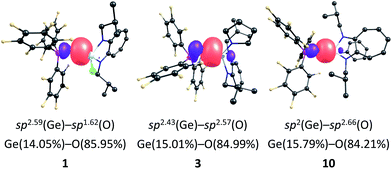Open Access Article
Open Access ArticleDonor–acceptor-stabilised germanium analogues of acid chloride, ester, and acyl pyrrole compounds: synthesis and reactivity†‡
Mahendra Kumar
Sharma
 a,
Soumen
Sinhababu
a,
Soumen
Sinhababu
 a,
Pritam
Mahawar
a,
Pritam
Mahawar
 a,
Goutam
Mukherjee
a,
Goutam
Mukherjee
 a,
Bhawana
Pandey
a,
Bhawana
Pandey
 b,
Gopalan
Rajaraman
b,
Gopalan
Rajaraman
 b and
Selvarajan
Nagendran
b and
Selvarajan
Nagendran
 *a
*a
aDepartment of Chemistry, Indian Institute of Technology Delhi, Hauz Khas, New Delhi 110 016, India. E-mail: sisn@chemistry.iitd.ac.in
bDepartment of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai 400076, India
First published on 18th February 2019
Abstract
Germaacid chloride, germaester, and N-germaacyl pyrrole compounds were not known previously. Therefore, donor–acceptor-stabilised germaacid chloride (i-Bu)2ATIGe(O)(Cl) → B(C6F5)3 (1), germaester (i-Bu)2ATIGe(O)(OSiPh3) → B(C6F5)3 (2), and N-germaacyl pyrrole (i-Bu)2ATIGe(O)(NC4H4) → B(C6F5)3 (3) compounds, with Cl–Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, Ph3SiO–Ge
O, Ph3SiO–Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C4H4N–Ge
O, and C4H4N–Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moieties, respectively, are reported here. Germaacid chloride 1 reacts with PhCCLi, KOt-Bu, and RLi (R = Ph, Me) to afford donor–acceptor-stabilised germaynone (i-Bu)2ATIGe(O)(CCPh) → B(C6F5)3 (4), germaester (i-Bu)2ATIGe(O)(Ot-Bu) → B(C6F5)3 (5), and germanone (i-Bu)2ATIGe(O)(R) → B(C6F5)3 (R = Ph 6, Me 7) compounds, respectively. Interconversion between a germaester and a germaacid chloride is achieved; reaction of germaesters 2 and 5 with TMSCl gave germaacid chloride 1, and 1 reacted with Ph3SiOLi and KOt-Bu to produce germaesters 2 and 5. Reaction of N-germaacyl pyrrole 3 with thiophenol produced a donor–acceptor-stabilised germaacyl thioester (i-Bu)2ATIGe(O)(SPh) → B(C6F5)3 (10). Furthermore, the attempted syntheses of germaamides and germacarboxylic acids are also discussed.
O moieties, respectively, are reported here. Germaacid chloride 1 reacts with PhCCLi, KOt-Bu, and RLi (R = Ph, Me) to afford donor–acceptor-stabilised germaynone (i-Bu)2ATIGe(O)(CCPh) → B(C6F5)3 (4), germaester (i-Bu)2ATIGe(O)(Ot-Bu) → B(C6F5)3 (5), and germanone (i-Bu)2ATIGe(O)(R) → B(C6F5)3 (R = Ph 6, Me 7) compounds, respectively. Interconversion between a germaester and a germaacid chloride is achieved; reaction of germaesters 2 and 5 with TMSCl gave germaacid chloride 1, and 1 reacted with Ph3SiOLi and KOt-Bu to produce germaesters 2 and 5. Reaction of N-germaacyl pyrrole 3 with thiophenol produced a donor–acceptor-stabilised germaacyl thioester (i-Bu)2ATIGe(O)(SPh) → B(C6F5)3 (10). Furthermore, the attempted syntheses of germaamides and germacarboxylic acids are also discussed.
Introduction
The carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in organic compounds such as ketones [RC(O)R], aldehydes [RC(O)H], acid halides [RC(O)X], esters [RC(O)OR], amides [RC(O)NR2], carboxylic acids [RC(O)OH], and acid anhydrides [RC(O)OC(O)R] is of great importance in organic chemistry (R = alkyl/aryl group; X = halogen). The significance of these carbon compounds provides inspiration for the synthesis of their heavier analogues,1–3 but the synthetic efforts are typically hampered by the lability of the M
O) in organic compounds such as ketones [RC(O)R], aldehydes [RC(O)H], acid halides [RC(O)X], esters [RC(O)OR], amides [RC(O)NR2], carboxylic acids [RC(O)OH], and acid anhydrides [RC(O)OC(O)R] is of great importance in organic chemistry (R = alkyl/aryl group; X = halogen). The significance of these carbon compounds provides inspiration for the synthesis of their heavier analogues,1–3 but the synthetic efforts are typically hampered by the lability of the M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (M = Si, Ge, Sn, Pb). The instability of this bond stems from the σ-bond polarisation and poor π-type overlap between M and O atoms, which usually leads to oligomerisation/polymerisation of compounds containing such M
O bond (M = Si, Ge, Sn, Pb). The instability of this bond stems from the σ-bond polarisation and poor π-type overlap between M and O atoms, which usually leads to oligomerisation/polymerisation of compounds containing such M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds.4–6 Strategies that utilise tailor-made ligands and/or provide donor–acceptor stabilisation to M/O atoms have been applied to address the aforementioned problems and have yielded various stable compounds containing M
O bonds.4–6 Strategies that utilise tailor-made ligands and/or provide donor–acceptor stabilisation to M/O atoms have been applied to address the aforementioned problems and have yielded various stable compounds containing M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds.7–11 Thus, silanones (silaketones) and germanones (germaketones) with formal Si
O bonds.7–11 Thus, silanones (silaketones) and germanones (germaketones) with formal Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and Ge
O and Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively, were successfully isolated, and the variety of silanones exceeds that of the germanones.7–10 In addition to silanones, silicon analogues of aldehyde, ester, amide, formyl chloride, carboxylic acid, and acid anhydride compounds were also synthesised via various methods mainly by the groups of Driess, Roesky, Baceiredo, and Kato.12 Very recently, Aldridge and co-workers reported the generation of a silicon analogue of an acid chloride [(N-nacnac)ArSi(Cl)
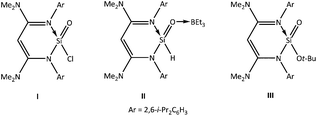
O bonds, respectively, were successfully isolated, and the variety of silanones exceeds that of the germanones.7–10 In addition to silanones, silicon analogues of aldehyde, ester, amide, formyl chloride, carboxylic acid, and acid anhydride compounds were also synthesised via various methods mainly by the groups of Driess, Roesky, Baceiredo, and Kato.12 Very recently, Aldridge and co-workers reported the generation of a silicon analogue of an acid chloride [(N-nacnac)ArSi(Cl)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (I)] through the reaction of the silylene (N-nacnac)ArSiCl with N2O (Chart 1) [(N-nacnac)Ar = HC{(Me2N)C(Ar)N}2; Ar = 2,6-i-Pr2C6H3]. The metathesis reactions of I with K[Et3BH] and KOt-Bu afforded a silaaldehyde [(N-nacnac)ArSi(H)
O (I)] through the reaction of the silylene (N-nacnac)ArSiCl with N2O (Chart 1) [(N-nacnac)Ar = HC{(Me2N)C(Ar)N}2; Ar = 2,6-i-Pr2C6H3]. The metathesis reactions of I with K[Et3BH] and KOt-Bu afforded a silaaldehyde [(N-nacnac)ArSi(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → BEt3 (II)] and a silaester [(N-nacnac)ArSi(Ot-Bu)
O → BEt3 (II)] and a silaester [(N-nacnac)ArSi(Ot-Bu)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (III)], respectively (Chart 1).12a Surprisingly, such analogues of germanium [LGe(O)Y] [L = a monoanionic ligand; Y = H (germaaldehyde), Cl (germaacid chloride), OR (germaester), NR2 (germaamide), OH (germacarboxylic acid), and (OGe(O)L) germaacid anhydride] are not yet known, perhaps due to the difficulty in adding an electron-withdrawing Y atom/group to the germanium atom in light of the already heavily polarised Ge
O (III)], respectively (Chart 1).12a Surprisingly, such analogues of germanium [LGe(O)Y] [L = a monoanionic ligand; Y = H (germaaldehyde), Cl (germaacid chloride), OR (germaester), NR2 (germaamide), OH (germacarboxylic acid), and (OGe(O)L) germaacid anhydride] are not yet known, perhaps due to the difficulty in adding an electron-withdrawing Y atom/group to the germanium atom in light of the already heavily polarised Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. Owing to our continued interest in the chemistry of germanium, we were able to isolate the Lewis acid (LA) complexes (i-Bu)2ATIGe(i-Pr)
O bond. Owing to our continued interest in the chemistry of germanium, we were able to isolate the Lewis acid (LA) complexes (i-Bu)2ATIGe(i-Pr)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → LA (LA = B(C6F5)3 (IV), ZnCl2 (V), SnCl2 (VI), and GeCl2 (VII)) of a germanone10 starting from a germanium-μ-oxo dimer [ATI = aminotroponiminate, a monoanionic bidentate ligand]. We now understand that this synthetic protocol is exploitable for the synthesis of hitherto unknown germaacid chlorides and germaesters. Consequently, we report in this article the isolation and reactivity of the first examples of a donor–acceptor-stabilised germaacid chloride (i-Bu)2ATIGe(O)(Cl) → B(C6F5)3 (1), germaester (i-Bu)2ATIGe(O)(OSiPh3) → B(C6F5)3 (2), and N-germaacyl pyrrole (i-Bu)2ATIGe(O)(NC4H4) → B(C6F5)3 (3). Compound 3 was obtained during our search for stable germaamides.
