Open Access Article
Open Access ArticlePhase-transfer extraction for the fast quantification of perchlorate anions in water†
J. Vázquez and
V. Šindelář
and
V. Šindelář *
*
Department of Chemistry, RECETOX, Faculty of Science, Masaryk University, Kamenice 5, 625 00 Brno, Czech Republic. E-mail: sindelar@chemi.muni.cz
First published on 1st November 2019
Abstract
Supramolecular approaches for the quantitative anion analysis in water remain scarce due to the lack of receptors that effectively bind anions in this medium. Herein, we present a novel, fast and easy, supramolecular approach for a selective and quantitative analysis of perchlorate anions in water, coupling the UV-Vis spectroscopic method and phase-transfer extraction of anions by a water-insoluble anion receptor.
During the past decades, supramolecular recognition of anions has developed rapidly, with an increasing number of reported receptors,1–4 applied also in analytical chemistry.5,6 These anion receptors often employ hydrogen bonding as the recognition element, and are seldom reported for aqueous solutions2 – a consequence of the challenging ion recognition due to the high solvation of anions as well the competition of water molecules for the binding site of the receptor. This may be circumvented by employing receptors that bare positive charges.7–12 However, due to the nondirectional electrostatic interactions and counterion competition for the binding site, neutral receptors incorporating weak interactions may offer a better alternative over charged receptors. In particular, neutral receptors relying on C–H hydrogen bond interactions in combination with the hydrophobic effect possess excellent anion binding properties in water. A few examples include cyclopeptide derived hosts by Kubik,13,14 biotinuril macrocycles by Pittelkow,15,16 indolocarbazole-based foldamer by Jeong,17 phenylethynyl hosts by Johnson and Haley,18 octa-acids by Gibb,19 and the bambusuril macrocycles, introduced by our group in 2010, which exhibit very high binding affinity towards many anions both in organic and aqueous media.20–23
Conventional methods for the detection and quantification of anions in water are often time consuming, require complicated instrumentation, or frequently necessitate troublesome sample pre-treatment and reagent preparations.24,25 Anions and cations in drinking water and environmental waters are mainly determined using the methods of capillary electrophoresis, ion chromatography, and mass spectrometry.24,26 However, these techniques exhibit disadvantages such as the presence of interferences, lengthy analysis, complex data interpretation, the usage of expensive stationary phases and/or large amounts of mobile phases.24,27,28 In this respect, a simple method that combines supramolecular anion recognition, phase-transfer extraction, and widely available spectroscopic technique (i.e. UV/Vis), would, in principle, offer a very convenient approach to the quantification of anions in water samples. To our surprise, such method was unprecedented up to now, even though neutral receptors, such as crown ethers or calix[n]pyrroles, have been widely used for the extraction or preconcentration of anions.29–31 In a few cases, ionic dyes facilitated the use of UV/Vis spectroscopy to follow the extraction process, albeit only in a qualitative sense.32–34
Herein, we present a novel, fast and simple analytical method for the quantification of perchlorate anions in water via supramolecular anion recognition. The method comprises a bi-phasic solution system of water-insoluble anion receptor, bambusuril (BnBU), and a cationic lucigenin dye (Luc(NO3)2, Scheme 1, Fig. 1), operating on the principle of selective extraction of Luc(ClO4)2 from the aqueous to the organic phase. This allowed a simple UV/Vis quantification of the remaining Luc2+ in water which is related to the perchlorate content.
