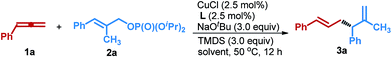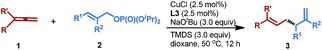Open Access Article
Open Access ArticleEnantioselective and site-specific copper-catalyzed reductive allyl–allyl cross-coupling of allenes†
Guoxing
Xu‡
,
Bin
Fu‡
,
Haiyan
Zhao
,
Yanfei
Li
,
Ge
Zhang
,
Ying
Wang
,
Tao
Xiong
 * and
Qian
Zhang
* and
Qian
Zhang
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, Department of Chemistry, Northeast Normal University, Changchun, 130024, Jilin, China. E-mail: xiongt626@nenu.edu.cn
First published on 4th December 2018
Abstract
A copper-catalyzed asymmetric reductive allyl–allyl cross-coupling reaction of allenes with allylic phosphates wherein allenes were used as allylmetal surrogates has been achieved for the first time. This protocol provides an efficient and straightforward route to optically active 1,5-dienes in a highly enantioselective and site-specific fashion. Furthermore, all-carbon quaternary stereogenic centers could also be constructed with this protocol. The versatility of the products is demonstrated through a diverse array of further transformations of the enantioenriched 1,5-dienes.
Introduction
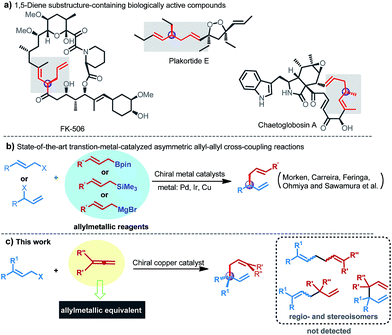
The development of highly efficient and straightforward methodologies for creation of configurationally well-defined stereocenters during the course of C–C bond formation is of paramount importance in organic synthesis.1 Among such developments, metal-catalyzed asymmetric allyl–allyl cross-coupling reactions between allylic electrophiles and allylmetal reagents have been regarded as particularly valuable methods, due to not only the ability to establish a new stereogenic center with concomitant introduction of two differential flexible allyl functional groups in one synthetic operation, but also providing a convenient route to access highly important chiral 1,5-diene structures, which serve as highly versatile building blocks in organic synthesis and are also found in many important biologically active molecules, such as plakortide E, FK-506, and chaetoglobosin (Fig. 1a).2 In this respect, several transition metal complexes of Pd, Au, Cu, and Ni had been successfully employed to catalyze such racemic transformations over the past few years;3 nevertheless, the development of an asymmetric version to expedite access to enantioenriched 1,5-dienes remains a long-standing challenge.Recently, Morken and co-workers described a Pd-catalyzed allyl–allyl cross-coupling of allylic electrophiles with allylboron reagents in a highly regio- and enantioselective manner through the use of a key chiral small bite-angle bidentate phosphine ligand.4 Subsequently, the group of Carreira also developed phosphoramidite-ligated Ir-catalyzed asymmetric coupling reactions between secondary allylic alcohols and allylsilanes.5 More recently, by employing Earth-abundant base metals as the catalysts, Feringa, Ohmiya and Sawamura independently reported Cu-catalyzed regio- and enantioselective allyl–allyl cross-coupling of allylic bromides or phosphates with corresponding allylic Grignard reagents or allylic boronates (Fig. 1b).6 In addition, Hoveyda and co-workers also elegantly disclosed an approach for the synthesis of chiral boron-containing 1,5-dienes bearing a tertiary stereogenic centre through Cu-catalyzed boron allylation of monoalkyl-substituted allenes.7 Notwithstanding these advances, stoichiometric preformed allylic metals have been generally indispensable in both racemic and asymmetric transformations to date,3–6 which significantly impedes further applications because of the intrinsic limitations associated with these reagents, such as the need for multistep operations of unsaturated hydrocarbons or organic halides, uncontrollable reactivity profiles, sensitivity toward water and poor functional group compatibility. Moreover, the more daunting issue of the enantioselective generation of all-carbon quaternary stereocenters during these processes remains an unmet synthetic challenge, owing to increased energy barriers as a result of the highly congested nature and the diminished steric bias compared with those of the corresponding chiral tertiary centers leading to more difficulty in discriminating enantiotopic faces.8 So far, to our knowledge, the sole reported example was demonstrated by Morken et al. through utilization of allylboron as the nucleophile with a precious Pd catalyst.4b Thus, the development of an alternative method for enantioselective allyl–allyl cross-coupling to furnish versatile 1,5-dienes that have the ability to create an all-carbon quaternary stereocenter with concomitant avoidance of the use of preformed allylic metals (or metalloids) and precious metal catalysts is highly desirable.
