Open Access Article
Open Access ArticleEfficient solar cells sensitized by a promising new type of porphyrin: dye-aggregation suppressed by double strapping†
Kaiwen
Zeng
a,
Yunyue
Lu
a,
Weiqiang
Tang
 b,
Shuangliang
Zhao
b,
Shuangliang
Zhao
 b,
Qingyun
Liu
b,
Qingyun
Liu
 c,
Weihong
Zhu
c,
Weihong
Zhu
 a,
He
Tian
a and
Yongshu
Xie
a,
He
Tian
a and
Yongshu
Xie
 *a
*a
aKey Laboratory for Advanced Materials and Feringa Nobel Prize Scientist Joint Research Center, School of Chemistry and Molecular Engineering, East China University of Science &Technology, 130 Meilong, Shanghai 200237, China. E-mail: yshxie@ecust.edu.cn
bSchool of Chemical Engineering and State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai, 200237, China
cCollege of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao, P. R. China
First published on 14th December 2018
Abstract
Porphyrin sensitizers play essential roles in the development of efficient dye-sensitized solar cells (DSSCs). To further improve power conversion efficiency (PCE), it is vital to reduce undesirable dye aggregation that causes serious charge recombination and lowered open-circuit voltages (Voc). To this end, we herein report a new class of porphyrin-based dyes XW40 and XW41, with the porphyrin cores strapped with two circle chains. Compared with the reference sensitizer XW10 which contains a porphyrin core wrapped in four dodecoxyl chains, the double strapping in XW40 not only effectively suppresses the dye aggregation but also improves the dye loading amount. As a result, the Voc and photocurrent (Jsc) were improved by 19 mV and 0.8 mA cm−2, respectively, compared with the corresponding values of XW10, and the efficiency was improved from 8.6% obtained for XW10 to 9.3% for XW40. To further extend the spectral response, an electron-withdrawing benzothiadiazole (BTD) unit was introduced as an auxiliary acceptor in XW41. Impressively, the onset wavelength of its IPCE spectrum was dramatically red-shifted to 830 nm. However, the extended π-conjugation framework results in aggravated dye aggregation, and thus a lowered efficiency of 8.2% was obtained for XW41. Through a combined approach of coadsorption and cosensitization, the efficiencies were dramatically enhanced to 10.6% and 10.2% for XW40 and XW41, respectively, as a result of simultaneously enhanced Voc and Jsc. The results of this work provide a novel strategy for developing efficient DSSCs by employing strapped porphyrin dyes.
Introduction
Because of the increasingly serious energy shortage problems associated with the exhaustion of fuel energy resources, inexhaustible clean energy sources are highly desired. In this respect, solar energy has been considered as the most viable option for sustainable development of human society. Among the photovoltaic devices, dye-sensitized solar cells have drawn considerable attention over the past two decades, showing advantages of relatively low-cost, eco-friendliness, and relatively high PCE.1 Since Grätzel and coworkers first reported a ruthenium dye based DSSC with a PCE of 7.1% in 1991,2 numerous efforts have been devoted to improve cell efficiency. For this purpose, porphyrin and their derivatives have been developed as promising sensitizers because of their facile structural modification and intrinsic strong absorption capability, showing an intense Soret band and moderate Q bands in the ranges of 400–450 nm and 550–650 nm, respectively.3–5Highly efficient porphyrin sensitizers usually feature a donor–π–acceptor (D–π–A) configuration.6 Such structures exhibit excellent intramolecular charge transfer (ICT) properties, and tunable optical, physical and electrochemical properties.7 However, simple porphyrin dyes usually exhibit poor absorption in the near infrared (NIR) region. To enhance light-harvesting ability in the NIR