA) under N2 atmosphere. First, the sample was heated to 80 °C at the heating rate of 10 °C min−1 to eliminate its thermal history, which was then cooled down to −80 °C at a rate of 10 °C min−1, then heated again to 80 °C at the same rate as cooling process.
The thermal properties were investigated by TGA analyser (PerkinElmer, USA) under N2 atmosphere. The measurements were carried out over a temperature range of 30 to 800 °C with the heating rate of 10 °C min−1.
The stress–strain curves were determined according to GB 528-92 (ISO/DIS 37-1990) using a tensile testing machine (Instron 3365, USA) at ambient temperature with a speed of 100 mm min−1. Shore hardness was tested according to GB/T531-1999 using Shore hardness tester. Rebound resilience was measured according to GB/T 1681-2009 by GT-7042-RE rebound tester.
The damping property was tested by DMA (DISCOVERY HR-2 rotary rheometer, America). The tests were carried out at a heating rate of 3 °C min−1 with a frequency of 10 Hz, and the temperature ranged from −100 to 80 °C.
3. Results and discussion
3.1 The cyclization of EUG
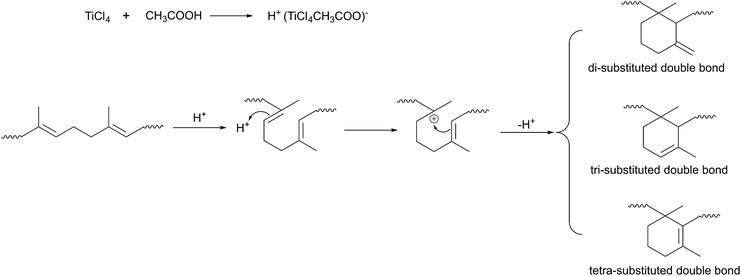
EUG was cyclized in xylene by using TiCl4/CH3COOH as catalysts for the first time. The structure of CEUG was characterized by 1H NMR and FT-IR.The 1H-NMR spectra of EUG and CEUG are shown in Fig. 1(a). The signals at 1.63, 2.0–2.1, 5.12 ppm are assigned to the methyl, methylene and olefinic proton of EUG, respectively.10 After cyclization, the integrals of the three peaks decreased, while some new peaks observed. The new signal at 0.8–1.0 corresponds to the methyl adjacent to saturate carbon, and the signals at 4.57, 5.33 ppm represent the di- and tri-substituted olefins in cyclized sequence respectively.19
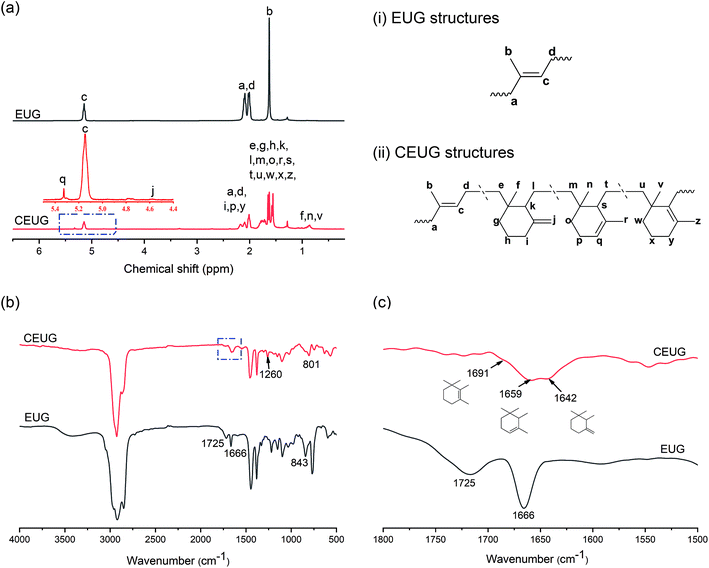 | ||
| Fig. 1 Structural characterization of EUG and CEUG. (a) 1H-NMR spectra, (b) FT-IR spectra, (c) expanded region of the carbon–carbon double bond stretching vibration. | ||
FT-IR was also employed to characterize the structure of CEUG. The FT-IR spectra of EUG and CEUG is shown in Fig. 1(b). The absorption peaks around 2900 cm−1 and 1400 cm−1 are attributed to the stretching and bending vibration of –CH3 and –CH2, respectively. The peak at 1724 cm−1 is attributed to the carbonyl group, since the molecular chain of EUG ended with hydrophilic ester groups. The absorption peak at 1666 cm−1 is assign to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C.10 In addition, the FT-IR spectra of EUG show the extra crystalline peaks at 875 cm−1, 798 cm−1, 758 cm−1, 595 cm−1, 465 cm−1 bands, respectively.4 After cyclization, the main characteristic peaks still existed, indicating that only partial cyclization has occurred. While the disappearance of the crystallization peak indicates that the cyclized structures could prevent crystallization. Besides, the new absorption peak at 801 cm−1 assigns to the C–H out-of-plane deformation of cyclized sequence, and the absorption peak at 1260 cm−1 assigns to the C–O–C stretching vibration produced by oxidation.26