Recently, an interesting protocol for utilizing readily available and stable unsaturated hydrocarbons as latent carbon nucleophiles in lieu of preformed organometallic reagents for coupling with various electrophiles in transition-metal catalysis has attracted tremendous attention.9 In this regard, Buchwald,10 Yun,11 Hoveyda12 and our group13 have disclosed copper hydride (CuH)-catalyzed regio- and enantioselective reductive allylation (or hydroallylation) reactions using olefins or alkynes as latent alkyl- or vinylmetal reagents. Inspired by these achievements, we envisioned whether the readily available allenes could serve as latent effective allylic metal equivalents to couple with allylic electrophiles leading to synthetically important 1,5-diene-containing molecules. In fact, allene-derived allylic nucleophiles that react with ketone, imine, alkyl triflates and CO2 under reductive conditions have been described by Tsuji, Buchwald and Lalic in the past few years.14 In comparison, the more intricate reductive allyl–allyl cross-coupling reaction remains unexploited to date, as exploiting such a reliable catalytic method that could well balance the reactivity profiles (the chemoselectivity of CuH species to allene versus allylic electrophile)13,15 regio- and stereocontrol, as well as having the power to establish an all-carbon quaternary stereocenter. Herein, we report the first Cu-catalyzed site-specific and enantioselective reductive allyl–allyl cross-coupling of allenes with allylic phosphates to afford enantioenriched 1,5-dienes without the use of any pre-formed allylic metals,16 highlighting the ability to construct more hindered all-carbon quaternary stereocenters by this approach (Fig. 1c).
Results and discussion
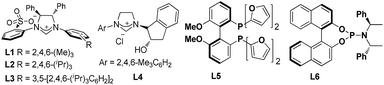
We initiated our study by choosing allene 1a as the latent allylic metal reagent to couple with allylic phosphate 2a under our previously reported hydroallylation conditions (Table 1).13 Pleasingly, the expected SN2′-type reductive allyl–allyl cross-coupling product 3a was obtained in a moderate yield with exclusive regioselectivity, albeit low enantioselectivity, under these catalytic conditions (entry 1). In view of the unique ability of chiral N-heterocyclic carbene (NHC) to facilitate Cu-catalyzed SN2′-type allylation,12,13,17 we therefore briefly surveyed various chiral NHCs, such as L2–L4, and L3 (ref. 17c and d) showed acceptable reactivity profiles and excellent enantiocontrol (entries 2–4). Likewise, chiral bisphosphine ligands L5 and L6, which exhibited excellent reactivity and enantioselectivity for the reported allyl–allyl cross-coupling reactions,4–6 however, were ineffective in this catalytic system (entries 5 and 6). To our delight, after subsequent solvent screening and switching the ratio of allene 1a and allylic phosphate 2a (entries 7–10), the desired product 3a could be isolated in 73% yield with 93% ee (entry 10). In addition, the other allylic electrophiles, including analogous allyl bromide and carbonate, were found to be completely ineffective under the present catalytic conditions (entry 11). Remarkably, under all conditions only regioisomer 3a was obtained, suggesting the exclusive regioselectivity. Further evaluation of various copper catalysts, ligands, bases, allylic phosphates and hydrosilanes is discussed in the ESI.†| Entry | Ligand | Solvent | Yieldb (%) | eec (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol), CuCl (2.5 mol%), ligand (2.5 mol%), NaOtBu (0.6 mmol) and TMDS (0.6 mmol) in 2 mL solvent at 50 °C in a N2 atmosphere unless otherwise stated. b Isolated yield. c ee determined by HPLC. d 1a (0.3 mmol) and 2a (0.2 mmol). TMDS = (Me2HSi)2O. e Using –Br or –OC(O)OtBu as the leaving group in the allyl electrophile. | ||||