region, porphyrin sensitizers with extended conjugation frameworks have been developed.8–10 However, this approach usually aggravates the undesired dye aggregation, which may result in serious charge recombination, low electron-injection efficiency, and poor cell performance.11–15 To overcome such a problem, efficient porphyrin dyes have been developed by wrapping the porphyrin macrocycle with four o-alkoxyl moieties on the meso-phenyl substituents.16–20 In this respect, we have designed XW10 using a phenothiazine-based donor, and an efficiency of 8.6% was achieved for the individual dye.33
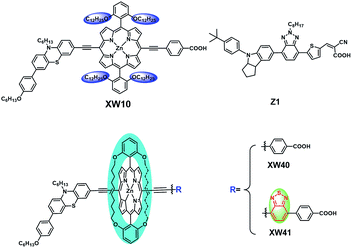
On the basis of XW10, with the purpose of further suppressing the dye aggregation effect, we herein report a new class of porphyrin sensitizers XW40 and XW41 (Fig. 1) with double straps. Compared with XW10, the straps in XW40 are favourable for suppressing dye aggregation, resulting in simultaneously improved Voc and Jsc and an efficiency of 9.3%, obviously higher than the 8.6% obtained for XW10. On this basis, XW41 was synthesized by introducing an auxiliary benzothiadiazole (BTD) moiety with the purpose of further extending the absorption spectrum.9,21–24 As a result, the onset wavelength of photocurrent response was red-shifted to 830 nm. Upon cosensitization with an organic dye, Z1, high efficiencies of 10.6% and 10.2% were achieved for XW40 and XW41, respectively, based on the traditional iodine electrolyte. Although circle chain strapping has been demonstrated to be effective in developing organic dyes,25,26 and strapped porphyrins have been reported for fabrication of OLEDs, and to achieve charge transfer and sensitization behavior on semiconductor films,27–29 this approach has not yet been employed in designing efficient porphyrin dyes. The results of this work provide an effective novel strategy for designing efficient porphyrin dyes by introducing double straps.
Results and discussion
Synthesis of dyes
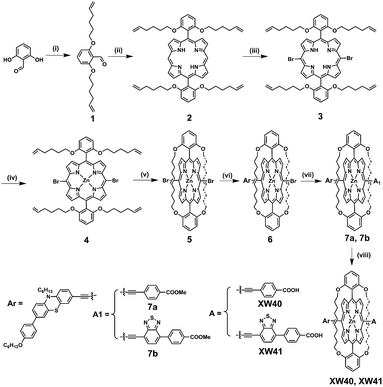
The synthesis routes are depicted in Scheme 1 and Scheme S1.† 2,6-bis(Hex-5-en-1-yloxy)benzaldehyde 1 was prepared from commercially available 2,6-dihydroxybenzaldehyde in 92% yield. Acid-catalysed condensation of 1 with dipyrromethane followed by DDQ oxidation provided 5,15-bis(2,6-bis(hex-5-en-1-yloxy)phenyl)porphyrin 2. Bromination of 2 with NBS followed by ZnII-ion insertion gave porphyrin 4, which was subjected to ring-closing metathesis (RCM) using the second-generation Grubbs catalyst, affording the key intermediate doubly strapped porphyrin 5. Then, it was successively reacted with the ethynyl derivatives of phenothiazine, and the acid ester of the acceptor through Sonogashira reactions to introduce the donor and the acid ester of the acceptor, respectively, in the meso-positions of the strapped porphyrin framework. Finally, the target dyes were obtained through hydrolysis of the corresponding esters. All the compounds were systematically characterized by 1H NMR, 13C NMR, and mass spectrometry (HRMS; ESI†). In addition, the key intermediate 5 was further characterized with single crystal X-ray diffraction analyses (Fig. S4†).Spectral properties
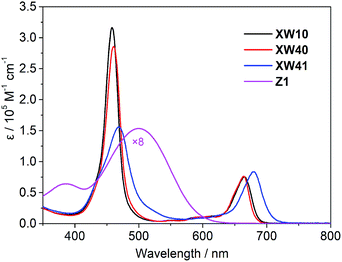
The electronic absorption spectra of the dyes in THF are shown in Fig. 2, and the corresponding absorption together with emission data are listed in Table 1.As expected, all the porphyrin dyes exhibit a typical intense Soret band around 460 nm and less intense Q bands in the wavelength range of 600–750 nm. Compared with XW10, doubly strapped porphyrin dye XW40 exhibits slightly red-shifted Soret band, and its Q bands are almost identical to those of XW10. As expected, the introduction of an auxiliary benzothiadiazole (BTD) group in XW41 dramatically improves the light-harvesting ability up to 730 nm.