O → LA (LA = B(C6F5)3 (IV), ZnCl2 (V), SnCl2 (VI), and GeCl2 (VII)) of a germanone10 starting from a germanium-μ-oxo dimer [ATI = aminotroponiminate, a monoanionic bidentate ligand]. We now understand that this synthetic protocol is exploitable for the synthesis of hitherto unknown germaacid chlorides and germaesters. Consequently, we report in this article the isolation and reactivity of the first examples of a donor–acceptor-stabilised germaacid chloride (i-Bu)2ATIGe(O)(Cl) → B(C6F5)3 (1), germaester (i-Bu)2ATIGe(O)(OSiPh3) → B(C6F5)3 (2), and N-germaacyl pyrrole (i-Bu)2ATIGe(O)(NC4H4) → B(C6F5)3 (3). Compound 3 was obtained during our search for stable germaamides.
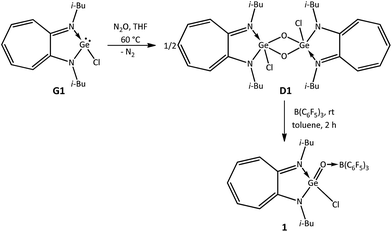
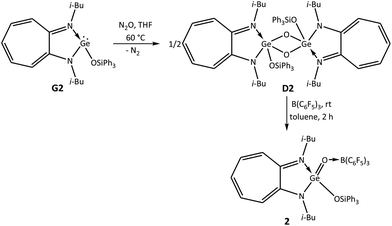
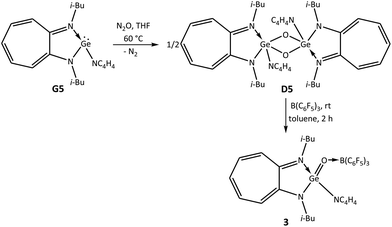
To synthesise a germaacid chloride, oxidation of the germylene monochloride13 (i-Bu)2ATIGeCl (G1) with N2O was carried out in tetrahydrofuran at room temperature. However, germylene G1 did not react with N2O at room temperature, and therefore, this reaction was performed at higher temperatures. Germylene G1 reacted with N2O at 60 °C in tetrahydrofuran and afforded the germanium μ-oxo dimer {(i-Bu)2ATIGe(Cl)(μ-O)}2 (D1) after 2 h as a yellow solid in 60% yield (Scheme 1).5,14 It appears that 60 °C is the optimum temperature for this reaction; higher temperatures afforded the ATI ligand salt [ATIH]+(Cl)−, and lower temperatures resulted in lower yields of μ-oxo dimer D1. Based on the successful conversion of a germanium μ-oxo dimer {(i-Bu)2ATIGe(i-Pr)(μ-O)}2 (D) containing Ge–C bonds into donor–acceptor-stabilised germanones IV–VII through the reaction of D with Lewis acids, we planned to react germanium μ-oxo dimer D1 containing Ge–Cl bonds with B(C6F5)3. To our surprise, treatment of μ-oxo dimer D1 with two equivalents of B(C6F5)3 in toluene for 2 h at room temperature yielded the first example of a donor–acceptor-stabilised germaacid chloride (i-Bu)2ATIGe(O)(Cl) → B(C6F5)3 (1) in quantitative yield (Scheme 1). This accomplishment inspired us to determine whether hitherto unknown germaesters and germaamides could also be isolated using this synthetic strategy of reacting suitable germanium μ-oxo dimers with Lewis acids. Thus, to synthesise a germaester, a germylene siloxide15 (i-Bu)2ATIGeOSiPh3 (G2) was reacted with N2O in tetrahydrofuran at 60 °C for 2 h to obtain the germanium μ-oxo dimer {(i-Bu)2ATIGe(OSiPh3)(μ-O)}2 (D2). The reaction of μ-oxo dimer D2 containing Ge–OSiPh3 bonds with two equivalents of B(C6F5)3 in toluene at room temperature afforded the first example of a donor–acceptor-stabilised germaester, namely, (i-Bu)2ATIGe(O)(OSiPh3) → B(C6F5)3 (2) (Scheme 2), and demonstrated the suitability of the germanium μ-oxo dimer route for the preparation of germaesters. To extend this route for the synthesis of germaamides, a germanium μ-oxo dimer with Ge–NR2 moieties is required. Two such germanium μ-oxo dimers, {(i-Bu)2ATIGeN(H)Ph(μ-O)}2 (D3) and {(i-Bu)2ATIGeN(Me)Ph(μ-O)}2 (D4), were obtained through the reaction of the amidogermylenes (i-Bu)2ATIGeN(H)Ph (G3) and (i-Bu)2ATIGeN(Me)Ph (G4) with N2O at 60 °C for 2 h in tetrahydrofuran (Scheme 3). However, the reaction of μ-oxo dimers D3 and D4 with two equivalents of B(C6F5)3 resulted in the amine → borane adducts PhNH2 → B(C6F5)3 and Ph(Me)NH → B(C6F5)3, respectively, along with an unidentified oily material instead of the expected germaamides (Scheme 3). These reactions suggest that the synthetic route discussed above is not suitable for the isolation of donor–acceptor-stabilised germaamides. On the basis of the products obtained, it was thought that the lone pairs of electrons on the nitrogen atoms of the NR2 moieties in D3 and D4 interfered with the expected reaction of these compounds (D3 and D4) with B(C6F5)3. To confirm this hypothesis, a germanium μ-oxo dimer containing amino functional groups with nitrogen atoms that cannot donate lone pairs of electrons to Lewis acids was synthesised and used. As a pyrrole substituent (Py; NC4H4) can satisfy the required criterion, the germanium μ-oxo dimer {(i-Bu)2ATIGe(NC4H4)(μ-O)}2 (D5) with two Ge–NC4H4 moieties was synthesised in quantitative yield by the reaction of the N-germylene pyrrole (i-Bu)2ATIGe(NC4H4) (G5) with N2O in tetrahydrofuran at 60 °C for 2 h (Scheme 4).16 Treatment of μ-oxo dimer D5 with two equivalents of B(C6F5)3 in toluene at room temperature resulted in the first donor–acceptor-stabilised N-germaacyl pyrrole, (i-Bu)2ATIGe(O)(NC4H4) → B(C6F5)3 (3) in quantitative yield (Scheme 4). The feasibility of isolating N-germaacyl pyrrole 3 as a stable species proves that the aforementioned hypothesis of the interference of lone pairs of electrons on the nitrogen atoms of the NR2 moieties in μ-oxo dimers D3 and D4 is factually valid.
 | ||
| Scheme 3 Attempted synthesis of donor–acceptor-stabilised germaamides that resulted in amine → borane adducts. | ||
In all the reactions, germanium μ-oxo dimers D1–D5 were reacted with the Lewis acid B(C6F5)3.17 To understand the utility of other Lewis acids for the successful conversion of germanium μ-oxo dimers D1, D2, and D5 to the corresponding donor–acceptor-stabilised germaacid chloride, germaester, and N-germaacyl pyrrole, a range of Lewis acids (such as BF3, GeCl2, and SnCl2) were screened. However, all of these reactions were typically unsuccessful until now (see the ESI‡ for details). Surprisingly, the germanium-μ-oxo dimer {(i-Bu)2ATIGe(i-Pr)(μ-O)}2 (D) with Ge–i-Pr bonds was insensitive to the nature of the Lewis acid used.10 Thus, it reacted smoothly with B(C6F5)3, ZnCl2, SnCl2, and GeCl2 to afford the donor–acceptor-stabilised germanones IV, V, VI, and VII, respectively.10
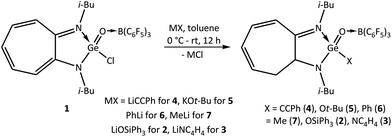
As the germanium analogues of acid halides, esters, and amides were previously unknown, there has been no reactivity study on them. Therefore, the reactivity of the donor–acceptor-stabilised germaacid chloride 1, germaester 2, and N-germaacyl pyrrole 3 was studied with great interest to understand how these compounds behave chemically. It was found that germaacid chloride 1 can react with various lithium salts and afford clean products. Thus, through reaction of 1 with lithium phenylacetylide in toluene for 12 h, a unique example of a germaynone (i-Bu)2ATIGe(O)(CCPh) → B(C6F5)3 (4) was obtained (Scheme 5). Notably, until now, there has been no example of a silaynone. Furthermore, this reaction reveals that the chloride attached to the germaacyl moiety can be replaced with other functional groups, a reactivity omnipresent among acid chlorides in organic chemistry. Germaacid chloride 1, a heavier analogue of acid halides, exhibits reactivity similar to that of acid halides and silaacid chloride;12a therefore, this reactivity of 1 was further exploited. The lithium and potassium salts of triphenylsilanol and t-butanol reacted with 1 to result in germaesters 2 and (i-Bu)2ATIGe(O)(Ot-Bu) → B(C6F5)3 (5), respectively (Scheme 5), which is another route for the isolation of germaesters in addition to that shown in Scheme 2.