| 1 | L1 | THF | 50 | 33 |
| 2 | L2 | THF | 0 | 0 |
| 3 | L3 | THF | 50 | 89 |
| 4 | L4 | THF | 0 | 0 |
| 5 | L5 | THF | 0 | 0 |
| 6 | L6 | THF | 0 | 0 |
| 7 | L3 | Toluene | 70 | 76 |
| 8 | L3 | Dioxane | 60 | 93 |
| 9 | L3 | Cyclohexane | 66 | 77 |
| 10d | L3 | Dioxane | 73 | 93 |
| 11e | L3 | THF | 0 | 0 |
|
||||
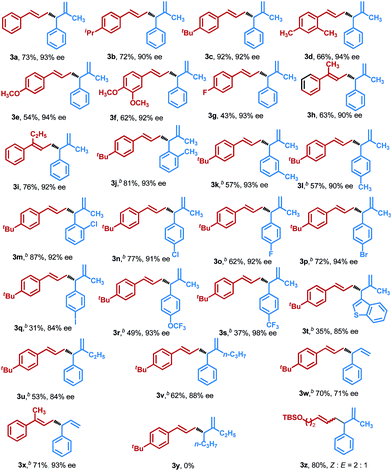
Having identified the optimal conditions, we explored the substrate scope with respect to various allenes 1 and allylic phosphates 2 (Table 2). The allenes bearing either aliphatic substituents, alkoxyl or halogen on the aryl rings, generally displayed high levels of reactivities and excellent enantioselectivities. A relatively lower efficiency was observed for the allene 1g, which is presumably ascribed to its relatively electron deficient nature, and this result is in accordance with a recent report by Hoveyda and co-workers.18 1,1-disubstituted alkenes 1h and 1i could also be efficiently converted into corresponding 1,5-dienes 3h and 3i in good yields with excellent enantioselectivities. We next assessed the scope of allylic phosphate components under these conditions. We found that a variety of functional groups were well tolerated, including alkyl, –F, –Cl, –Br, –I, –OCF3 and –CF3, providing the expected chiral 1,5-dienes 3j–3s in moderate to good yields with generally excellent enantiocontrol. We noted that the substituents at either ortho-, meta- or para-positions of the aromatic rings in the allylic phosphates, such as 3j–3l and 3m–3n, had a negligible effect on enantiocontrol, while ortho-substituted allylic phosphates showed higher reactivities. Furthermore, the introduction of halogens in the aryls in 3m–3q could provide more opportunities for further elaboration, particularly for the high reactivity of aryl-I, which is rarely tolerated in CuH-catalyzed transformations. Heteroaromatic ring-containing allylic phosphates and β-ethyl-, propyl- or H-substituted allylic phosphates also enabled the reaction to proceed smoothly, furnishing chiral 1,5-dienes 3t–3w in acceptable yields with good enantioselectivities. Additionally, 1,1-disubstituted allene was also a valid substrate for this transformation and the corresponding product 3x was obtained with excellent enantioselectivity, but alkyl substituted allylic phosphate was not suitable for this reductive cross-coupling reaction. By comparison, in the case of alkyl substituted allene, the corresponding product 1,5-diene 3z could be efficiently formed, although a mixture of alkene isomers was observed, and this result is in line with the more recent findings of Tsuji.16
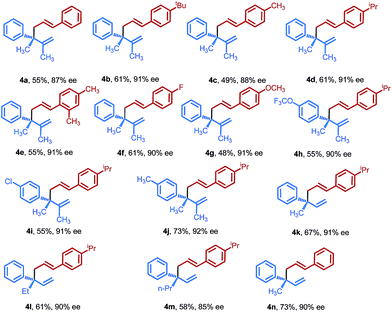