The absorption spectra of XW40 and XW41 on 3 μm thick TiO2 films are shown in Fig. S1.† Compared with the corresponding absorption spectra in THF, all the spectra acquired on the films show broadened absorption, beneficial for enhancing the light-harvesting ability, and the Q peaks are hypsochromically shifted, which can be ascribed to the deprotonation of the carboxylic acid moiety.30–32 Notably, the weak blue shoulder of the Soret band observed for XW10 was not discernible in XW40, indicating that the H-type aggregation of porphyrins on TiO2 might be insignificant. However, the re-appearance of the obvious blue-shoulder of the Soret band in the XW41/TiO2 spectrum indicates severe aggregation due to the introduction of the BTD group,16,30 which is detrimental to DSSC photovoltaic performance.
Electrochemical studies
Cyclic voltammetry (Fig. S3†) was carried out to investigate the thermodynamic driving force of electron injection from the photoexcited dyes to the conduction band of TiO2, and dye regeneration by the redox electrolyte. The obtained HOMO levels corresponding to the ground state oxidation potentials of the two dyes are 0.71 and 0.72 V (vs. NHE), respectively (Table 2). Their HOMO levels are well below the redox potential of the iodide/triiodide couple (∼0.4 V vs. NHE), indicating thermodynamically feasible regeneration processes for the sensitizers, and the respective LUMO levels of −1.15 and −1.08 V for XW40 and XW41 lie well above the conduction band edge of TiO2 (−0.5 V vs. NHE), indicating sufficient driving forces for the electron injection processes (Fig. 3).| Dyes | HOMOa/V (NHE) | E 0–0 /V | LUMOc/V (NHE) |
|---|---|---|---|
| a HOMO levels were obtained from the first oxidation potential measured in acetonitrile using 0.1 M tetrabutylammonium hexafluorophosphate (TBAPF6) as the electrolyte (working electrode: FTO/TiO2/dye; reference electrode: SCE; calibrated with ferrocene/ferrocenium (Fc/Fc+) as an external reference. Counter electrode: Pt). b Estimated from the wavelength at the intersection (λinter) of normalized and emission spectra with the equation E0–0 = 1240/λinter. c The LUMO was calculated from the equation LUMO = HOMO − E0–0. | |||
| XW40 | 0.71 | 1.86 | −1.15 |
| XW41 | 0.72 | 1.80 | −1.08 |
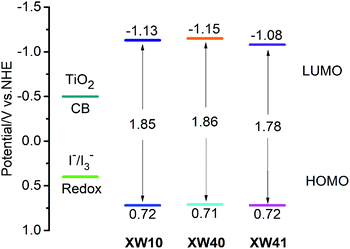 | ||
| Fig. 3 Schematic energy-level diagram of XW10,33XW40 and XW41. | ||
Theoretical calculations
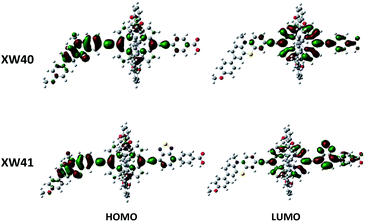
To gain insight into the electron distribution of distinct porphyrin dyes, density functional theory (DFT) calculations were performed using the Gaussian 09 program package. As presented in Fig. 4, the HOMO orbitals of porphyrin dyes XW40 and XW41 are distributed over the phenothiazine donors and the porphyrin frameworks. The LUMO orbitals of XW40 are predominantly delocalized over the acceptor and porphyrin moiety, while the LUMO orbitals of XW41 are mainly delocalized over the porphyrin framework, the BTD unit and the carboxyphenyl acceptor. The obvious overlap between the HOMO and LUMO orbitals facilitates the efficient intramolecular charge-transfer from the donor to the acceptor and subsequent electron-injection from the excited dyes to the TiO2 film.34–36Photovoltaic performance
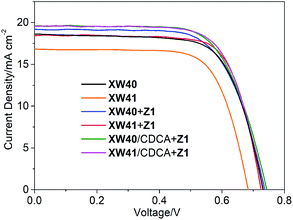