In a similar fashion, alternate synthetic protocols can be suggested for N-germaacyl pyrrole 3 and germanones. For example, treatment of 1 with lithium pyrrol-1-ide and phenyl/methyl lithium yielded N-germaacyl pyrrole 3 and the germanones (i-Bu)2ATIGe(O)(Ph) → B(C6F5)3 (6)/(i-Bu)2ATIGe(O)(Me) → B(C6F5)3 (7) as products, respectively (Scheme 5). Thus, from germaacid chloride 1, germaesters, N-germaacyl pyrrole, and germanones can be derived without the need to isolate the corresponding germanium-μ-oxo dimers. This route was also attempted for the possible isolation of germaamides, and the reactions of germaacid chloride 1 with the lithium salts PhN(H)Li and PhN(Me)Li were carried out. However, these reactions faced the same fate as that of the abovementioned reactions carried out for the isolation of germaamides (shown in Scheme 3) by yielding amine → borane adducts only.
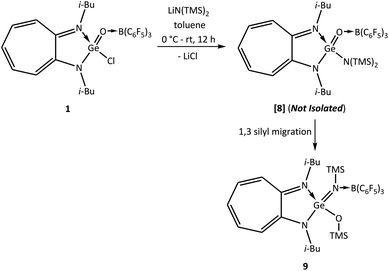
However, another reaction of germaacid chloride 1 with lithium bis(trimethylsilyl)amide, which aimed again at obtaining the elusive germaamide, occurred differently and resulted in the germaimine (i-Bu)2ATIGe(NTMS)(OTMS) → B(C6F5)3 (9) in quantitative yield (Scheme 6). This result reveals that the desired germaamide [8] was formed as an intermediate, which then underwent 1,3-silyl migration to form the stable compound 9 (Scheme 6).
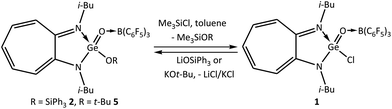
Reactivity studies with donor–acceptor-stabilised germaesters 2 and 5 demonstrated that an interconversion between these germaesters and germaacid chloride 1 is achievable. Germaesters 2 and 5 reacted with a slight excess of Me3SiCl in toluene at room temperature and offered germaacid chloride 1 (Scheme 7). As mentioned above (Scheme 5), reactions of germaacid chloride 1 with one equivalent of LiOSiPh3 and KOt-Bu in toluene at room temperature generated the germaesters 2 and 5, respectively (Scheme 7). This type of interconversion is not known among the analogous silicon compounds.
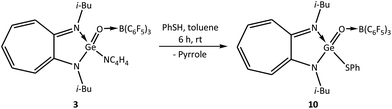
The reactivity studies on N-germaacyl pyrrole 3 demonstrated that the thiophenoxide moiety of thiophenol can substitute the pyrrolide of 3. Accordingly, the reaction of N-germaacyl pyrrole 3 with thiophenol at room temperature in toluene for 6 h resulted in the first example of a germaacyl thioester (i-Bu)2ATIGe(O)(SPh) → B(C6F5)3 (10) in quantitative yield (Scheme 8).
Considering this reaction, the feasibility of substituting the pyrrolide of 3 with hydroxide from a suitable precursor was investigated, as this might lead to the first example of a donor–acceptor-stabilised germacarboxylic acid. However, the reaction of 3 with water in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio for 2 h in toluene resulted in [ATIH]+[(OH)(B(C6F5)3)]− and not the expected germacarboxylic acid (Scheme S1; see the ESI‡). The commonality in all of the abovementioned reactions of donor–acceptor-stabilised germaacid chloride 1, germaester 2, and N-germaacyl pyrrole 3 is that these reactants undergo nucleophilic substitution in the presence of suitable substrates without any damage to the Ge
1 molar ratio for 2 h in toluene resulted in [ATIH]+[(OH)(B(C6F5)3)]− and not the expected germacarboxylic acid (Scheme S1; see the ESI‡). The commonality in all of the abovementioned reactions of donor–acceptor-stabilised germaacid chloride 1, germaester 2, and N-germaacyl pyrrole 3 is that these reactants undergo nucleophilic substitution in the presence of suitable substrates without any damage to the Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 moiety.
O → B(C6F5)3 moiety.
The germanium-μ-oxo dimers D1 and D3–D5, germaacid chloride 1, germaesters 2 and 5, N-germaacyl pyrrole 3, germaynone 4, germanones 6 and 7, and germaacyl thioester 10 are stable at room temperature in an inert atmosphere of dinitrogen. All these compounds are freely soluble in common organic solvents, such as toluene, chloroform, and dichloromethane. Though the germanium-μ-oxo dimers D1–D5 are also freely soluble in tetrahydrofuran, products 1–7 and 10, containing a Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 moiety, decompose even in tetrahydrofuran dried over a potassium mirror to afford [ATIH]+[(OH)(B(C6F5)3)]−.
O → B(C6F5)3 moiety, decompose even in tetrahydrofuran dried over a potassium mirror to afford [ATIH]+[(OH)(B(C6F5)3)]−.
Compounds D1, D3–D5, 1–7, and 10 were characterised through multinuclear NMR spectroscopic (1H, 11B, 13C, 19F, and 29Si) and single-crystal X-ray diffraction studies in the solution and solid states, respectively (see the ESI‡ for details). In the 1H NMR spectra of D1 and D5, all the resonances are shifted slightly downfield in comparison to those of the precursor molecules, germylene monochloride G1 and N-germylene pyrrole G5, respectively. This shifting is due to the attachment of germanium atoms to electronegative oxygen atoms and the concomitant increase in the formal oxidation state of germanium atoms from +2 to +4. The resonances of the seven-membered ring protons in 1–7 and 10 are shifted downfield in comparison to the corresponding protons in germanium-μ-oxo dimer D1. Owing to the increased electrophilicity of the germanium atom in the Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 moiety (of 1–7 and 10) in comparison to the germanium atoms in the Ge(μ-O)2Ge moiety of D1, these shifts are expected. In the 13C NMR spectra of D1, D3–D5, 1–7, and 10, the expected numbers of signals were observed. In the 11B NMR spectra of 1–6, and 10, singlet resonances at −2.46, −2.61, −2.72, −2.79, −2.44, −3.12, and −2.73 ppm were observed, respectively (Table 1). In comparison, B(C6F5)3 and the donor–acceptor-stabilised germanone (i-Bu)2ATIGe(O)(i-Pr) → B(C6F5)3 (IV) showed singlet resonances at −2.30 ppm18,19 and −4.52 ppm,10 respectively. These data reveal that the resonances in 1–6 and 10 are in between the resonances of B(C6F5)3 and IV. These results suggest that the electron donation by the germaacyl oxygen atom to the boron atom in 1–6, and 10 is reduced relative to that in IV due to the electron-withdrawing effect of the Cl, OSiPh3, NC4H4, CCPh, Ot-Bu, Ph, and SPh atom/group on the germanium atom, respectively (IV has an electron-donating i-Pr group on the germanium atom). The donor–acceptor-stabilised silaaldehyde L′Si(H)
O → B(C6F5)3 moiety (of 1–7 and 10) in comparison to the germanium atoms in the Ge(μ-O)2Ge moiety of D1, these shifts are expected. In the 13C NMR spectra of D1, D3–D5, 1–7, and 10, the expected numbers of signals were observed. In the 11B NMR spectra of 1–6, and 10, singlet resonances at −2.46, −2.61, −2.72, −2.79, −2.44, −3.12, and −2.73 ppm were observed, respectively (Table 1). In comparison, B(C6F5)3 and the donor–acceptor-stabilised germanone (i-Bu)2ATIGe(O)(i-Pr) → B(C6F5)3 (IV) showed singlet resonances at −2.30 ppm18,19 and −4.52 ppm,10 respectively. These data reveal that the resonances in 1–6 and 10 are in between the resonances of B(C6F5)3 and IV. These results suggest that the electron donation by the germaacyl oxygen atom to the boron atom in 1–6, and 10 is reduced relative to that in IV due to the electron-withdrawing effect of the Cl, OSiPh3, NC4H4, CCPh, Ot-Bu, Ph, and SPh atom/group on the germanium atom, respectively (IV has an electron-donating i-Pr group on the germanium atom). The donor–acceptor-stabilised silaaldehyde L′Si(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 (VIII),12g silaformyl chloride IPr·SiH(Cl)
O → B(C6F5)3 (VIII),12g silaformyl chloride IPr·SiH(Cl)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 (IX),12c silaacid anhydride [{PhC(t-BuN)2}Si{
O → B(C6F5)3 (IX),12c silaacid anhydride [{PhC(t-BuN)2}Si{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O→B(C6F5)3}O–Si(H){