C.10 In addition, the FT-IR spectra of EUG show the extra crystalline peaks at 875 cm−1, 798 cm−1, 758 cm−1, 595 cm−1, 465 cm−1 bands, respectively.4 After cyclization, the main characteristic peaks still existed, indicating that only partial cyclization has occurred. While the disappearance of the crystallization peak indicates that the cyclized structures could prevent crystallization. Besides, the new absorption peak at 801 cm−1 assigns to the C–H out-of-plane deformation of cyclized sequence, and the absorption peak at 1260 cm−1 assigns to the C–O–C stretching vibration produced by oxidation.26
The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration region is expanded in Fig. 1(c) to analyse cyclized structure in detail. The peak at 1666 cm−1 separated into three peaks though cyclization. The peak at 1691 cm−1, 1642 cm−1 is attributed to the C
C stretching vibration region is expanded in Fig. 1(c) to analyse cyclized structure in detail. The peak at 1666 cm−1 separated into three peaks though cyclization. The peak at 1691 cm−1, 1642 cm−1 is attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of tetra- and di-substituted olefin in cyclized sequence respectively. While the peak at 1659 cm−1 is attributed to the C
C stretching vibration of tetra- and di-substituted olefin in cyclized sequence respectively. While the peak at 1659 cm−1 is attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of tri-substituted olefin and EUG.26 The characterization data from both FT-IR and 1H NMR techniques agreed well, indicating EUG was cyclized successfully.
C stretching vibration of tri-substituted olefin and EUG.26 The characterization data from both FT-IR and 1H NMR techniques agreed well, indicating EUG was cyclized successfully.
CEUG has a very broad molecular weight distribution after cyclization with the molecular weight decreased and polymer dispersity index (PDI) increased (shown in Table 1), suggesting that the cyclization reaction was accompanied by the scission of the chains.
3.2 Effect of cyclized conditions
Cyclization reaction mainly produced di-, tri-, and tetra-substituted olefinic cyclized structures by consuming double bonds. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C consumption and degree of cyclization will be affected by various reaction conditions, such as reaction temperature, time and the catalyst dosage.
C consumption and degree of cyclization will be affected by various reaction conditions, such as reaction temperature, time and the catalyst dosage.