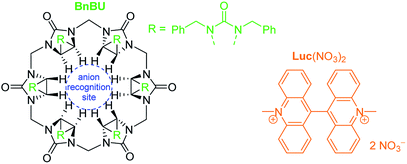 | ||
| Scheme 1 The bambusuril anion receptor (BnBU) and the lucigenin dye (Luc(NO3)2) used for perchlorate quantification by phase-transfer extraction. | ||
 | ||
| Fig. 1 Extraction experiments of the Luc2+ dye from water to dichloromethane (DCM), showing that both BnBU and perchlorate are necessary to extract Luc2+. | ||
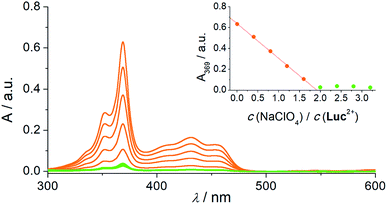
The extraction of Luc2+ from water to the dichloromethane (DCM) solution of BnBU, upon adding NaClO4, was in agreement with the disappearance of UV/Vis absorption bands of Luc2+ (Fig. 2). Control experiments showed that, in the absence of NaClO4, only 5% of Luc2+ is extracted to the BnBU DCM solution (Fig. S1†). However, no extraction occurred in the absence of BnBU. This observation is likely explained by the extraction of nitrate via complexation to BnBU, accompanied by the Luc2+ counter-ions. The association constant for the BnBU·NO3− complex is more than one order of magnitude lower compared to that one of the BnBU·ClO4− complex in chloroform,21 which results in a poor extraction of Luc2+ in the form of a nitrate salt, but much greater extraction in the form of a perchlorate salt. Moreover, Luc2+ was still being extracted as a preferred counter-ion even in the competing environment having Na+, K+, Mg2+, or Ca2+ cations (Fig. S2–S5†), commonly found in drinking water.
Furthermore, sodium salts of other anions, namely, Cl−, I−, H2PO4−, SO42−, HCO3−, and NO3− were also studied in the extraction experiments (Fig. S7–S12†) as these anions are naturally present in waters and biological fluids. No extraction was observed for the Cl−, H2PO4−, and SO42− (Fig. S7–S9†) because these anions bind to BnBU with low affinity due to their high solvation by water molecules. NO3− and HCO3− anions extracted only at much higher concentrations (>2 mM, Fig. S10 and S11†) compared to perchlorate (<50 μM). The extraction of NO3− is due to its higher chaotropic nature while the Luc2+ extraction via HCO3− was only partially achieved up to ≈30% even with a large excess of HCO3− (Fig. S10†). This may indicate additional acid–base equilibrium in the extractions process of HCO3−. Only I− anion exhibited a Luc2+ extraction to a significant extent at concentrations comparable to that of perchlorate (<50 μM, Fig. S12†). The affinity of tested anions towards BnBU is significantly weaker in comparison to perchlorate, with the exception of iodide, that binds with a similar strength.21 This indicates that the anion affinity towards BnBU plays an important role in the extraction process and it is following the order of these anions in the Hofmeister series.35 Therefore we decided to study the extraction of Luc2+ in presence of other chaotropic anions such as BF4− and PF6−. The results showed a rather quantitative extraction of Luc2+ (Fig. S13 and S14†), suggesting that, even though chaotropic anions are far less common in aqueous solutions, they should be taken in consideration, since they might as well interfere with the methodology herein presented.
Spectral changes in the course of the extraction experiments, followed by UV/Vis spectroscopy (Fig. 2), exhibited a linear dependence of the absorbance versus perchlorate concentration, up to two-fold excess of NaClO4 with respect to Luc2+. This strongly indicates that the Luc2+ dication is extracted exclusively in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry (Luc2+
2 stoichiometry (Luc2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) perchlorate). Since the absorbance is linearly proportional to the concentration of perchlorate, the titration curve could be used for the quantitative analysis of perchlorate in water (Fig. S6†).
perchlorate). Since the absorbance is linearly proportional to the concentration of perchlorate, the titration curve could be used for the quantitative analysis of perchlorate in water (Fig. S6†).
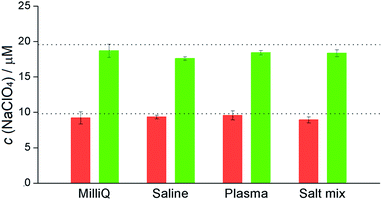
We utilized the aforementioned findings for the determination of perchlorates in different aqueous media, containing 10 or 20 μM of NaClO4: (i) deionized water (MilliQ quality), (ii) saline water (c(NaCl) = 500 mM), and (iii) a solution mimicking conditions in blood plasma (c(NaCl) = 100 mM, c(NaHCO3) = 20 mM). The UV/Vis spectra of the water phases were recorded after the extraction procedure, and the perchlorate concentrations for each sample was calculated from the difference in the Luc2+ absorbance in the aqueous phase. The measurements, repeated in triplicates, were in very good agreement with the real perchlorate content (Fig. 3), with absolute errors of up to −10% (saline media with 20 μM of NaClO4).