O→B(C6F5)3}O–Si(H){![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O→B(C6F5)3}(Nt-Bu)(HNt-Bu)CPh] (X),12d monoalumoxane L*Al
O→B(C6F5)3}(Nt-Bu)(HNt-Bu)CPh] (X),12d monoalumoxane L*Al![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 (XI),20 and boraacid chloride IPr → B(Cl)
O → B(C6F5)3 (XI),20 and boraacid chloride IPr → B(Cl)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 (XII)21 have B(C6F5)3 as the acceptor in the M
O → B(C6F5)3 (XII)21 have B(C6F5)3 as the acceptor in the M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 moiety (M = Si VIII, IX, X; Al XI; B XII) [L′ = HC[CMeN(Ar)]2 IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene, L* = Et2NCH2CH2NC(Me)CHC(Me)NCH2CH2NEt2]. It may therefore be appropriate to compare the boron and fluorine resonances of these compounds with those of 1–6 and 10 (Table 1). These resonances in compounds VIII, IX, X, XI, and XII are shifted upfield with respect to the corresponding resonances of B(C6F5)3 (Table 1), which indicates the shielding of boron and fluorine atoms due to electron donation by oxygen atoms. This result is similar to that observed for compounds 1–6 and 10, containing a Ge
O → B(C6F5)3 moiety (M = Si VIII, IX, X; Al XI; B XII) [L′ = HC[CMeN(Ar)]2 IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene, L* = Et2NCH2CH2NC(Me)CHC(Me)NCH2CH2NEt2]. It may therefore be appropriate to compare the boron and fluorine resonances of these compounds with those of 1–6 and 10 (Table 1). These resonances in compounds VIII, IX, X, XI, and XII are shifted upfield with respect to the corresponding resonances of B(C6F5)3 (Table 1), which indicates the shielding of boron and fluorine atoms due to electron donation by oxygen atoms. This result is similar to that observed for compounds 1–6 and 10, containing a Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 moiety (Table 1), but as revealed by the 11B NMR spectral data (Table 1), the magnitude of the shielding in these compounds is lower than that in compounds VIII, IX, X, and XI. In the 29Si NMR spectra of germaester 2, a signal at −13.62 ppm for the SiPh3 group is shifted downfield in comparison to that in germylene G2 (−24.72 ppm).15
O → B(C6F5)3 moiety (Table 1), but as revealed by the 11B NMR spectral data (Table 1), the magnitude of the shielding in these compounds is lower than that in compounds VIII, IX, X, and XI. In the 29Si NMR spectra of germaester 2, a signal at −13.62 ppm for the SiPh3 group is shifted downfield in comparison to that in germylene G2 (−24.72 ppm).15
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 moiety(s) (M = Ge, Si, Al, B)
O → B(C6F5)3 moiety(s) (M = Ge, Si, Al, B)
| S. no. | Compound | 11B NMR chemical shift (ppm) | 19F NMR chemical shift (ppm) | O–B bond length (Å) | Reference |
|---|---|---|---|---|---|
| a In CDCl3. b In CD2Cl2. c In THF-d8. d In C6D6/THF-d8. e In C6D6. | |||||
| 1 | Germanone, (i-Bu)2ATIGe(i-Pr)(O) → B(C6F5)3 (IV) | −4.52a | (−134, −161, and −166)a | 1.473(4) | 10 |
| 2 | Silaaldehyde, L′Si(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 (VIII) O → B(C6F5)3 (VIII) |
−4.70b | (−132, −162, and −165)b | 1.503(3) | 12g |
| 3 | Silaformyl chloride, IPr·SiH(Cl)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 (IX) O → B(C6F5)3 (IX) |
−5.28c | (−134, −163, and −168)c | 1.492(3) | 12c |
| 4 | Silaacid anhydride, [{PhC(t-BuN)2}Si{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O→B(C6F5)3}O–Si(H) { O→B(C6F5)3}O–Si(H) {![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O→B(C6F5)3}(Nt-Bu)(HNt-Bu)CPh] (X) O→B(C6F5)3}(Nt-Bu)(HNt-Bu)CPh] (X) |
(−3.99, and −5.46)c | (−134, −135, −164, −165, −167, and −168)c | 1.493(3), and 1.488(3) | 12d |
| 5 | Monoalumoxane, L*Al![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5) (XI) O → B(C6F5) (XI) |
-4.83d | (-134, -164, and -166)d | 1.444(3) | 20 |
| 6 | Boraacid chloride, IPr → B(Cl)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5) (XII) O → B(C6F5) (XII) |
-2.7e | (-131, -160, and -165)e | 1.518(3) | 21 |
| 7 | B(C6F5)3 | −2.30a | (−127, −143, and −160)a | — | 19 |
| 8 | Germaacid chloride, (i-Bu)2ATIGe(O)(Cl) → B(C6F5)3 (1) | −2.46a | (-133, -159, and -165)a | 1.493(5) | This work |
| 9 | Germaester, (i-Bu)2ATIGe(O)(OSiPh3) → B(C6F5)3 (2) | −2.61a | (-132, -160, and -165)a | 1.497(3) | This work |
| 10 | N-Germaacyl pyrrole, (i-Bu)2ATIGe(O)(NC4H4) → B(C6F5)3 (3) | −2.72a | (−133, −159, and −165)a | 1.494(6) | This work |
| 11 | Germaynone, (i-Bu)2ATIGe(O)(CCPh) → B(C6F5)3 (4) | −2.79a | (−133, −161, and −165)a | 1.489(4) | This work |
| 12 | Germaester, (i-Bu)2ATIGe(O)(Ot-Bu) → B(C6F5)3 (5) | −2.44a | (−132, −160, and −165)a | 1.505(3) and 1.502(3) | This work |
| 13 | Germanone, (i-Bu)2ATIGe(O)(Ph) → B(C6F5)3 (6) | −3.12a | (−133, −160, and −165)a | 1.481(3) | This work |
| 14 | Germaacyl thioester, (i-Bu)2ATIGe(O)(SPh) → B(C6F5)3 (10) | −2.73a | (−133, −160, and −165)a | 1.501(5) | This work |
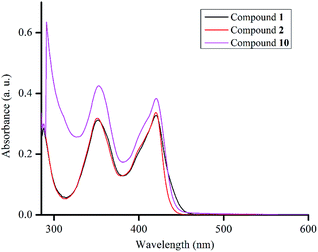
In a preliminary study of optical properties, the UV-vis spectra of compounds 1, 2, and 10 were recorded in toluene at room temperature. Compounds 1, 2, and 10 showed an absorption maximum in the visible region at approximately 420 nm (Fig. 1). Theoretical studies suggested that these absorptions in compounds 1, 2, and 10 are essentially due to 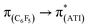 ,
, 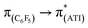 , and
, and 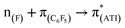 transitions, respectively (Table S1; see the ESI‡ for details). Furthermore, there are two high-energy transitions in each of these compounds with λmax values of approximately 350 and 285 nm (Fig. 1), which are due to multiple transitions (Table S1; see the ESI‡ for details). The optical properties of compounds with formal M
transitions, respectively (Table S1; see the ESI‡ for details). Furthermore, there are two high-energy transitions in each of these compounds with λmax values of approximately 350 and 285 nm (Fig. 1), which are due to multiple transitions (Table S1; see the ESI‡ for details). The optical properties of compounds with formal M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → LA moieties (M = Ge, Si) have rarely been studied. For germanone VII with a Ge
O → LA moieties (M = Ge, Si) have rarely been studied. For germanone VII with a Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → GeCl2 moiety, optical properties have been reported. In comparison to compounds 1, 2, and 10, the absorption maximum of VII in the visible region (437 nm) is slightly redshifted, and this absorption is due to a
O → GeCl2 moiety, optical properties have been reported. In comparison to compounds 1, 2, and 10, the absorption maximum of VII in the visible region (437 nm) is slightly redshifted, and this absorption is due to a 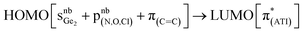 transition. Most likely, a different Lewis acid in compound VII altered the composition of the HOMO.
transition. Most likely, a different Lewis acid in compound VII altered the composition of the HOMO.
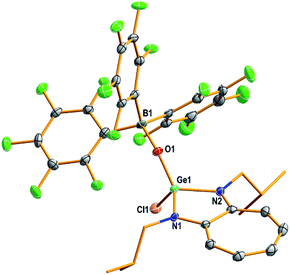
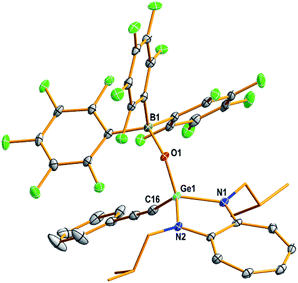
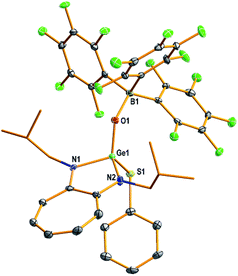
The structures of compounds D1, D3–D5, 1–7, 9, and 10 in the solid state were determined by single-crystal X-ray diffraction analysis (Fig. 2–4 and S53–S62, Tables S2–S5, and Experimental section; see the ESI‡).22 Compounds 1–4 and 6 crystallised in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Tables S3 and S4; see the ESI‡). Compounds 5, 7, and 10 crystallised in the monoclinic, orthorhombic, and monoclinic space groups P21/n, P212121, and P21/c, respectively (Table S4; see the ESI‡).
(Tables S3 and S4; see the ESI‡). Compounds 5, 7, and 10 crystallised in the monoclinic, orthorhombic, and monoclinic space groups P21/n, P212121, and P21/c, respectively (Table S4; see the ESI‡).