The porphyrin dyes were evaluated as the sensitizers for fabricating DSSCs based on the traditional I−/I3− electrolyte. Fig. 5 shows the photocurrent–voltage (J–V) curves of the DSSCs, and the photovoltaic parameters are listed in Tables 3 and S1.†| Dye | V oc/mV | J sc/mA cm−2 | FF | PCE (%) | Dye loading amount (× 10−7 mol cm−2) | |
|---|---|---|---|---|---|---|
| Porphyrin | Z1 | |||||
| a The data were reported in our previous work.33 b The measurement procedure was adapted from the reported work.37 c The cosensitization was performed through an optimized stepwise approach: the TiO2 electrode was first dipped in 0.2 mM porphyrin dye in chloroform/ethanol (v/v, 3/2) for 12 h, rinsed with ethanol, and then immersed in a 0.3 mM solution of Z1 in chloroform/ethanol (v/v, 1/1) for 1.5 h (XW40 + Z1) or 0.75 h (XW41 + Z1). d The approach is similar to the simple cosensitization approach, except that the TiO2 electrode was first dipped in a cocktail solution of porphyrin (0.2 mM) and CDCA (1.0 mM), then in Z1 (0.3 mM) for 1.5 h for the optimized conditions. | ||||||
| XW10 | 711 ± 5 | 17.90 ± 0.04 | 0.684 ± 0.005 | 8.60 ± 0.10 | 1.62 | \ |
| XW40 | 730 ± 3 | 18.67 ± 0.75 | 0.683 ± 0.021 | 9.31 ± 0.12 | 1.99 | \ |
| XW41 | 695 ± 2 | 16.77 ± 0.31 | 0.701 ± 0.006 | 8.16 ± 0.10 | 2.78 | \ |
| Z1 | 788 ± 3 | 13.30 ± 0.15 | 0.781 ± 0.004 | 8.23 ± 0.03 | \ | 6.47 |
| XW40 + Z1c | 742 ± 1 | 19.36 ± 0.49 | 0.694 ± 0.013 | 9.97 ± 0.06 | 1.62 | 3.67 |
| XW41 + Z1c | 728 ± 3 | 18.32 ± 0.13 | 0.728 ± 0.003 | 9.71 ± 0.01 | 2.65 | 1.96 |
| XW40/CDCA + Z1d | 748 ± 2 | 19.59 ± 0.21 | 0.719 ± 0.004 | 10.55 ± 0.11 | 1.64 | 2.73 |
| XW41/CDCA + Z1d | 726 ± 2 | 19.63 ± 0.31 | 0.715 ± 0.003 | 10.19 ± 0.21 | 2.39 | 3.06 |
The DSSCs based on XW40 exhibit a PCE of 9.3%, higher than the 8.6% obtained for XW10, with Voc and Jsc improved to 730 mV and 18.90 mA cm−2, respectively, compared to the respective values of 711 mV and 17.90 mA cm−2 obtained for XW10.33 These observations indicate that the porphyrin core strapping is favorable for suppressing the aggregation effect and reducing the charge recombination, and thus the Voc of XW40 was significantly enhanced by 19 mV, compared to that of XW10. On the other hand, the Jsc of XW40 was also improved despite the fact that XW40 and XW10 possess identical π-conjugation backbones and very similar absorption spectra. To further understand the enhancement of Jsc, we measured the adsorption amounts of the dyes (Table 3). The loading amount of XW40 was found to be 1.99 × 10−7 mol cm−2, 23% higher than the 1.62 × 10−7 mol cm−2 measured for XW10. Hence, the higher Jsc obtained for XW40 can be rationalized by its larger dye adsorption amount, which may be ascribed to the fact that the doubly strapped porphyrin molecule may occupy a smaller area, because the doubly strapped structure is more compact than the quadruply wrapped structure. Compared to XW40, although XW41 absorbs light over a broader range due to the presence of an extra BTD unit, it exhibits a lower PCE of 8.2%, which may be ascribed to the lower Voc of 695 mV associated with the aggravated dye aggregation effect induced by the extended conjugation framework. However, the efficiency of XW41 is still higher than the 7.8% obtained for XW11,33 in agreement with the superior anti-aggregation abilities of the doubly strapped porphyrins relative to the quadruply wrapped porphyrins.