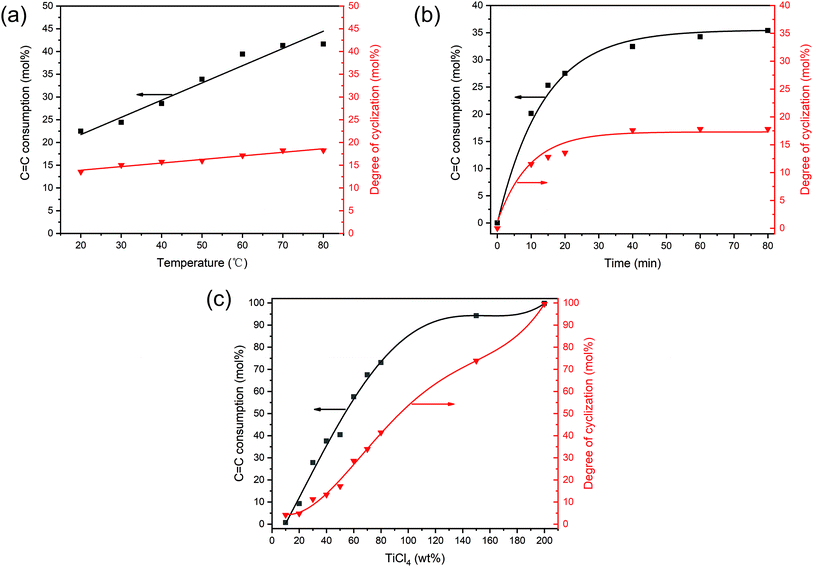
EUG was cyclized by using 40 wt% of TiCl4 and 1 ml g−1-EUG of CH3COOH as catalysts at various temperatures with reaction time of 30 min. Fig. 2(a) shows the effect of temperature on cyclization. It was found that cyclization could take place at low temperatures. With the increase of temperature, the degree of cyclization increased slowly. This is because the mechanism of cyclization is similar to that of cationic polymerization,20,21 and the reaction activation energy of this process is low, so the temperature has a little effect on it.27,28 However, as the temperature increases, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C consumption increased. This may be because high temperatures are more conducive to the formation of polycyclic structures. As EUG was easy to crystallize at room temperature, it was not easy to dissolve in the solution without heating. So, the appropriate reaction temperature was 60 °C, at which EUG was easy to dissolve and less heat energy was consumed for cyclization.
C consumption increased. This may be because high temperatures are more conducive to the formation of polycyclic structures. As EUG was easy to crystallize at room temperature, it was not easy to dissolve in the solution without heating. So, the appropriate reaction temperature was 60 °C, at which EUG was easy to dissolve and less heat energy was consumed for cyclization.
The effect of reaction time on cyclization was discussed with 40 wt% of TiCl4 and 1 ml g−1-EUG of CH3COOH at 60 °C, which is shown in Fig. 2(b). The cyclization reaction was very rapid, and it only took 10 minutes to achieve a high degree of cyclization. This was due to the initiation and propagation rates of cationic cyclization were very fast.20,28 However, termination reaction was difficult to control, so the degree of cyclization would increase with the increase of time. But it was not the case that the longer the reaction time, the higher the degree of cyclization. Because long reaction time would lead to the transfer of the carbene to the macromolecular chain, resulting in gel. So, the appropriate reaction time was 30 min, at which time no gel was produced.
Fig. 2(c) shows the effect of the TiCl4 dosage on cyclization with 1 ml g−1-EUG of CH3COOH at 60 °C for 30 min. Increasing the TiCl4 concentration could increase the probability of catalyst contacting molecular chains and lead to producing more carbocation, thus improving the degree of cyclization. So, the degree of cyclization can be adjusted by controlling the TiCl4 dosage under the appropriate reaction conditions of reaction temperature of 60 °C for 30 min.