As presented in previous paragraphs, individual anion/cation influence on the extraction of Luc2+ indicated potentially selective behaviour of perchlorate. Therefore, the determination of perchlorate content in the presence of different anions and cations was further probed. Indeed, in a multiple salt mixture, namely, Mg(ClO4)2, Na2SO4, CaCl2, KH2PO4, NaHCO3, and NaNO3 (a detailed composition can be found in ESI, Fig. S18†), the extraction experiments gave very good agreement of the perchlorate content for both 10 and 20 μM concentrations of perchlorate anions (Fig. 3). Therefore, the proposed extraction method is indeed suitable for the quantitative analysis of perchlorate content in water. The presence of iodide, however, is expected to be a relevant interference in the determination of perchlorate. In this case, the iodide and perchlorate contributions could be easily assessed by 1H NMR. The ability of bambusurils to differentiate anions on NMR has been already reported previously by our group.21 The extraction experiment of a sample containing both ClO4− and I−, resulted in a Luc2+ extraction corresponding to the total concentration of these anions, the ratio of which was accurately determined by 1H NMR (Fig. S19 and S20†).
In summary, a new supramolecular approach for the quantification of perchlorate in water has been demonstrated. Bambusuril anion receptor was employed in the phase-transfer extraction of anions into an organic solvent, based on their relative binding strength with the receptor. The organic dye, lucigenin, was used as an indicator of the anion extraction process, as it was selectively extracted as a counter-ion. The presented methodology is invariant towards common anionic or cationic interfering species. Very good results were obtained for the analysis of perchlorate anions in different matrices (pure water, saline, and plasma blood conditions). We believe the methodology could be generally applied for quantification of various water-soluble analytes, using different water insoluble receptors. The presented method may in principle be applied for simple routine analysis of perchlorates, as it shows superiority towards the usual analytical methods in both simplicity and speed, with satisfying accuracy.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Czech Science Foundation (18-21801S) and the RECETOX Research Infrastructure (LM2015051 and CZ.02.1.01/0.0/0.0/16_013/0001761). We thank Zoran Kokan for the valuable remarks and discussions.Notes and references
- A. Brown and P. D. Beer, Chem. Commun., 2016, 52, 8645–8658 RSC.
- M. J. Langton, C. J. Serpell and P. D. Beer, Angew. Chem., Int. Ed., 2016, 55, 1974–1987 CrossRef CAS.
- Q. He, P. Tu and J. L. Sessler, Chem, 2018, 4, 46–93 CAS.
- M. Zhang, R.-L. Lin, W.-Q. Sun and J.-X. Liu, J. Inclusion Phenom. Macrocyclic Chem., 2018, 90, 173–187 CrossRef CAS.
- S. Li and W. C. Purdy, Chem. Rev., 1992, 92, 1457–1470 CrossRef CAS.
- L. Szente and J. Szemán, Anal. Chem., 2013, 85, 8024–8030 CrossRef CAS.
- M. A. Saeed, F. R. Fronczek and M. A. Hossain, Chem. Commun., 2009, 6409–6411, 10.1039/b916099j.
- K. R. Dey, T. Horne, F. R. Fronczek and M. A. Hossain, Inorg. Chem. Commun., 2010, 13, 1515–1518 CrossRef CAS PubMed.
- R. Kumar, S. Kumar, P. Singh, G. Hundal, M. S. Hundal and S. Kumar, Analyst, 2012, 137, 4913–4916 RSC.
- J. Cai, B. P. Hay, N. J. Young, X. Yang and J. L. Sessler, Chem. Sci., 2013, 4, 1560–1567 RSC.
- H. Zhou, Y. Zhao, G. Gao, S. Li, J. Lan and J. You, J. Am. Chem. Soc., 2013, 135, 14908–14911 CrossRef CAS.
- V. Amendola, G. Bergamaschi, M. Boiocchi, R. Alberto and H. Braband, Chem. Sci., 2014, 5, 1820–1826 RSC.
- S. Kubik, R. Goddard, R. Kirchner, D. Nolting and J. Seidel, Angew. Chem., Int. Ed., 2001, 40, 2648–2651 CrossRef CAS.
- F. Sommer and S. Kubik, Org. Biomol. Chem., 2014, 12, 8851–8860 RSC.
- M. Lisbjerg, B. M. Jessen, B. Rasmussen, B. E. Nielsen, A. Ø. Madsen and M. Pittelkow, Chem. Sci., 2014, 5, 2647–2650 RSC.
- M. Lisbjerg, B. E. Nielsen, B. O. Milhøj, S. P. A. Sauer and M. Pittelkow, Org. Biomol. Chem., 2015, 13, 369–373 RSC.
- J.-m. Suk and K.-S. Jeong, J. Am. Chem. Soc., 2008, 130, 11868–11869 CrossRef CAS.
- O. B. Berryman, C. A. Johnson II, L. N. Zakharov, M. M. Haley and D. W. Johnson, Angew. Chem., Int. Ed., 2008, 47, 117–120 CrossRef CAS.
- P. Sokkalingam, J. Shraberg, S. W. Rick and B. C. Gibb, J. Am. Chem. Soc., 2016, 138, 48–51 CrossRef CAS.
- J. Svec, M. Necas and V. Sindelar, Angew. Chem., Int. Ed., 2010, 49, 2378–2381 CrossRef CAS.
- V. Havel and V. Sindelar, ChemPlusChem, 2015, 80, 1601–1606 CrossRef CAS.
- M. A. Yawer, V. Havel and V. Sindelar, Angew. Chem., Int. Ed., 2015, 54, 276–279 CrossRef CAS.
- J. Vazquez and V. Sindelar, Chem. Commun., 2018, 54, 5859–5862 RSC.
- M. L. Magnuson, E. T. Urbansky and C. A. Kelty, Anal. Chem., 2000, 72, 25–29 CrossRef CAS.
- Y. Yang, F. Huo, J. Zhang, Z. Xie, J. Chao, C. Yin, H. Tong, D. Liu, S. Jin, F. Cheng and X. Yan, Sens. Actuators, B, 2012, 166–167, 665–670 CrossRef CAS.
- R. Srinivasan and G. A. Sorial, Sep. Purif. Technol., 2009, 69, 7–21 CrossRef CAS.
- A. Padarauskas, V. Olsauskaite and V. Paliulionyte, J. Chromatogr. A, 1998, 829, 359–365 CrossRef CAS.
- F. A. Mellon, in Encyclopedia of Food Sciences and Nutrition, ed. B. Caballero, Academic Press, Oxford, 2nd edn, 2003, pp. 3739–3749, DOI:10.1016/B0-12-227055-X/00746-X.
- Q. He, Z. Zhang, J. T. Brewster, V. M. Lynch, S. K. Kim and J. L. Sessler, J. Am. Chem. Soc., 2016, 138, 9779–9782 CrossRef CAS.
- H. Zhou, Y. Wang, X. Guo, Y. Dong, X. Su and X. Sun, J. Mol. Liq., 2018, 254, 414–420 CrossRef CAS.
- W. Zhu, Y. Jia, Q. Zhang, J. Sun, Y. Jing and J. Li, J. Mol. Liq., 2019, 285, 75–83 CrossRef CAS.
- I. Carreira-Barral, M. Mato-Iglesias, A. de Blas, C. Platas-Iglesias, P. A. Tasker and D. Esteban-Gómez, Dalton Trans., 2017, 46, 3192–3206 RSC.
- S. Mahajan, N. Singh, J. P. Kushwaha and A. Rajor, Chem. Eng. Commun., 2019, 206, 697–707 CrossRef CAS.
- G. A. Marcelo, S. M. G. Pires, M. A. F. Faustino, M. M. Q. Simões, M. G. P. M. S. Neves, H. M. Santos, J. L. Capelo, J. P. Mota, C. Lodeiro and E. Oliveira, Dyes Pigm., 2019, 161, 427–437 CrossRef CAS.
- F. Hofmeister, Arch. Exp. Pathol. Pharmakol., 1888, 24, 247–260 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, UV/Vis and NMR graphs. See DOI: 10.1039/c9ra08602a |
| This journal is © The Royal Society of Chemistry 2019 |