The incident photon-to-current conversion efficiency (IPCE) spectra for the dyes were also measured to probe the efficiencies for the conversion of the absorbed sunlight to electrical current at various wavelengths. As shown in Fig. 6, the bandwidths of IPCE spectra for XW40 are almost the same as those of XW10,33 but the plateau is slightly higher than that of XW10, which is consistent with the improved Jsc. Impressively, the introduction of the auxiliary BTD acceptor unit in XW41 successfully expanded the light responsive wavelengths into the NIR region with a striking onset wavelength of 830 nm, but the plateau is obviously lowered, leading to a lowered Jsc of 16.77 mA cm−2.
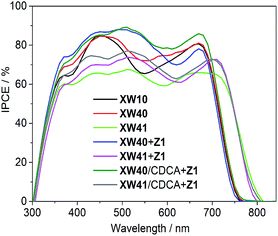 | ||
| Fig. 6 IPCE action spectra for the DSSCs based on XW10,33Z1, XW40 and XW41. | ||
It is obvious that both XW40 and XW41 exhibit obvious valleys within 500–600 nm in the IPCE spectra. To fill up the valleys, an organic dye, Z1 (Fig. 1, Table S2†), was thus synthesized and employed for co-sensitization with XW40 and XW41. Upon co-sensitization, the valleys in the IPCE spectra were filled up by the contribution from the absorption of Z1, and thus better photovoltaic performance was achieved for both XW40 and XW41, with higher efficiencies of 10.0% and 9.7%, respectively, as a result of simultaneously improved Jsc and Voc. The enhancement of Jsc can be readily rationalized by the fact that the IPCE spectra are obviously improved upon cosensitization. On the other hand, the improved Voc values achieved for the cosensitization systems lie between the corresponding values obtained for the individual porphyrin dyes and the cosensitizer. These observations agree well with previous results of cosensitization systems.33,38–40
Generally, the utilization of CDCA as a coadsorbent is effective for suppressing dye aggregation and enhancing the photovoltaic performance. Hence, a combined strategy of coadsorption and cosensitization was employed with the purpose of further enhancing the photovoltaic performance of DSSCs. Thus, the DSSCs were fabricated by immersing TiO2 films in a cocktail solution containing the individual porphyrin dye and CDCA for 12 h, followed by dipping in a solution containing Z1. Compared with XW40 + Z1 and XW41 + Z1, the Jsc values obtained for XW40/CDCA + Z1 and XW41/CDCA + Z1 were improved to 19.59 and 19.63 mAcm−2, respectively, with the efficiencies enhanced to 10.6% and 10.2%, respectively. The improved Jsc values are consistent with the improved IPCE responses (Fig. 6). As we know, the presence of CDCA can suppress dye aggregation, decrease the rate of aggregation-induced non-radiative energy transfer, enhance electron injection yield, and thus improve the IPCE response.7 For XW40/CDCA + Z1, the improved IPCE response around 500 nm was offset by the decreased loading amount of Z1 which features a broad absorption around 500 nm (Fig. S5†). Meanwhile, the loading amount of XW40 remains almost unchanged. As a result, the IPCE response was improved mainly in the Q band region. For XW41/CDCA + Z1, the loading amounts of XW41 and Z1 were decreased and increased, respectively, compared with the corresponding values for the cells based on XW41 + Z1. Consistently, the IPCE response was improved mainly within the wavelength range of 400–600 nm.