3.3 Crystallizing behaviour and thermal properties of CEUG
Crystallizing behaviour will be influenced by structural integrity of materials. Fig. 3(a) and (b) shows the DSC curves of EUG and CEUG with various degree of cyclization. Compared with EUG, the melting point (Tm), crystallization temperature (Tc) and degree of crystallinity of CEUG decreased, while the glass transition temperature (Tg) increased greatly (shown in Table 1), suggesting that cyclized sequence can reduce the regularity and flexibility of molecular chains. Above all, when the degree of cyclization reached 11.2%, both the crystallization peak and the melting peak disappeared, indicating that it is no longer crystallized and turned into elastomer. The plastic–rubber transformation process will be discussed in the next section.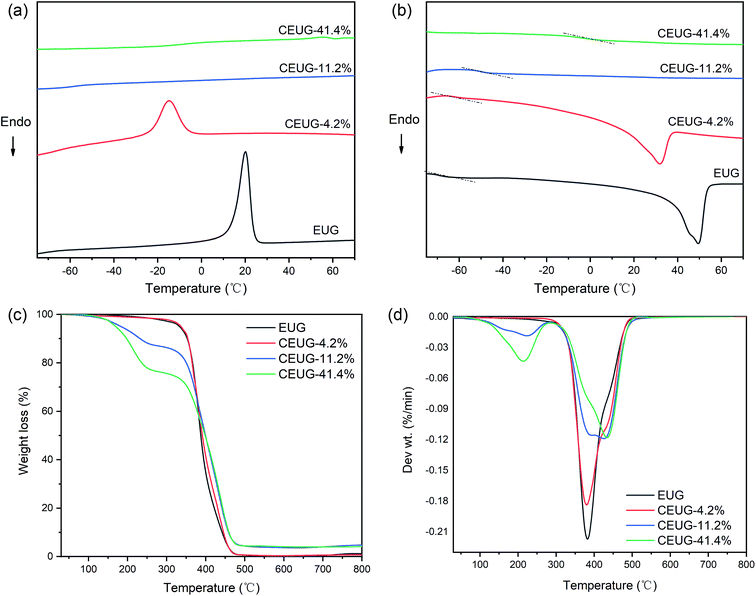 | ||
| Fig. 3 Crystallizing behaviour and thermal properties of EUG and CEUG with various degree of cyclization: DSC curves of (a) heating scan and (b) cooling scan; (c) TG and (d) DTG curves. | ||
The crystal form was characterized by XRD. Fig. S1† shows the XRD spectra of EUG and CEUG with various degree of cyclization within 5° < 2θ < 35° by crystallizing at room temperature from the molten state. The peaks 1, 2, 3, 4, 5, 6, 7, 8 are the characteristic peaks of the α-form, while peaks 3 and 5 are the characteristic peaks of the β-form. Both crystals are present in EUG, but it tends to grow more β-form.6 After slight cyclization, the peaks of the α-form increased. The reason is that the cyclization leads the crystallization temperature decreased, which has been confirmed by DSC, so the crystal is more favourable to grow and form α-form at room temperature. Further increasing the degree of cyclization, the crystal peak became weak due to the decreasing of regularity. When the degree of cyclization reached 11.2%, the crystal peak disappeared completely, which is consistent with DSC results.
The thermogravimetric analysis (TG) and differential thermal gravity curves (DTG) curves of EUG and CEUG with various degree of cyclization are shown in Fig. 3(c) and (d). EUG showed a single thermal gravity stage with maximum temperature degradation (Tmax) at 381 °C, while CEUG showed four thermal gravity stages. The first and second stages ranged at 100–200 °C, which mainly caused by the degradation of low-molecular weight substances and oxides generated by side reactions of cyclization. The Tmax of the third stage at 381 °C, which was triggered by the pyrolysis of carbon–carbon double bonds. The fourth stage occurred at about 427 °C, which result from the degradation of cyclized structure. Although the side action of cyclization would lead the initial weight loss temperature decreased, in general, cyclization could reduce the content of carbon–carbon double bonds, which was conducive to the improvement of thermal stability.