We considered that 1,5-dienes bearing all-carbon quaternary stereocenters widely distributed in many important biologically active molecules have significant value in organic synthesis,8,19 while the synthesis of such highly congested stereocenter-containing molecules remains an unmet challenge. Thus, we were particularly interested in whether this protocol would be applicable for the construction of an all-carbon quaternary stereogenic center. After briefly screening the reaction conditions, to our delight, the anticipated quaternary carbon center-containing chiral 1,5-diene 4 could be readily obtained with exclusive regioselectivity and excellent enantioselectivity. As depicted in Table 3, various aliphatic substituents, –OCH3 and –F, on the aromatic rings of the allenes could be efficiently transformed into the corresponding 1,5-dienes 4a–4g in moderate to good yields with generally excellent enantiocontrol. Additionally, a number of allylic electrophiles, including different substituents on the aryl ring, and β- or γ-position of allylic phosphates, could also be cross-coupled efficiently, affording optically active 1,5-dienes 4h–4n with high enantioselectivities. Finally, the absolute configuration of the major enantiomer was determined as (R) by comparison of the optical rotation of 4n with a reported value.4b
| a Reaction conditions: 1 (0.3 mmol), 2 (0.2 mmol), CuCl (2.5 mol%), L3 (2.5 mol%), NaOtBu (0.6 mmol) and TMDS (0.6 mmol) in 2 mL dry dioxane at 50 °C under a N2 atmosphere unless otherwise stated. Isolated yield. |
|---|
|
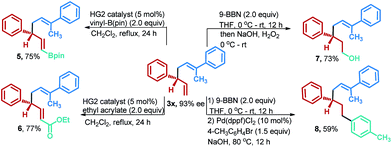
To showcase the synthetic utility of chiral 1,5-dienes, the reductive allyl–allyl cross-coupling product 3x could be expediently converted into a series of synthetically valuable optically active compounds 5–8 with exclusive chemoselectivity (Fig. 2). For example, chiral 1,5-diene 3x was subjected to olefin cross-metathesis conditions with vinylB(pin), providing the versatile building block chiral vinyl borate 5 in good yields. In addition, 3x could also be efficiently transformed into α, β-unsaturated ester 6 in good yields under the same reaction conditions. Furthermore, chiral alcohol 7 and multi-aromatic compound 8 could also be efficiently obtained via the hydroboration/oxidation or hydroboration/Suzuki reaction.
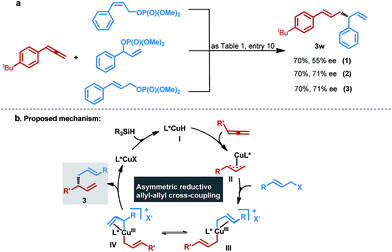
To gain some insight into the mechanism, we assessed the impact of different allylic phosphate isomers on the reactivity, and regio- and enantioselectivity. As depicted in Fig. 3a, besides (E)-allylic phosphate, (Z)-allylic phosphate and racemic secondary allylic phosphate were also valid substrates and exhibited identical reactivity and exclusive regioselectivity, despite the relatively lower enantiocontrol of (Z)-allylic phosphate (Fig. 3a, eqn (1)–(3)). Moreover, according to previous reports6 and regioconvergent outcomes, which suggested that these transformations might produce the same allylcopper(III) intermediate III prior to reductive elimination to form the branched chiral 1,5-diene 3w (vide infra). Based on this experimental result and the previously proposed mechanism for Cu-catalyzed allylic substitutions,20 a possible mechanism was described in Fig. 3b. Initially, chemoselective insertion of the terminal allene into catalytic ligated L*CuH species I, which is derived from the in situ generated L*CuOR with hydrosilane through a direct metathesis process, could catalytically form allylcopper species II. Subsequently, oxidative addition of intermediate II with an allylic electrophile would furnish 16-electron allylcopper(III) intermediates III and IV, which probably equilibrate via σ–π–σ isomerization. Finally, the chiral regioisomer 1,5-diene 3 was generated via reductive elimination and simultaneous release of the Cu(I) species, which then reacts with a hydridic silane via a σ-bond metathesis pathway, regenerating the L*CuH catalyst.