The molecular structures of compounds 1–7 and 10 [Fig. 2 (1), 3 (4), 4 (10), S57 (2), S58 (3), S59 (5), S60 (6), and S61 (7)‡] confirmed the presence of a (Y)Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 moiety [Y = Cl (1), OSiPh3 (2), NC4H4 (3), CCPh (4), Ot-Bu (5), Ph (6), Me (7), and SPh (10)]. In these compounds, the germanium atom has a distorted tetrahedral geometry with two ATI ligand nitrogens, one germaacyl oxygen, and one Cl (1), O (2), N (3), C (4), O (5), C (6), C (7), or S (10) atom. The average length of the Ge–Nligand bonds in compounds 1 (1.838 Å), 2 (1.848 Å), and 3 (1.843 Å) is shorter than that in their precursors D1 (1.931 Å), D2 (1.946 Å), and D5 (1.942 Å), respectively. Similarly, the Ge–Y bond in compounds 1 (2.117(1) Å; Y = Cl), 2 (1.719(2) Å; Y = OSiPh3), and 3 (1.820(4) Å; Y = NC4H4) is also shorter than that in compounds D1 (2.20(8) Å), D2 (1.767(3) Å), and D5 (1.892(3) Å), respectively. These differences are due to the electrophilicity of the oxygen atom in the Ge
O → B(C6F5)3 moiety [Y = Cl (1), OSiPh3 (2), NC4H4 (3), CCPh (4), Ot-Bu (5), Ph (6), Me (7), and SPh (10)]. In these compounds, the germanium atom has a distorted tetrahedral geometry with two ATI ligand nitrogens, one germaacyl oxygen, and one Cl (1), O (2), N (3), C (4), O (5), C (6), C (7), or S (10) atom. The average length of the Ge–Nligand bonds in compounds 1 (1.838 Å), 2 (1.848 Å), and 3 (1.843 Å) is shorter than that in their precursors D1 (1.931 Å), D2 (1.946 Å), and D5 (1.942 Å), respectively. Similarly, the Ge–Y bond in compounds 1 (2.117(1) Å; Y = Cl), 2 (1.719(2) Å; Y = OSiPh3), and 3 (1.820(4) Å; Y = NC4H4) is also shorter than that in compounds D1 (2.20(8) Å), D2 (1.767(3) Å), and D5 (1.892(3) Å), respectively. These differences are due to the electrophilicity of the oxygen atom in the Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 moiety of compounds 1, 2, and 3 being higher than that of the oxygen atoms in the Ge(μ-O)2Ge moiety of D1, D2, and D5, which makes the germanium atom in the former set of compounds more electrophilic than that in the latter set. Though these effects are observed in germanone IV, in comparison to the electron-donating i-Pr group bound to the germanium atom of germanone IV, the Cl, OSiPh3, NC4H4, CCPh, and SPh atom/group bound to the germanium atom in germaacid chloride 1, germaester 2, N-germaacyl pyrrole 3, germaynone 4, and germaacyl thioester 10, respectively, exert electron-withdrawing (+I) effects and compete for the germanium atom's electron density, thus increasing the interaction between the germanium and oxygen atoms of the Ge
O → B(C6F5)3 moiety of compounds 1, 2, and 3 being higher than that of the oxygen atoms in the Ge(μ-O)2Ge moiety of D1, D2, and D5, which makes the germanium atom in the former set of compounds more electrophilic than that in the latter set. Though these effects are observed in germanone IV, in comparison to the electron-donating i-Pr group bound to the germanium atom of germanone IV, the Cl, OSiPh3, NC4H4, CCPh, and SPh atom/group bound to the germanium atom in germaacid chloride 1, germaester 2, N-germaacyl pyrrole 3, germaynone 4, and germaacyl thioester 10, respectively, exert electron-withdrawing (+I) effects and compete for the germanium atom's electron density, thus increasing the interaction between the germanium and oxygen atoms of the Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. Therefore, the length of the formal Ge
O bond. Therefore, the length of the formal Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in compounds 1 (1.698(2) Å), 2 (1.696(2) Å), 3 (1.695(3) Å), 4 (1.708(2) Å), and 10 (1.698(3) Å) is shorter than that in germanones IV (1.718(2) Å), V (1.724(2) and 1.728(2) Å), VI (1.728(5) Å), and VII (1.718(2) Å).10 These data also reveal that relative to the polarisation of the Ge
O bond in compounds 1 (1.698(2) Å), 2 (1.696(2) Å), 3 (1.695(3) Å), 4 (1.708(2) Å), and 10 (1.698(3) Å) is shorter than that in germanones IV (1.718(2) Å), V (1.724(2) and 1.728(2) Å), VI (1.728(5) Å), and VII (1.718(2) Å).10 These data also reveal that relative to the polarisation of the Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in germanone IV,10 the same bonds in germaacid chloride 1, germaester 2, N-germaacyl pyrrole 3, germaynone 4, and germaacyl thioester 10 are less polarised due to the electron-withdrawing effect of the Cl, OSiPh3, NC4H4, CCPh, and SPh atoms/groups bound to the germanium atom, respectively. A consequence of the increased interaction between the germanium and oxygen atoms of the germaacyl bond in these compounds is the reduced Lewis basicity of the oxygen atom. This result is reflected in the interaction of this oxygen atom with the Lewis acid B(C6F5)3, where the O → B bond in compounds 1 (1.493(5) Å), 2 (1.497(3) Å), 3 (1.494(6) Å), 4 (1.489(4) Å), and 10 (1.501(5) Å) is longer than the corresponding bond in germanone IV (1.473(4) Å).10 The O → B bond lengths observed in these compounds are similar to those observed in analogous silicon derivatives (VIII 1.503(3), IX 1.492(3), and X 1.493(3) and 1.488(3); M = Si) and boraacid chloride (XII 1.518(3); M = B) with an M
O bond in germanone IV,10 the same bonds in germaacid chloride 1, germaester 2, N-germaacyl pyrrole 3, germaynone 4, and germaacyl thioester 10 are less polarised due to the electron-withdrawing effect of the Cl, OSiPh3, NC4H4, CCPh, and SPh atoms/groups bound to the germanium atom, respectively. A consequence of the increased interaction between the germanium and oxygen atoms of the germaacyl bond in these compounds is the reduced Lewis basicity of the oxygen atom. This result is reflected in the interaction of this oxygen atom with the Lewis acid B(C6F5)3, where the O → B bond in compounds 1 (1.493(5) Å), 2 (1.497(3) Å), 3 (1.494(6) Å), 4 (1.489(4) Å), and 10 (1.501(5) Å) is longer than the corresponding bond in germanone IV (1.473(4) Å).10 The O → B bond lengths observed in these compounds are similar to those observed in analogous silicon derivatives (VIII 1.503(3), IX 1.492(3), and X 1.493(3) and 1.488(3); M = Si) and boraacid chloride (XII 1.518(3); M = B) with an M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 bond (Table 1).12g,12c,12d,21 However, in the monoalumoxane20XI with an Al
O → B(C6F5)3 bond (Table 1).12g,12c,12d,21 However, in the monoalumoxane20XI with an Al![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 bond, the O → B bond is shorter (1.444(3) Å) than those in compounds 1–4, 10, VIII, IX, X, and XII. All the bonding aspects discussed here are supported by theoretical studies (vide infra). Furthermore, the Ge
O → B(C6F5)3 bond, the O → B bond is shorter (1.444(3) Å) than those in compounds 1–4, 10, VIII, IX, X, and XII. All the bonding aspects discussed here are supported by theoretical studies (vide infra). Furthermore, the Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (vide supra) in compounds 1–4 and 10 is slightly longer than the Ge
O bond (vide supra) in compounds 1–4 and 10 is slightly longer than the Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in the base-stabilised germanones[L′′LDGe
O bond in the base-stabilised germanones[L′′LDGe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] (L′′ = [CH{(C
O] (L′′ = [CH{(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(CMe)(NAr)2}]; LD = [{(Me)CN(Me)}2C] (XIII), [{(Me)CN(i-Pr)}2C] (XIV), 4-(Me2N)–C5H4N (XV)) without an acceptor at an oxygen atom (1.646(2)-1.672(3) Å)8 and shorter than the Ge–O single bonds in germanium-μ-oxo dimers D1, D2, and D5 (1.848(2)–1.787(3) Å).
CH2)(CMe)(NAr)2}]; LD = [{(Me)CN(Me)}2C] (XIII), [{(Me)CN(i-Pr)}2C] (XIV), 4-(Me2N)–C5H4N (XV)) without an acceptor at an oxygen atom (1.646(2)-1.672(3) Å)8 and shorter than the Ge–O single bonds in germanium-μ-oxo dimers D1, D2, and D5 (1.848(2)–1.787(3) Å).
The nature of the Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in compounds 1–3 and 10 was analysed through natural bond orbital (NBO)23,24 studies, and the details are provided in Table S6 (see the ESI‡). The Ge–O σ-bond in compounds 1 and 10 is formed by the overlap of the sp2.59 and sp2 hybrid orbitals of germanium with the sp1.62 and sp2.66 hybrid orbitals of oxygen, respectively (Fig. 5 and Table S6; see the ESI‡). In compounds 2 and 3, the sp2.53 and sp2.43 hybrid orbitals of germanium overlap with the sp2.89 and sp2.57 hybrid orbitals of oxygen to form the Ge–O bond, respectively (Fig. 5 and Table S6; see the ESI‡). MO calculations also reveal the presence of Ge–O bonds in compounds 1–3 and 10, and these bonds are deeply buried (Figure S63, see the ESI‡).
O bond in compounds 1–3 and 10 was analysed through natural bond orbital (NBO)23,24 studies, and the details are provided in Table S6 (see the ESI‡). The Ge–O σ-bond in compounds 1 and 10 is formed by the overlap of the sp2.59 and sp2 hybrid orbitals of germanium with the sp1.62 and sp2.66 hybrid orbitals of oxygen, respectively (Fig. 5 and Table S6; see the ESI‡). In compounds 2 and 3, the sp2.53 and sp2.43 hybrid orbitals of germanium overlap with the sp2.89 and sp2.57 hybrid orbitals of oxygen to form the Ge–O bond, respectively (Fig. 5 and Table S6; see the ESI‡). MO calculations also reveal the presence of Ge–O bonds in compounds 1–3 and 10, and these bonds are deeply buried (Figure S63, see the ESI‡).
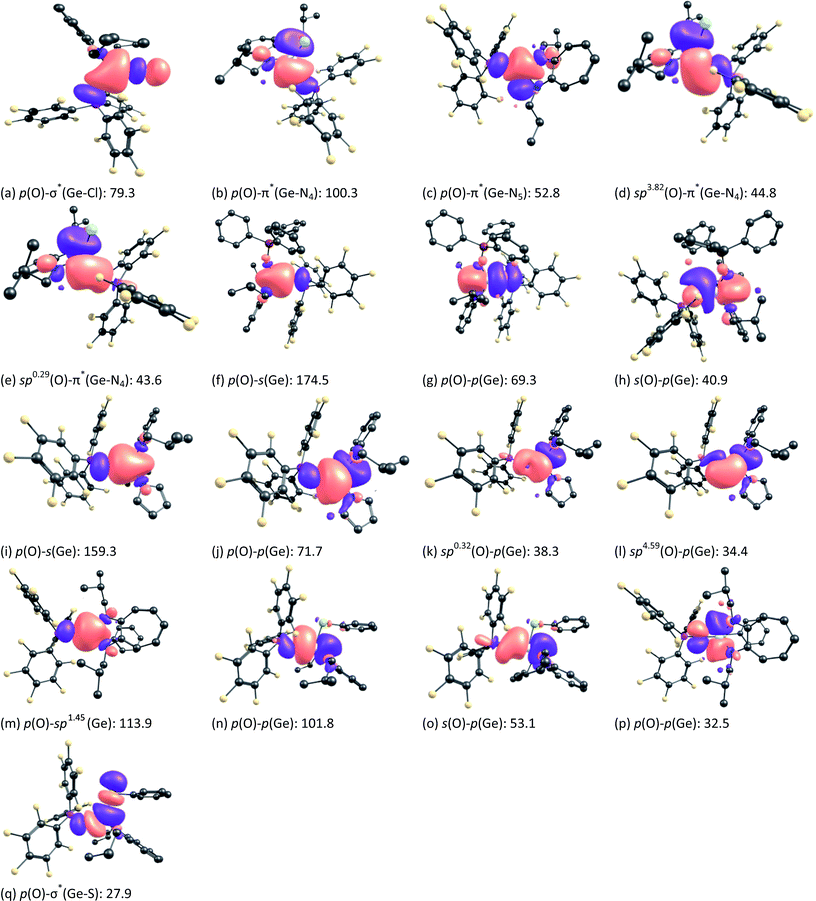
NBO second-order perturbation theory analysis reveals that in germaacid chloride 1, the sigma bond between germanium and oxygen is formed by the donation of the lone pair of electrons on the oxygen atom to the σ* orbital of the Ge–Cl bond (Fig. 6a; 79.3 kcal mol−1). The lone pair of electrons on the oxygen atom also interacts with the π* orbitals of the Ge–NATI bonds (Fig. 6b; 100.3 kcal mol−1 and Fig. 6c; 52.8 kcal mol−1). However, in addition to these interactions, there are two strong stabilising interactions between the sp3.82 (Fig. 6d; 44.8 kcal mol−1) and sp0.29 (Fig. 6e; 43.6 kcal mol−1) orbitals of oxygen and the π* orbital of the Ge–N4 bond. Compounds 2, 3, and 10, instead of showing the aforementioned n (lone pair of electrons on oxygen) to σ*/π* orbital interactions, showed strong NBO donor–acceptor interactions from the s, p or spx orbitals of oxygen atoms to vacant s, p or spx orbitals of the germanium atoms [Fig. 6f–h (2), Fig. 6i–l (3), and Fig. 6m–p (10)]. However, in compound 10, a moderately strong NBO donor–acceptor interaction was found between the p orbital of oxygen and the σ* orbital of the Ge–S bond (27.9 kcal mol−1) (Fig. 6q). In comparison, germanone IV showed three σ interactions: two O → Ge interactions and one O → σ*(Ge–Ci-Pr) interaction; these interactions result in a total stabilisation energy of 236.3 kcal mol−1.10 Thus, the total stabilisation energy due to the donor–acceptor interactions in compounds 1 (320.8 kcal mol−1), 2 (284.7 kcal mol−1), 3 (303.7 kcal mol−1), and 10 (329.2 kcal mol−1) is higher than that in germanone IV, which is due to the difference in the nature of the atoms/moieties bound to germanium atom in these compounds (–Cl, –OSiPh3, –NC4H4, and –SPh, respectively) instead of an i-Pr group. The Wiberg bond index (WBI) calculations for compounds 1, 3, and 10 also showed a slightly increased bond order for the Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (0.74–0.76) relative to that in germanone IV (0.70)10 (Table S6; see the ESI‡). A similar bond order (0.7955) was calculated for silaaldehyde II (with BEt3 as an acceptor bound to the oxygen atom); for silaacid chloride I and silaester III (without any acceptor bound to the oxygen atom), the calculated WBI values are 1.0993 and 1.0441, respectively.12a In compounds 1, 2, and 10, the HOMO is localised on the phenyl ring of the B(C6F5)3 moiety (Fig. S64; see the ESI‡), and in compound 3, it is localised on the pyrrole ring, which also reveals the stabilisation of the formal Ge
O bond (0.74–0.76) relative to that in germanone IV (0.70)10 (Table S6; see the ESI‡). A similar bond order (0.7955) was calculated for silaaldehyde II (with BEt3 as an acceptor bound to the oxygen atom); for silaacid chloride I and silaester III (without any acceptor bound to the oxygen atom), the calculated WBI values are 1.0993 and 1.0441, respectively.12a In compounds 1, 2, and 10, the HOMO is localised on the phenyl ring of the B(C6F5)3 moiety (Fig. S64; see the ESI‡), and in compound 3, it is localised on the pyrrole ring, which also reveals the stabilisation of the formal Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in these compounds (Fig. S64; see the ESI‡). Furthermore, NBO donor–acceptor interactions between oxygen and boron atoms can be observed in all these compounds (Fig. S65; see the ESI‡); the stabilisation energies due to these interactions are 280.3 kcal mol−1, 315.6 kcal mol−1, 296.3 kcal mol−1, and 294.6 kcal mol−1 in compounds 1 (Fig. S65a‡), 2 (Fig. S65b‡), 3 (Fig. S65c‡), and 10 (Fig. S65d‡), respectively. All these stabilisation energies are lower than that observed in germanone IV (334.9 kcal mol−1),10 indicating the reduced electron donation from oxygen atoms to boron atoms in compounds 1–3, and 10.
O bonds in these compounds (Fig. S64; see the ESI‡). Furthermore, NBO donor–acceptor interactions between oxygen and boron atoms can be observed in all these compounds (Fig. S65; see the ESI‡); the stabilisation energies due to these interactions are 280.3 kcal mol−1, 315.6 kcal mol−1, 296.3 kcal mol−1, and 294.6 kcal mol−1 in compounds 1 (Fig. S65a‡), 2 (Fig. S65b‡), 3 (Fig. S65c‡), and 10 (Fig. S65d‡), respectively. All these stabilisation energies are lower than that observed in germanone IV (334.9 kcal mol−1),10 indicating the reduced electron donation from oxygen atoms to boron atoms in compounds 1–3, and 10.