Electrochemical impedance spectroscopy analysis
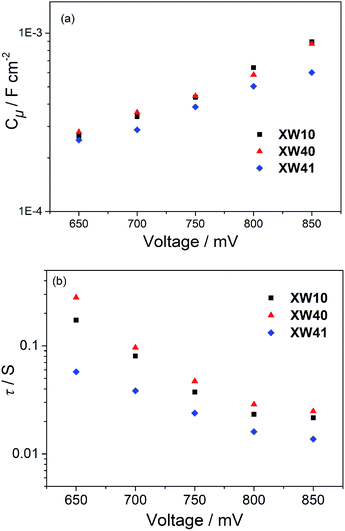
The Voc for a DSSC is dependent on the TiO2 conduction band edge (ECB) and the charge recombination process, which can be inferred from the chemical capacitance (Cμ) and electron lifetime (τ), respectively. To provide further insight into the improved photovoltaic behavior of the doubly strapped porphyrin dyes, electrochemical impedance spectroscopy (EIS) was conducted under dark conditions. Thereby, the Cμ and τ values were obtained through fitting the EIS spectra.As shown in Fig. 7a, the devices based on XW40 showed similar Cμ values with those of XW10 at a given Voc. However, the electron lifetimes of XW40 were found to be obviously longer than those of XW10 (Fig. 7b), indicating a slower interfacial charge recombination process, which is consistent with the significantly enhanced Voc for the doubly strapped porphyrin dyes. On the other hand, the Cμ values of XW41 are lower than those of XW40, which is favorable for improving the Voc. However, the electron lifetimes for XW41 are much shorter than those of XW40 which is unfavorable for the Voc. As a result of the contradictory effects, XW41 exhibits a lower Voc than XW40, indicating that the Voc values are predominantly governed by the electron lifetimes. These results are consistent with the more severe dye aggregation of XW41 associated with the extended conjugation framework due to the presence of the additional BDT unit.
Photostability
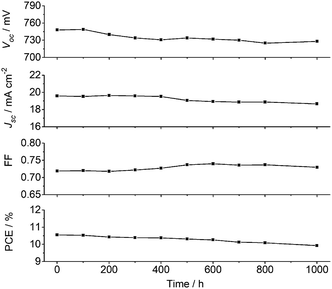
In addition to high efficiency, long-term stability is a critical requirement for practical use of DSSCs. Here, the Voc, Jsc, FF and PCE values for the XW40/CDCA + Z1 cells were recorded over a period of 1000 h under one sun soaking at 60 °C (Fig. 8). After 1000 h light soaking, more than 90% of the initial PCE value was retained, indicating that the DSSCs based on XW40/CDCA + Z1 are photostable.Conclusions
In summary, a new class of doubly strapped porphyrin sensitizers has been designed and successfully synthesized as DSSC sensitizers. Compared to the dyes with the porphyrin core wrapped with four long alkoxyl chains, the circle chain strapping can further suppress the dye aggregation and charge recombination. Thus, the Voc was greatly improved from 711 mV (XW10) to 730 mV (XW40). Moreover, the much more compact structures of the strapped porphyrins are favorable for improving the dye loading amount, leading to the improvement of Jsc. With the simultaneously improved Voc and Jsc, the efficiency of 9.3% achieved for the strapped porphyrin was found to be significantly higher than the 8.6% obtained for the wrapped dye XW10. The auxiliary electron-withdrawing unit BTD in XW41 successfully extended the response spectral range with a striking onset wavelength of 830 nm in the IPCE spectra, but the extended π-conjugation framework resulted in a lowered efficiency of 8.2% because of the aggravated dye aggregation. After applying a combined approach of coadsorption with CDCA and cosensitization with Z1, high efficiencies of 10.6% and 10.2% were achieved for XW40 and XW41, respectively. These results provide a novel strategy for developing efficient DSSCs by employing strapped porphyrin dyes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Shanghai Municipal Science and Technology Major Project (Grant No. 2018SHZDZX03) and the international cooperation program of the Shanghai Science and Technology Committee (17520750100), NSFC (21472047, 21772041, 201702062, 21811530005, 21878078), Program for Professor of Special Appointment (Eastern Scholar, GZ2016006) at Shanghai Institutions of Higher Learning, Shanghai Pujiang Program (17PJ1401700), Fundamental Research Funds for the Central Universities (WK1616004, 222201717003), and Program of Introducing Talents of Discipline to Universities (B16017). The authors thank the Research Center of Analysis and Test of the East China University of Science and Technology for the help with the characterization. W. T. is grateful to the China Scholarship Council and British Council for the visiting fellowship.Notes and references
- C. C. Chou, F. C. Hu, H. H. Yeh, H. P. Wu, Y. Chi, J. N. Clifford, E. Palomares, S. H. Liu, P. T. Chou and G. H. Lee, Angew. Chem., Int. Ed., 2014, 53, 178–183 CrossRef CAS.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- C. Li, J. Zhang, J. Song, Y. Xie and J. Jiang, Sci. China: Chem., 2018, 61, 511–514 CrossRef CAS.
- A. S. Hart, B. K. Chandra, H. B. Gobeze, L. R. Sequeira and F. D'Souza, ACS Appl. Mater. Interfaces, 2013, 5, 5314–5323 CrossRef CAS.
- T. Higashino, Y. Kurumisawa, N. Cai, Y. Fujimori, Y. Tsuji, S. Nimura, D. M. Packwood, J. Park and H. Imahori, ChemSusChem, 2017, 10, 3347–3351 CrossRef CAS.
- T. Higashino and H. Imahori, Dalton Trans., 2015, 44, 448–463 RSC.
- L. L. Li and E. W. Diau, Chem. Soc. Rev., 2013, 42, 291–304 RSC.
- J. Luo, M. Xu, R. Li, K. W. Huang, C. Jiang, Q. Qi, W. Zeng, J. Zhang, C. Chi, P. Wang and J. Wu, J. Am. Chem. Soc., 2014, 136, 265–272 CrossRef CAS.
- C. L. Wang, M. Zhang, Y. H. Hsiao, C. K. Tseng, C. L. Liu, M. Xu, P. Wang and C. Y. Lin, Energy Environ. Sci., 2016, 9, 200–206 RSC.
- J. M. Ball, N. K. S. Davis, J. D. Wilkinson, J. Kirkpatrick, J. Teuscher, R. Gunning, H. L. Anderson and H. J. Snaith, RSC Adv., 2012, 2, 6846–6853 RSC.
- C. L. Mai, W. K. Huang, H. P. Lu, C. W. Lee, C. L. Chiu, Y. R. Liang, E. W. Diau and C. Y. Yeh, Chem. Commun., 2010, 46, 809–811 RSC.
- J. W. Shiu, Y. C. Chang, C. Y. Chan, H. P. Wu, H. Y. Hsu, C. L. Wang, C. Y. Lin and E. W. G. Diau, J. Mater. Chem. A, 2015, 3, 1417–1420 RSC.
- F. M. Jradi, D. O'Neil, X. Kang, J. Wong, P. Szymanski, T. C. Parker, H. L. Anderson, M. A. El-Sayed and S. R. Marder, Chem. Mater., 2015, 27, 6305–6313 CrossRef CAS.
- C. Y. Lin, Y. C. Wang, S. J. Hsu, C. F. Lo and E. W. G. Diau, J. Phys. Chem. C, 2010, 114, 687–693 CrossRef CAS.
- Enzymes in Industry: Production and Applications, ed. W. Aehle, 3rd edn, Wiley-VCH, Weinheim, 2007 Search PubMed.
- C. L. Wang, C. M. Lan, S. H. Hong, Y. F. Wang, T. Y. Pan, C. W. Chang, H. H. Kuo, M. Y. Kuo, E. W. G. Diau and C. Y. Lin, Energy Environ. Sci., 2012, 5, 6933–6940 RSC.
- Q. Chai, W. Li, Y. Wu, K. Pei, J. Liu, Z. Geng, H. Tian and W. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 14621–14630 CrossRef CAS.
- A. Yella, C. L. Mai, S. M. Zakeeruddin, S. N. Chang, C. H. Hsieh, C. Y. Yeh and M. Gratzel, Angew. Chem., Int. Ed., 2014, 53, 2973–2977 CrossRef CAS.
- Y. K. Eom, S. H. Kang, I. T. Choi, Y. Yoo, J. Kim and H. K. Kim, J. Mater. Chem. A, 2017, 5, 2297–2308 RSC.
- H. Song, X. Li, H. Ågren and Y. Xie, Dyes Pigm., 2017, 137, 421–429 CrossRef CAS.
- P. Brogdon, H. Cheema and J. H. Delcamp, ChemSusChem, 2018, 11, 86–103 CrossRef CAS PubMed.