Conclusion
In summary, a copper-catalyzed asymmetric reductive allyl–allyl cross-coupling reaction of allenes with allylic phosphates was achieved for the first time. This protocol exhibited good functional group tolerance, exclusive regioselectivity and highly enantioselective control, and provides a facile route to access chiral 1,5-dienes bearing tertiary or more hindered all-carbon quaternary stereocenters. Studies on further expanding this strategy including utilizing different coupling partners for enantioselective construction of C–C bonds are being carried out in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the NSFC (21672033, 21831002), the Jilin Province Natural Science Foundation (20160520140JH and 20160519003JH), and the Ten Thousand Talents Program for generous financial support.Notes and references
- (a) N. Jacobsen, A. Pfaltz and H. Yamamoto, Comprehensive Asymmetric Catalysis: Suppl. 2, Springer-Verlag, Berlin, 2004 Search PubMed; (b) B. M. Trost, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5348 CrossRef CAS PubMed; (c) Z. Lu and S. Ma, Angew. Chem., Int. Ed., 2008, 47, 258 CrossRef CAS PubMed; (d) G. Helmchem, U. Kazmaier and S. Förster, in Catalytic Asymmetric Synthesis, John Wiley & Sons, Hoboken, NJ, 2010 Search PubMed; (e) E. Negishi, Angew. Chem., Int. Ed., 2011, 50, 6738 CrossRef CAS PubMed.
- (a) E. Breitmaier, Terpenes, Flavors, Fragrances, Pharmaca, Pheromones, Wiley-VCH, Weinheim, 2006 Search PubMed; (b) P. M. Dewick, Medicinal Natural Products: A Biosynthetic Approach, Wiley, Chichester, 2002 Search PubMed.
- For selected examples, see: Pd-catalyzed allylation: (a) H. Nakamura, M. Bao and Y. Yamamoto, Angew. Chem., Int. Ed., 2001, 40, 3208 CrossRef CAS; (b) E. F. Flegeau, U. Schneider and S. Kobayashi, Chem.–Eur. J., 2009, 15, 12247 CrossRef CAS PubMedAu-catalyzed allylation: (c) S. Porcel, V. López-Carrillo, C. García-Yebra and A. M. Echavarren, Angew. Chem., Int. Ed., 2008, 47, 1883 CrossRef CAS PubMedCu-catalyzed allylation: (d) A. Yanagisawa, N. Nomura and H. Yamamoto, Tetrahedron, 1994, 50, 6017 CrossRef CAS; (e) A. S. E. Karlström and J.-E. Bäckvall, Chem.–Eur. J., 2001, 7, 1981 CrossRefNi-catalyzed allylation: (f) Y. Sumida, S. Hayashi, K. Hirano, H. Yorimitsu and K. Oshima, Org. Lett., 2008, 10, 1629 CrossRef CAS PubMed; (g) R. Matsubara and T. F. Jamison, J. Am. Chem. Soc., 2010, 132, 6880 CrossRef CAS PubMed; (h) A. Jiménez-Aquino, E. F. Flegeau, U. Schneider and S. Kobayashi, Chem. Commun., 2011, 47, 9456 RSC.