As none of the monoanionic ligands, such as β-diketiminate and amidinate ligands, are known to stabilise compounds with formal Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, it is of interest to examine how the bulky monoanionic aminotroponiminate (ATI) ligand used in the present study helps to stabilise various compounds with formal Ge
O bonds, it is of interest to examine how the bulky monoanionic aminotroponiminate (ATI) ligand used in the present study helps to stabilise various compounds with formal Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds. NBO second-order perturbation theory analysis reveals the existence of donor–acceptor interactions between (a) spx orbitals of nitrogen atoms of the ATI ligand to vacant s, p or spx orbitals of germanium in compounds 1–3 and 10 (Fig. S66a, b, S67a–d, S68a–d, and S69a–d; see the ESI‡); (b) NATI orbitals to the σ* orbital of the Ge–Cl bond in compound 1 (Fig. S66c and d; see the ESI‡) and NATI orbitals to the σ* orbital of the Ge–S bond in compound 10 (Fig. S69e and f; see the ESI‡); and (c) s or p orbitals of the chlorine atom to π* orbitals of Ge–NATI bonds in compound 1 (Fig. S66e and f; see the ESI‡). Owing to the interactions of types (b) and (c), the energies of the σ* orbital of the Ge–Cl bond in compound 1, π* orbitals of the Ge–NATI bonds in compound 1, and the σ* orbital of the Ge–S bond in compound 10 are lower, and these orbitals are available for accepting electrons donated by the O atom of the Ge
O bonds. NBO second-order perturbation theory analysis reveals the existence of donor–acceptor interactions between (a) spx orbitals of nitrogen atoms of the ATI ligand to vacant s, p or spx orbitals of germanium in compounds 1–3 and 10 (Fig. S66a, b, S67a–d, S68a–d, and S69a–d; see the ESI‡); (b) NATI orbitals to the σ* orbital of the Ge–Cl bond in compound 1 (Fig. S66c and d; see the ESI‡) and NATI orbitals to the σ* orbital of the Ge–S bond in compound 10 (Fig. S69e and f; see the ESI‡); and (c) s or p orbitals of the chlorine atom to π* orbitals of Ge–NATI bonds in compound 1 (Fig. S66e and f; see the ESI‡). Owing to the interactions of types (b) and (c), the energies of the σ* orbital of the Ge–Cl bond in compound 1, π* orbitals of the Ge–NATI bonds in compound 1, and the σ* orbital of the Ge–S bond in compound 10 are lower, and these orbitals are available for accepting electrons donated by the O atom of the Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. Further, energy decomposition analysis (EDA)25 was performed using {Y–Ge
O bond. Further, energy decomposition analysis (EDA)25 was performed using {Y–Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3} (Y = Cl (1), OSiPh3 (2), NC4H4 (3), SPh (10)) as one fragment and the {ATI} ligand as another fragment with frozen geometries obtained from DFT calculations; the results are summarised in Table S7 (see the ESI‡). The large interaction energy (Eint) observed for these compounds arises essentially due to the favourable ΔEorb term that describes the stabilising interaction between the ATI ligand and the Y–Ge
O → B(C6F5)3} (Y = Cl (1), OSiPh3 (2), NC4H4 (3), SPh (10)) as one fragment and the {ATI} ligand as another fragment with frozen geometries obtained from DFT calculations; the results are summarised in Table S7 (see the ESI‡). The large interaction energy (Eint) observed for these compounds arises essentially due to the favourable ΔEorb term that describes the stabilising interaction between the ATI ligand and the Y–Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 moiety (Y = Cl (1), OSiPh3 (2), NC4H4 (3), SPh (10)).
O → B(C6F5)3 moiety (Y = Cl (1), OSiPh3 (2), NC4H4 (3), SPh (10)).
Conclusions
Donor–acceptor-stabilised germaacyl chloride (i-Bu)2ATIGe(O)(Cl) → B(C6F5)3 (1), germaester (i-Bu)2ATIGe(O)(OSiPh3) → B(C6F5)3 (2), and N-germaacyl pyrrole (i-Bu)2ATIGe(O)(NC4H4) → B(C6F5)3 (3) compounds were successfully isolated as stable species for the first time. Compounds 1, 2, and 3 can undergo nucleophilic substitution reactions without any disturbance to the Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → B(C6F5)3 moiety to afford germaynone (i-Bu)2ATIGe(O)(CCPh) → B(C6F5)3 (4), germaester (i-Bu)2ATIGe(O)(Ot-Bu) → B(C6F5)3 (5), germanone (i-Bu)2ATIGe(O)(R) → B(C6F5)3 (R = Ph 6, Me 7), and germaacyl thioester (i-Bu)2ATIGe(O)(SPh) → B(C6F5)3 (10) compounds in good yields. Interestingly, through the reactivity of 1 and 2, the feasibility to interconvert germaesters and germaacid chlorides is exposed. Attempts were also made to synthesise germaamides and germacarboxylic acids, and it is anticipated that the wisdom obtained during these endeavours will offer new directions to the isolation of these compounds as stable species in the near future.
O → B(C6F5)3 moiety to afford germaynone (i-Bu)2ATIGe(O)(CCPh) → B(C6F5)3 (4), germaester (i-Bu)2ATIGe(O)(Ot-Bu) → B(C6F5)3 (5), germanone (i-Bu)2ATIGe(O)(R) → B(C6F5)3 (R = Ph 6, Me 7), and germaacyl thioester (i-Bu)2ATIGe(O)(SPh) → B(C6F5)3 (10) compounds in good yields. Interestingly, through the reactivity of 1 and 2, the feasibility to interconvert germaesters and germaacid chlorides is exposed. Attempts were also made to synthesise germaamides and germacarboxylic acids, and it is anticipated that the wisdom obtained during these endeavours will offer new directions to the isolation of these compounds as stable species in the near future.
Author contributions
M. K. S. carried out all the experimental studies and drafted the manuscript. S. S. and P. M. helped M. K. S. with some of the experimental studies. The theoretical studies were carried out by G. M., who also wrote the theoretical section of the manuscript. B. P. assisted G. M. with some of the theoretical calculations/write-up. S. N. and G. R. corrected the experimental and theoretical write-ups of the manuscript, respectively.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. K. S., P. M. and S. S. thank the Indian Institute of Technology Delhi (IITD), New Delhi, India, and the University Grants Commission (UGC), New Delhi, India, for research fellowships. S. N. thanks the SERB, Department of Science and Technology (DST), New Delhi, India, for funding (EMR/2017/005519) and DST-FIST for establishing single-crystal X-ray diffraction (SR/FST/CSII-027/2014) and HRMS (SR/FST/CS-1-195/2008) facilities in the Department of Chemistry, IIT Delhi.Notes and references
- (a) Y. Xiong, S. Yao and M. Driess, Angew. Chem., Int. Ed., 2013, 52, 4302 CrossRef CAS PubMed and references cited therein; (b) M. Asay, C. Jones and M. Driess, Chem. Rev., 2011, 111, 354 CrossRef CAS PubMed; (c) C. R. Fischer and P. P. Power, Chem. Rev., 2010, 110, 3877 CrossRef PubMed; (d) Y. Mizuhata, T. Sasamori and N. Tokitoh, Chem. Rev., 2009, 109, 3479 CrossRef CAS PubMed; (e) S. Nagendran and H. W. Roesky, Organometallics, 2008, 27, 457 CrossRef CAS; (f) R. Okazaki and N. Tokitoh, Acc. Chem. Res., 2000, 33, 625 CrossRef CAS PubMed; (g) P. P. Power, Chem. Rev., 1999, 99, 3463 CrossRef CAS PubMed; (h) J. Barrau and G. Rima, Coord. Chem. Rev., 1998, 178–180, 593 CrossRef CAS.
- (a) E. Bonnefille, S. Mazières, C. Bibal, N. Saffon, H. Gornitzka and C. Couret, Eur. J. Inorg. Chem., 2008, 4242 CrossRef CAS; (b) L. Pu, N. J. Hardman and P. P. Power, Organometallics, 2001, 20, 5105 CrossRef CAS; (c) M. Veith and A. Rammo, Z. Anorg. Allg. Chem., 1997, 623, 861 CrossRef CAS; (d) P. Jutzi, H. Schmidt, B. Neumann and H.-G. Stammler, Organometallics, 1996, 15, 741 CrossRef CAS; (e) N. Tokitoh, T. Matsumoto and R. Okazaki, Chem. Lett., 1995, 1087 CrossRef CAS.
- G. L. Wegner, R. J. F. Berger, A. Schier and H. Schmidbaur, Organometallics, 2001, 20, 418 CrossRef CAS.
- (a) R. Tacke, C. Kobelt, J. A. Baus, R. Bertermann and C. Burschka, Dalton Trans., 2015, 44, 14959 RSC; (b) K. Junold, M. Nutz, J. A. Baus, C. Burschka, C. Fonseca Guerra, F. M. Bickelhaupt and R. Tacke, Chem.–Eur. J., 2014, 20, 9319 CrossRef CAS PubMed; (c) R. Azhakar, R. S. Ghadwal, H. W. Roesky, H. Wolf and D. Stalke, Chem. Commun., 2012, 48, 4561 RSC; (d) A. Jana, R. Azhakar, S. P. Sarish, P. P. Samuel, H. W. Roesky, C. Schulzke and D. Koley, Eur. J. Inorg. Chem., 2011, 5006 CrossRef CAS; (e) S. S. Sen, G. Tavčar, H. W. Roesky, D. Kratzert, J. Hey and D. Stalke, Organometallics, 2010, 29, 2343 CrossRef CAS.
- (a) D. Ellis, P. B. Hitchcock and M. F. Lappert, J. Chem. Soc., Dalton Trans., 1992, 3397 RSC; (b) H. Wang and Z. Xie, Eur. J. Inorg. Chem., 2017, 4430 CrossRef CAS.
- T. Chlupatý, Z. Padělková, F. DeProft, R. Willem and A. Růzička, Organometallics, 2012, 31, 2203 CrossRef.