3.4 Plastic–rubber transformation of CEUG
When materials change from plastic to rubber, it's physical properties, such as hardness, will change radically, in other words, the percolation will occur.29 The percolation threshold of CEUG is near 8.2% (Fig. 4(a)). When the degree of cyclization was near 8.2%, the hardness of CEUG decreased suddenly as crystals disappeared, indicating the plastic–rubber transformation of CEUG. However, with the increase of the degree of cyclization, the glass transition temperature would increase. When the degree of cyclization was near 70.0%, CEUG lost elasticity and turns into leather. Further increasing the degree of cyclization, it turned into plastic powders finally.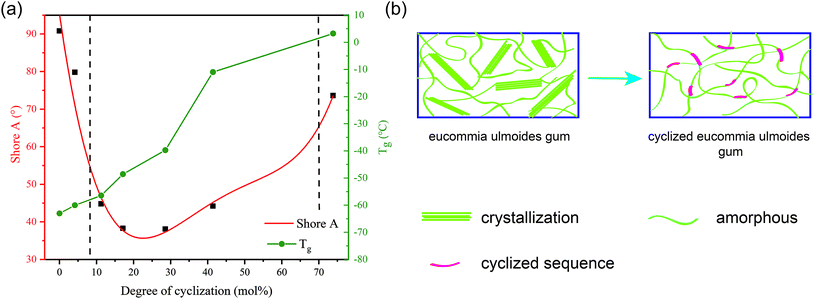 | ||
| Fig. 4 Plastic–rubber transformation of CEUG. (a) Shore A and Tg of CEUG with various degree of cyclization; (b) illustration of plastic–rubber transformation. | ||
The plastic–rubber transformation process of CEUG was illustrated in Fig. 4(b). EUG is a hard plastic at room temperature because it is easy to crystallize. After cyclization, the cyclized sequence would be randomly distributed on the molecular chain, making the molecular chain less orderly. When reached the percolation threshold, the crystal structure disappeared, and the CEUG showed the original flexibility, thus turning into elastomer. However, cyclization sequence is a rigid structure, too high degree of cyclization would make it less elastic. Therefore, the appropriate degree of cyclization should be limited to 8.2–70.0%.
3.5 Mechanical and damping properties of CEUG vulcanizates
The stress–strain curves of EUG and CEUG with various degree of cyclization are shown in Fig. 5(a) and summarized in Table 2. EUG had high tensile strength, low rebound resilience and showed significant stress yield due to crystallization at room temperature. The low cyclization sequence could not completely break the crystallization, so the stress yield phenomenon still observed and the rebound resilience was only slightly increased. When the cyclization degree was 7.2%. Once the crystallization disappeared, the tensile strength would decrease significantly due to the absence of self-reinforcing force, while the rebound resilience increased prominently, indicating the cyclized EUG is already an elastomer. After that, the tensile strength would increase with the increase of the degree of cyclization, which was mainly attributed to the rigidity of the cyclization sequence. When the degree of cyclization reached at 29.1%, it had optimal tensile strength and elongation at break, which were 13.2 MPa and 283% respectively. If we continued to increase the degree of cyclization to 38.4%, the tensile strength will stop increasing, and the elongation at break will reduce, which was mainly caused by the reduction of molecular weight.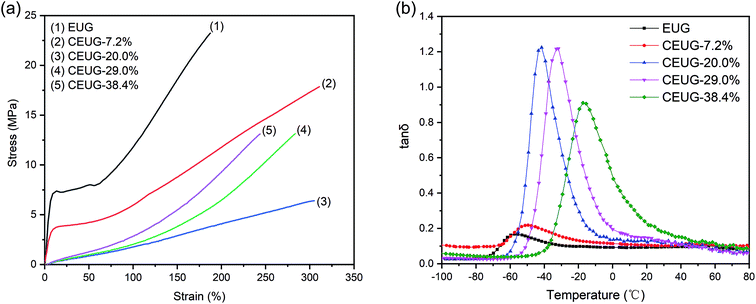 | ||
Fig. 5 Mechanical and damping properties of EUG and CEUG vulcanizates. (a) Stress–strain curves, (b) tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ–temperature curves. δ–temperature curves. | ||
| Sample | σa (MPa) | εb (%) | Rec (%) | tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δmaxd δmaxd |
DTRe (°C) |
|---|---|---|---|---|---|
| a Tensile strength.b Tensile strain at break.c Rebound resilience.d Damping peak.e Effective damping temperature range. | |||||
| EUG | 23.2 | 188 | 5 | 0.2 | — |
| CEUG-7.2% | 17.9 | 311 | 7 | 0.9 | −33.6 to −1.4 |
| CEUG-20.0% | 6.4 | 305 | 36 | 1.2 | −53.0 to −19.2 |
| CEUG-29.0% | 13.5 | 283 | 33 | 1.2 | −45.5 to −7.4 |
| CEUG-38.4% | 13.1 | 244 | 31 | 0.9 | −30.9 to 14.8 |
The damping properties of CEUG were tested by DMA. The tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ–temperature curves of EUG and CEUG with various degree of cyclization are presented in Fig. 5(b). After cyclization, the tan
δ–temperature curves of EUG and CEUG with various degree of cyclization are presented in Fig. 5(b). After cyclization, the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ increased and the peak position moved towards the high temperature direction. The damping peak and the effective damping temperature range (tan
δ increased and the peak position moved towards the high temperature direction. The damping peak and the effective damping temperature range (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ > 0.3) are given in Table 2. The tan
δ > 0.3) are given in Table 2. The tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δmax was very low at low degree of cyclization because of crystallization. When it converted to elastomer, the tan
δmax was very low at low degree of cyclization because of crystallization. When it converted to elastomer, the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δmax would be increased to 1.2 and showed excellent damping performance, which arise from the viscoelastic movement of segments in polymer chains and the friction between cyclized sequences. Above all, the tan
δmax would be increased to 1.2 and showed excellent damping performance, which arise from the viscoelastic movement of segments in polymer chains and the friction between cyclized sequences. Above all, the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δmax of CEUG-38.4% was 0.9, and the effective damping temperature range was between −30.9 °C and 14.8 °C, near room temperature, whose damping performance is comparable with commonly used damping materials, such as NR, NBR and SBR.14
δmax of CEUG-38.4% was 0.9, and the effective damping temperature range was between −30.9 °C and 14.8 °C, near room temperature, whose damping performance is comparable with commonly used damping materials, such as NR, NBR and SBR.14
The damping temperature range of elastomer was usually near Tg. DSC results have shown that Tg would increase with the increase of the degree of cyclization. So, the effective damping temperature range could be adjusted by controlling the degree of cyclization. As showed in Table 2, the effective damping temperature could cover −53 °C to 14.8 °C by adjusted the degree of cyclization from 20.0% to 38.4%, which means that CEUG has promising application in the field of damping materials.
4. Conclusions
The cyclized EUG elastomer are successfully produced by the cyclization of unsaturation rubber. It was confirmed that di-, tri-, and tetra-substituted olefins in cyclized sequence were formed by FT-IR and 1H NMR. Various cyclization conditions were studied, and the degree of cyclization can be adjusted by controlling the TiCl4 dosage under the appropriate reaction conditions of reaction temperature of 30 °C and reaction time of 30 min. DSC and XRD results indicated that cyclized sequence could inhibit crystallization and influence crystal form. When the degree of cyclization reached 8.2%, crystals disappeared and CEUG transformed into elastomer. With increasing of the degree of cyclization, the glass transition temperature increased and the thermal stability enhanced, which is due to the decrease of unsaturation, while the molecular weight decreased because of the scission of the chains during cyclization. When the degree of cyclization reached 29.1%, it had optimal tensile strength and elongation at break, which were 13.2 MPa and 283%, respectively. DMA results showed that the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δmax can reach 1.2, and the effective damping temperature could cover −53 °C to 14.8 °C by adjusted the degree of cyclization from 20.0% to 38.4%, which means that CEUG has promising application in the field of damping materials.
δmax can reach 1.2, and the effective damping temperature could cover −53 °C to 14.8 °C by adjusted the degree of cyclization from 20.0% to 38.4%, which means that CEUG has promising application in the field of damping materials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by State Key Laboratory of Organic–Inorganic Composites, Beijing University of Chemical Technology.References
- R. A. Sheldon, Green Chem., 2014, 16, 950–963 RSC.
- Z. Wang, L. Yuan and C. Tang, Acc. Chem. Res., 2017, 50, 1762–1773 CrossRef CAS PubMed.
- Z. Sun, F. Li, H. Du, J. Zhu and Y. P. Wang, Ind. Crops Prod., 2013, 42, 216–222 CrossRef CAS.
- G. Liu, X. Zhang, T. Zhang, J. Zhang, P. Zhang and W. Wang, Polym. Test., 2017, 63, 582–586 CrossRef CAS.
- X. Zhang, C. Cheng, M. Zhang, X. Lan, Q. Wang and S. Han, J. Agric. Food Chem., 2008, 56, 8936–8943 CrossRef CAS PubMed.
- J. Zhang and Z. Xue, Polym. Test., 2011, 30, 753–759 CrossRef.
- Q. Fang, X. Jin, F. Yang, C. Ma, Y. Gao and N. Wang, Polym. Bull., 2016, 73, 357–367 CrossRef CAS.
- H. Kang, L. Yao, Y. Li, X. Hu, F. Yang, Q. Fang and L. Zhang, J. Appl. Polym. Sci., 2018, 135, 1–8 Search PubMed.
- Y. Wang, L. Xia and Z. Xin, Polym. Adv. Technol., 2018, 29, 190–197 CrossRef CAS.
- F. Yang, Q. Liu, X. Li, L. Yao and Q. Fang, Polym. Bull., 2017, 74, 3657–3672 CrossRef CAS.
- L. Xia, Y. Wang, Z. Ma, A. Du, G. Qiu and Z. Xin, Polym. Adv. Technol., 2017, 28, 94–101 CrossRef CAS.
- H. Rahmani, S. H. M. Najafi, S. Saffarzadeh-Matin and A. Ashori, J. Reinf. Plast. Compos., 2014, 33(8), 733–740 CrossRef.
- B. Liu, X. Gao, Y. Zhao, L. Dai, Z. Xie and Z. Zhang, J. Mater. Sci., 2017, 52, 1–15 CrossRef.
- K. Liu, Q. Lv and J. Hua, Polym. Test., 2017, 60, 321–325 CrossRef CAS.
- D. I. G. Jones, J. Sound Vib., 1974, 33, 451–470 CrossRef.
- K. Urayama, T. Miki, T. Takigawa and S. Kohjiya, Chem. Mater., 2004, 16, 173–178 CrossRef CAS.
- C. Wang, Mater. Chem. Phys., 2005, 89, 116–121 CrossRef CAS.
- C. Wang, X. Huang and J. Yang, J. Appl. Polym. Sci., 2002, 86, 2227–2231 CrossRef CAS.
- S. A. Riyajan, D. J. Liaw, Y. Tanaka and J. T. Sakdapipanich, J. Appl. Polym. Sci., 2007, 105, 664–672 CrossRef CAS.
- G. J. Van Veersen, Rubber Chem. Technol., 1951, 24(4), 957–969 CrossRef CAS.
- F. Cataldo, Polym. Degrad. Stab., 2003, 81, 249–260 CrossRef CAS.
- C. Wang, X. Huang and J. Yang, Eur. Polym. J., 2001, 37, 1895–1899 CrossRef CAS.
- A. Nakahara, K. Satoh and M. Kamigaito, Macromolecules, 2009, 42, 620–625 CrossRef CAS.
- Y. Shinke, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 841–847 CrossRef CAS.
- S. Ouardad, A. Deffieux and F. Peruch, Pure Appl. Chem., 2012, 84, 2065–2080 CAS.
- D. J. Patterson and J. L. Koenig, Die Makromol. Chemie, 1987, 188, 2325–2337 CrossRef CAS.
- E. J. Goethals and F. Du Prez, Prog. Polym. Sci., 2007, 32, 220–246 CrossRef CAS.
- D. Baskaran and A. H. E. Müller, Prog. Polym. Sci., 2007, 32, 173–219 CrossRef CAS.
- S. Stankovich, D. A. Dikin, G. H. B. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner, S. B. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282–286 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra07856h |
| This journal is © The Royal Society of Chemistry 2019 |