- (a) P. Zhang, L. A. Brozek and J. P. Morken, J. Am. Chem. Soc., 2010, 132, 10686 CrossRef CAS PubMed; (b) P. Zhang, H. Le, R. E. Kyne and J. P. Morken, J. Am. Chem. Soc., 2011, 133, 9716 CrossRef CAS PubMed; (c) L. A. Brozek, M. J. Ardolino and J. P. Morken, J. Am. Chem. Soc., 2011, 133, 16778 CrossRef CAS PubMed; (d) H. Le, R. E. Kyne, L. A. Brozek and J. P. Morken, Org. Lett., 2013, 15, 1432 CrossRef CAS PubMed; (e) H. Le, A. Batten and J. P. Morken, Org. Lett., 2014, 16, 2096 CrossRef CAS PubMed; (f) M. J. Arolino and J. P. Morken, J. Am. Chem. Soc., 2014, 136, 7092 CrossRef PubMed.
- J. Y. Hamilton, N. Hauser, D. Sarlah and E. M. Carreira, Angew. Chem., Int. Ed., 2014, 53, 10759 CrossRef CAS PubMed.
- (a) V. Hornillos, M. Pérez, M. Fañanás-Mastral and B. L. Feringa, J. Am. Chem. Soc., 2013, 135, 2140 CrossRef CAS PubMed; (b) Y. Yasuda, H. Ohmiya and M. Sawamura, Angew. Chem., Int. Ed., 2016, 55, 10816 CrossRef CAS PubMed.
- F. Meng, K. McGrath and A. H. Hoveyda, Nature, 2014, 513, 367 CrossRef CAS PubMed.
- K. W. Quasdorf and L. E. Overman, Nature, 2014, 516, 181 CrossRef CAS PubMed.
- For selected reviews, see: (a) H.-Y. Jang and M. J. Krische, Acc. Chem. Res., 2004, 37, 653 CrossRef CAS PubMed; (b) H.-Y. Jang and M. J. Krische, Eur. J. Org. Chem., 2004, 3953 CrossRef CAS; (c) H. Nishiyama and T. Shiomi, Top. Curr. Chem., 2007, 279, 105 CrossRef CAS; (d) J. F. Bower, I. S. Kim, R. L. Patman and M. J. Krische, Angew. Chem., Int. Ed., 2009, 48, 34 CrossRef CAS PubMed; (e) J. M. Ketcham, I. Shin, T. P. Montgomery and M. J. Krische, Angew. Chem., Int. Ed., 2014, 53, 9142 CrossRef CAS PubMed; (f) S. K. Murphy and V. M. Dong, Chem. Commun., 2014, 50, 13645 RSC; (g) K. D. Nguyen, B. Y. Park, T. Luong, H. Sato, V. J. Garza and M. J. Krische, Science, 2016, 354, aah5133 CrossRef PubMed; (h) M. T. Pirnot, Y.-M. Wang and S. L. Buchwald, Angew. Chem., Int. Ed., 2016, 55, 48 CrossRef CAS PubMed.
- Y.-M. Wang and S. L. Buchwald, J. Am. Chem. Soc., 2016, 138, 5024 CrossRef CAS PubMed.
- J. T. Han, W. J. Jang, N. Kim and J. Yun, J. Am. Chem. Soc., 2016, 138, 15146 CrossRef CAS PubMed.
- J. Lee, S. Torker and A. H. Hoveyda, Angew. Chem., Int. Ed., 2017, 56, 821 CrossRef CAS PubMed.
- G. Xu, H. Zhao, B. Fu, A. Cang, G. Zhang, Q. Zhang, T. Xiong and Q. Zhang, Angew. Chem., Int. Ed., 2017, 56, 13130 CrossRef CAS PubMed.