- For selected references see: (a) I. Alvarado-Beltran, A. Rosas-Sánchez, A. Baceiredo, N. Saffon-Merceron, V. Branchadell and T. Kato, Angew. Chem., Int. Ed., 2017, 56, 10481 CrossRef CAS PubMed; (b) M. M. Linden, H. P. Reisenauer, D. Gerbig, M. Karni, A. Schäfer, T. Müller, Y. Apeloig and P. R. Schreiner, Angew. Chem., Int. Ed., 2015, 54, 12404 CrossRef CAS PubMed; (c) S. U. Ahmad, T. Szilvási, E. Irran and S. Inoue, J. Am. Chem. Soc., 2015, 137, 5828 CrossRef CAS PubMed; (d) T. Muraoka, K. Abe, H. Kimura, Y. Haga, K. Ueno and Y. Sunada, Dalton Trans., 2014, 43, 16610 RSC; (e) A. C. Filippou, B. Baars, O. Chernov, Y. N. Lebedev and G. Schnakenburg, Angew. Chem., Int. Ed., 2014, 53, 565 CrossRef CAS PubMed; (f) S. S. Sen, Angew. Chem., Int. Ed., 2014, 53, 8820–8822 CrossRef CAS PubMed; (g) R. Rodriguez, T. Troadec, D. Gau, N. Saffon-Merceron, D. Hashizume, K. Miqueu, J. Sotiropoulos, A. Baceiredo and T. Kato, Angew. Chem., Int. Ed., 2013, 52, 4426 CrossRef CAS PubMed; (h) Y. Xiong, S. Yao and M. Driess, Angew. Chem., Int. Ed., 2013, 52, 4302 CrossRef CAS PubMed; (i) T. Muraoka, K. Abe, Y. Haga, T. Nakamura and K. Ueno, J. Am. Chem. Soc., 2011, 133, 15365 CrossRef CAS PubMed; (j) Y. Gao, H. Hu and C. Cui, Chem.–Eur. J., 2011, 17, 8803 CrossRef CAS PubMed; (k) Y. Xiong, S. Yao and M. Driess, Dalton Trans., 2010, 39, 9282 RSC; (l) S. Yao, Y. Xiong and M. Driess, Chem.–Eur. J., 2010, 16, 1281 CrossRef CAS PubMed; (m) Y. Xiong, S. Yao, R. Müller, M. Kaupp and M. Driess, Nat. Chem., 2010, 2, 577 CrossRef CAS PubMed; (n) J. D. Epping, S. Yao, M. Karni, Y. Apeloig and M. Driess, J. Am. Chem. Soc., 2010, 132, 5443 CrossRef CAS PubMed; (o) Y. Xiong, S. Yao and M. Driess, J. Am. Chem. Soc., 2009, 131, 7562 CrossRef CAS PubMed.
- (a) S. Yao, Y. Xiong, W. Wang and M. Driess, Chem.–Eur. J., 2011, 17, 4890 CrossRef CAS PubMed; (b) S. Yao, Y. Xiong and M. Driess, Chem. Commun., 2009, 6466 RSC.
- L. Li, T. Fukawa, T. Matsuo, D. Hashizume, H. Fueno, K. Tanaka and K. Tamao, Nat. Chem., 2012, 4, 361 CrossRef CAS PubMed.
- S. Sinhababu, D. Yadav, S. Karwasara, M. K. Sharma, G. Mukherjee, G. Rajaraman and S. Nagendran, Angew. Chem., Int. Ed., 2016, 55, 7742 CrossRef CAS PubMed.
- A. V. Zabula, T. Pape, A. Hepp, F. M. Schappacher, U. C. Rodewald, R. Pöttgen and F. E. Hahn, J. Am. Chem. Soc., 2008, 130, 5648 CrossRef CAS PubMed.
- (a) D. C. H. Do, A. V. Protchenko, M. Ángeles Fuentes, J. Hicks, E. L. Kolychev, P. Vasko and S. Aldridge, Angew. Chem., Int. Ed., 2018, 57, 13907 CrossRef CAS PubMed; (b) R. Rodriguez, D. Gau, T. Troadec, N. Saffon-Merceron, V. Branchadell, A. Baceiredo and T. Kato, Angew. Chem., Int. Ed., 2013, 52, 8980 CrossRef CAS PubMed; (c) R. S. Ghadwal, R. Azhakar, H. W. Roesky, K. Pröpper, B. Dittrich, C. Goedecke and G. Frenking, Chem. Commun., 2012, 48, 8186 RSC; (d) R. S. Ghadwal, R. Azhakar, H. W. Roesky, K. Propper, B. Dittrich, S. Klein and G. Frenking, J. Am. Chem. Soc., 2011, 133, 17552 CrossRef CAS PubMed; (e) Y. Xiong, S. Yao, R. Müller, M. Kaupp and M. Driess, J. Am. Chem. Soc., 2010, 132, 6912 CrossRef CAS PubMed; (f) Y. Xiong, S. Yao and M. Driess, Angew. Chem., Int. Ed., 2010, 49, 6642 CrossRef CAS PubMed; (g) S. Yao, M. Brym, C. Wullen and M. Driess, Angew. Chem., Int. Ed., 2007, 46, 4159 CrossRef CAS PubMed; (h) S. Yao, Y. Xiong, M. Brym and M. Driess, J. Am. Chem. Soc., 2007, 129, 7268 CrossRef CAS PubMed.
- (a) S. Sinhababu, R. K. Siwatch, G. Mukherjee, G. Rajaraman and S. Nagendran, Inorg. Chem., 2012, 51, 9240 CrossRef CAS PubMed; (b) R. K. Siwatch, S. Kundu, D. Kumar and S. Nagendran, Organometallics, 2011, 30, 1998 CrossRef CAS; (c) H. V. R. Dias, Z. Wang and W. Jin, Coord. Chem. Rev., 1998, 176, 67 CrossRef CAS; (d) H. V. R. Dias and Z. Wang, J. Am. Chem. Soc., 1997, 119, 4650 CrossRef CAS.
- (a) D. Yang, J. Guo, H. Wu, Y. Ding and W. Zheng, Dalton Trans., 2012, 41, 2187 RSC; (b) S. M. I. Al-Rafia, P. A. Lummis, M. J. Ferguson, R. McDonald and E. Rivard, Inorg. Chem., 2010, 49, 9709 CrossRef CAS PubMed; (c) M. Veith, S. Becker and V. Huch, Angew. Chem., Int. Ed., 1989, 28, 1237 ( Angew. Chem. , 1989 , 101 , 1287 ) CrossRef.
- S. Karwasara, R. K. Siwatch, C. K. Jha and S. Nagendran, Organometallics, 2015, 34, 3246 CrossRef CAS.
- S. Karwasara, M. K. Sharma, R. Tripathi and S. Nagendran, Organometallics, 2013, 32, 3830 CrossRef CAS.
- (a) M. A. Beckett, D. S. Brassington, S. J. Coles and M. B. Hursthouse, Inorg. Chem. Commun., 2000, 3(10), 530 CrossRef CAS; (b) M. A. Beckett and G. C. Strickland, Polymer, 1996, 37, 4629 CrossRef CAS.
- M. Hoshi, K. Shirakawa and M. Okimoto, Tetrahedron Lett., 2007, 48, 8475 CrossRef CAS.
- A. Bähr, L. C. Wilkins, K. Ollegott, B. M. Kariuki and R. L. Melen, Molecules, 2015, 20, 4530 CrossRef PubMed.
- D. Neculai, H. W. Roesky, A. M. Neculai, J. Magull, B. Walfort and D. Stalke, Angew. Chem., Int. Ed., 2002, 41, 4294 CrossRef CAS PubMed.
- A. K. Swarnakar, C. Hering-Junghans, M. J. Ferguson, R. McDonald and E. Rivard, Chem.–Eur. J., 2017, 23, 8628 CrossRef CAS PubMed.
- CCDC 1564828–1564834, 1564836, and 1851011–1851015 contains the crystallographic data for this paper.‡.
- F. Wheinhold and C. Landis, Valency and Bonding, Cambridge, 2005 Search PubMed.
- (a) A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899 CrossRef CAS; (b) E. D. Glendening, A. E. Reed, J. E. Carpenter and F. Weinhold, NBO Version 3.1 Search PubMed.
- (a) S. I. Gorelsky, AOMix: Program for Molecular Orbital Analysis, version 6.6, University of Ottawa, Ottawa, 2010, http://www.sg-chem.net/ Search PubMed; (b) S. I. Gorelsky, S. Ghosh and E. I. Solomon, J. Am. Chem. Soc., 2006, 128, 278 CrossRef CAS PubMed; (c) T. Ziegler and A. Rauk, Theor. Chem. Acc., 1977, 46, 1 Search PubMed; (d) K. Kitaura and K. Morokuma, Int. J. Quantum Chem., 1976, 10, 325 CrossRef CAS.
Footnotes |
| † Dedicated to Prof. V. Chandrasekhar on the occasion of his 60th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental section, UV-vis spectra of compounds 1, 2, and 10; molecular structure determination of compounds D1, D3–D5, 1–7, 9, and 10; computational details (PDF). CIFs for compounds D1, D3–D5, 1–7, 9, and 10, are deposited with the Cambridge Structural Database (CSD). CCDC 1564828–1564834, 1564836, and 1851011–1851015. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc05380d |
| This journal is © The Royal Society of Chemistry 2019 |