- H. Li, M. Fang, R. Tang, Y. Hou, Q. Liao, A. Mei, H. Han, Q. Li and Z. Li, J. Mater. Chem. A, 2016, 4, 16403–16409 RSC.
- Y. Ren, D. Sun, Y. Cao, H. N. Tsao, Y. Yuan, S. M. Zakeeruddin, P. Wang and M. Gratzel, J. Am. Chem. Soc., 2018, 140, 2405–2408 CrossRef CAS PubMed.
- Y. Liu, Y. Cao, W. Zhang, M. Stojanovic, M. I. Dar, P. Péchy, Y. Saygili, A. Hagfeldt, S. M. Zakeeruddin and M. Grätzel, Angew. Chem., Int. Ed., 2018, 57, 14125–14128 CrossRef CAS.
- J. Liu, Y. Numata, C. Qin, A. Islam, X. Yang and L. Han, Chem. Commun., 2013, 49, 7587–7589 RSC.
- J. Liu, X. Yang, A. Islam, Y. Numata, S. Zhang, N. T. Salim, H. Chen and L. Han, J. Mater. Chem. A, 2013, 1, 10889–10897 RSC.
- M. Ikai, F. Ishikawa, N. Aratani, A. Osuka, S. Kawabata, T. Kajioka, H. Takeuchi, H. Fujikawa and Y. Taga, Adv. Funct. Mater., 2006, 16, 515–519 CrossRef CAS.
- V. Rauch, J. Conradt, M. Takahashi, M. Kanesato, J. A. Wytko, Y. Kikkawa, H. Kalt and J. Weiss, J. Porphyrins Phthalocyanines, 2013, 18, 67–75 CrossRef.
- C. H. Lee and E. Galoppini, J. Org. Chem., 2010, 75, 3692–3704 CrossRef CAS.
- S. Fan, K. Lv, H. Sun, G. Zhou and Z. S. Wang, J. Power Sources, 2015, 279, 36–47 CrossRef CAS.
- M. W. Lee, J. Y. Kim, H. J. Son, J. Y. Kim, B. Kim, H. Kim, D. K. Lee, K. Kim, D. H. Lee and M. J. Ko, Sci. Rep., 2015, 5, 7711 CrossRef PubMed.
- Y. Gao, X. Li, Y. Hu, Y. Fan, J. Yuan, N. Robertson, J. Hua and S. R. Marder, J. Mater. Chem. A, 2016, 4, 12865–12877 RSC.
- Y. Xie, Y. Tang, W. Wu, Y. Wang, J. Liu, X. Li, H. Tian and W. H. Zhu, J. Am. Chem. Soc., 2015, 137, 14055–14058 CrossRef CAS PubMed.
- Q. Xu, G. Yang, Y. Ren, F. Lu, N. Zhang, M. Qamar, M. Yang, B. Zhang and Y. Feng, Phys. Chem. Chem. Phys., 2017, 19, 28867–28875 RSC.
- X. Li, X. Zhang, J. Hua and H. Tian, Mol. Syst. Des. Eng., 2017, 2, 98–122 RSC.
- J. Luo, X. Wang, L. Fan, G. Li, Q. Qi, K. W. Huang, T. L. Dexter Tam, J. Zhang, Q. Wang and J. Wu, J. Mater. Chem. C, 2016, 4, 3709–3714 RSC.
- Y. Cui, Y. Wu, X. Lu, X. Zhang, G. Zhou, F. B. Miapeh, W. Zhu and Z. S. Wang, Chem. Mater., 2011, 23, 4394–4401 CrossRef CAS.
- Y. Tang, Y. Wang, X. Li, H. Ågren, W. H. Zhu and Y. Xie, ACS Appl. Mater. Interfaces, 2015, 7, 27976–27985 CrossRef CAS.
- J. Liu, B. Liu, Y. Tang, W. Zhang, W. Wu, Y. Xie and W. H. Zhu, J. Mater. Chem. C, 2015, 3, 11144–11150 RSC.
- Y. Wang, B. Chen, W. Wu, X. Li, W. Zhu, H. Tian and Y. Xie, Angew. Chem., Int. Ed., 2014, 53, 10779–10783 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8sc04969f |
| This journal is © The Royal Society of Chemistry 2019 |