- For a review on allenes as the allylic nucleophiles in copper catalysis, see: (a) A. P. Pulis, K. Yeung and D. J. Procter, Chem. Sci., 2017, 8, 5240 RSCFor recent examples of CuH catalysis, see: (b) Y. Tani, K. Kuga, T. Fujihara, J. Terao and Y. Tsuji, Chem. Commun., 2015, 51, 13020 RSC; (c) R. Y. Liu, Y. Yang and S. L. Buchwald, Angew. Chem., Int. Ed., 2016, 55, 14077 CrossRef CAS PubMed; (d) M. Lee, M. Nguyen, C. Brandt, W. Kaminsky and G. Lalic, Angew. Chem., Int. Ed., 2017, 56, 15703 CrossRef CAS PubMed; (e) E. Y. Tsai, R. Y. Liu, Y. Yang and S. L. Buchwald, J. Am. Chem. Soc., 2018, 140, 2007 CrossRef CAS PubMed.
- T. N. Nguyen, N. O. Thiel, F. Pape and J. F. Teichert, Org. Lett., 2016, 18, 2455 CrossRef CAS PubMed.
- More recently, the racemic version was reported by Tsuji and co-workers, see: T. Fujihara, K. Yokota, J. Terao and Y. Tsuji, Chem. Commun., 2017, 53, 7898 RSC.
- For selected recent examples, see: (a) R. Shintani, K. Takatsu, M. Takeda and T. Hayashi, Angew. Chem., Int. Ed., 2011, 50, 8656 CrossRef CAS PubMed; (b) A. Harada, Y. Makida, T. Sato, H. Ohmiya and M. Sawamura, J. Am. Chem. Soc., 2014, 136, 13932 CrossRef CAS PubMed; (c) K. Akiyama, F. Gao and A. H. Hoveyda, Angew. Chem., Int. Ed., 2010, 49, 419 CrossRef CAS PubMed; (d) Y. Shi, B. Jung, S. Torker and A. H. Hoveyda, J. Am. Chem. Soc., 2015, 137, 8948 CrossRef CAS PubMed; (e) Z.-Q. Zhang, B. Zhang, X. Lu, J.-H. Liu, X.-Y. Lu, B. Xiao and Y. Fu, Org. Lett., 2016, 18, 952 CrossRef CAS PubMed; (f) S. Guduguntla, J.-B. Gualtierotti, S. S. Goh and B. L. Feringa, ACS Catal., 2016, 6, 6591 CrossRef CAS.
- J. Lee, S. Radomkit, S. Torker, J. del Pozo and A. H. Hoveyda, Nat. Chem., 2018, 10, 99 CrossRef CAS PubMed.
- (a) P. G. Cozzi, R. Hilgraf and N. Zimmermann, Eur. J. Org. Chem., 2007, 36, 5869 Search PubMed; (b) B. M. Trost and C. Jiang, Synthesis, 2006, 369 CrossRef CAS; (c) J. P. Das and I. Marek, Chem. Commun., 2011, 47, 4593 RSC; (d) K. W. Quasdorf and L. E. Overman, Nature, 2014, 516, 181 CrossRef CAS; (e) J. Feng, M. Holmes and M. J. Krische, Chem. Rev., 2017, 117, 12564 CrossRef CAS PubMed.
- For selected recent reviews on Cu-mediated allylic substitutions, see: (a) A. H. Hoveyda, A. W. Hird and M. A. Kacprzynski, Chem. Commun., 2004, 1779 RSC; (b) H. Yorimitsu and K. Oshima, Angew. Chem., Int. Ed., 2005, 44, 4435 CrossRef CAS PubMed; (c) A. Alexakis, J. E. Bäckvall, N. Krause, O. Pàmies and M. Diéguez, Chem. Rev., 2008, 108, 2796 CrossRef CAS PubMed; (d) S. R. Harutyunyan, den T. Hartog, K. Geurts, A. J. Minnaard and B. L. Feringa, Chem. Rev., 2008, 108, 2824 CrossRef CAS PubMed; (e) A. H. Cherney, N. T. Kadunce and S. E. Reisman, Chem. Rev., 2015, 115, 9587 CrossRef CAS PubMed; (f) R. Shintani, Synthesis, 2016, 48, 1087 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Optimization tables, experimental procedures, and characterization data. For other electronic format see DOI: 10.1039/c8sc04505d |
| ‡ G. X. and B. F. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |