Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A synthetic cyclitol-nucleoside conjugate polyphosphate is a highly potent second messenger mimic†
Wolfgang
Dohle‡
a,
Xiangdong
Su‡
a,
Stephen J.
Mills‡
a,
Ana M.
Rossi
b,
Colin W.
Taylor
b and
Barry V. L.
Potter
 *a
*a
aMedicinal Chemistry & Drug Discovery, Department of Pharmacology, University of Oxford, Mansfield Road, Oxford, OX1 3QT, UK. E-mail: barry.potter@pharm.ox.ac.uk; Tel: +44-1865-271945
bDepartment of Pharmacology, University of Cambridge, Tennis Court Road, Cambridge, CB2 1PD, UK
First published on 23rd April 2019
Abstract
Reactions that form sec–sec ethers are well known, but few lead to compounds with dense functionality around the O-linkage. Replacement of the α-glucopyranosyl unit of adenophostin A, a potent D-myo-inositol 1,4,5-trisphosphate (IP3R) agonist, with a D-chiro-inositol surrogate acting substantially as a pseudosugar, leads to “D-chiro-inositol adenophostin”. At its core, this cyclitol-nucleoside trisphosphate comprises an ether linkage between the axial 1-hydroxyl position of D-chiro-inositol and the 3′-hydroxyl group of an adenosine ribose sugar. A divergent synthesis of D-chiro-inositol adenophostin has been achieved. Key features of the synthetic strategy to produce a triol for phosphorylation include a new selective mono-tosylation of racemic 1,2:4,5-di-O-isopropylidene-myo-inositol using tosyl imidazole; subsequent conversion of the product into separable camphanate ester derivatives, one leading to a chiral myo-inositol triflate used as a synthetic building block and the other to L-1-O-methyl-myo-inositol [L-(+)-bornesitol] to assign the absolute configuration; the nucleophilic coupling of an alkoxide of a ribose pent-4-ene orthoester unit with a structurally rigid chiral myo-inositol triflate derivative, representing the first sec–sec ether formation between a cyclitol and ribose. Reaction of the coupled product with a silylated nucleobase completes the assembly of the core structure. Further protecting group manipulation, mixed O- and N-phosphorylation, and subsequent removal of all protecting groups in a single step achieves the final product, avoiding a separate N6 protection/deprotection strategy. D-chiro-Inositol adenophostin evoked Ca2+ release through IP3Rs at lower concentrations than adenophostin A, hitherto the most potent known agonist of IP3Rs.
Introduction
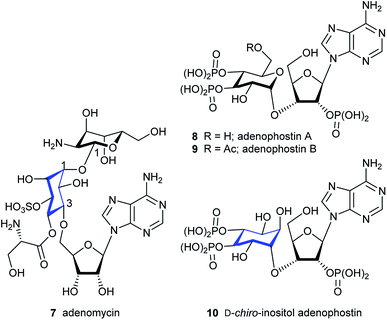
Inositols are present in cells from every domain of life (archaea, bacteria and eukaryotes), with myo-inositol the most abundant of the nine inositol stereoisomers.1 Polyphosphorylated derivatives of myo-inositol are common in biology and have many functions,2 including roles in regulating ion-channels,3 phosphate levels,4 metabolic flux,5 transcription, mRNA export and translation,6 insulin signalling, embryonic development7 and stress responses.8D-myo-Inositol 1,4,5-trisphosphate (IP3, 1, Fig. 1), which is an intracellular messenger that evokes Ca2+ signals after binding to its own receptor (IP3R)1a,9 and higher myo-inositol polyphosphates are subjects of intense interest.1a,10 Such compounds are implicated in diverse areas of biology and disease.11myo-Inositol is also a component of glycosyl-phosphatidylinositol (GPIs, see compound 2, Fig. 1),12 which anchor proteins to the cell surface and are present in all eukaryotic cells and some bacteria.13 Other stereoisomers of inositol also have biological activities,1a but these are less common or have not been widely investigated.14 Beyond GPIs, derivatives of myo-inositol can make simple conjugated derivatives with sugars; these are much less common and only a few natural products are known. In general, an equatorial hydroxyl group of an inositol is linked to the glycosidic C1 of a sugar. Examples of the rarer inositol derivatives are fagopyritol (3 & 4) and pinitol derivatives (5 & 6) which contain a D-chiro-inositol-galactose conjugate, and are isolated from various plant sources (Fig. 1).15 Most of the conjugates have a galactose unit connected via its glycosidic C1 to an equatorially configured oxygen at either C3 (3), C2 (4 & 5) or C5 (not shown) of the D-chiro-inositol unit, with further conjugates sometimes added at the primary C6 hydroxyl group of galactose (see compounds 3 & 4, Fig. 1). Only one example was found where the axial configured oxygen at C1 of D-chiro-inositol forms an axial/axial configured glycosidic bond (see compound 6, Fig. 1).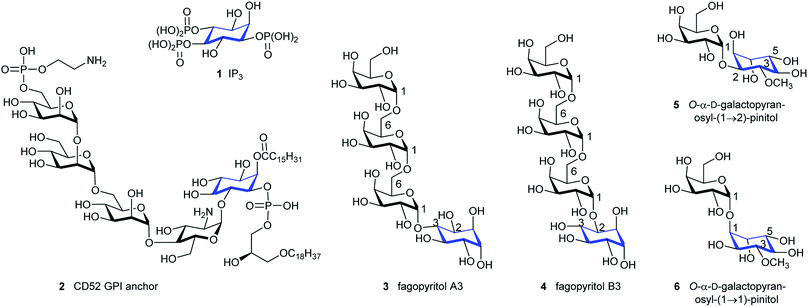 | ||
| Fig. 1 D-myo-Inositol 1,4,5-trisphosphate (IP3) 1, CD52 GPI anchor 2 and natural products 3–6 containing D-myo- and D-chiro-inositol sugar conjugates (inositol component shown in blue). | ||
Complex, but feasible, synthetic routes to such compounds and GPIs require conjugation at the sugar C1 glycosidic centre. GPIs are sugar conjugates, where a D-myo-inositol derivative is usually oxygen-linked from its C6 position to a D-glucosamine unit at its glycosidic C1 position. A phospholipid moiety at C1 of the D-myo-inositol unit enables GPIs to insert into the plasma membrane. The difficulty of isolating and purifying GPIs from biological sources lead to the development of methodologies for the synthesis of GPIs, GPI-anchored proteins and glycoproteins.16–19 To our knowledge, sec–sec inositol-hexose sugar conjugates are rare, apart from those at the glycosidic centre. Only one example of a pseudo-disaccharide could be found20 and there were no examples of inositol-pentose sugar conjugates. However, one example of a prim-sec ether linkage is known, for the antibiotic adenomycin 7 (Fig. 2).21 This compound contains one adenosine unit linked through its primary C5′ hydroxyl group to the C3 position of an L-chiro-inositol derivative to form a prim-sec ether bond. Additionally, a hemi-acetal is located at the glycosidic C1 of an L-gulosamine linked to the L-chiro-inositol C1. Interestingly, contrary to glycosidic connections in GPIs and in 3–6, the latter linkage in 7 is reported to be β-glycosidic.21 Given the extensive biology of myo-inositol sugar conjugates and growing interest in the other eight inositol isomers,1a there is a need to generate analogues to construct prim-sec or more particularly sec–sec ether linkages. In the latter case, an example of particular interest can be drawn from the Ca2+-signalling field in the form of the adenophostins 8 & 9 (Fig. 2).22 These are naturally occurring fungal metabolites with a phosphorylated glucose component that resembles part of the IP3 pharmacophore and which are more potent than IP31 in evoking Ca2+ release through IP3 receptors (IP3Rs).
IP3Rs are intracellular Ca2+ channels that allow IP3, produced when extracellular stimuli promote hydrolysis of phosphatidylinositol 4,5-bisphosphate, to release Ca2+ from intracellular stores.23 The resulting Ca2+ signals regulate diverse cellular activities.24 Opening of the Ca2+-permeable channel of the IP3R is initiated when IP3 binds to the IP3-binding core (IBC, residues 224–604) of each of the four subunits of the tetrameric IP3R (Fig. 3A and B).25,26 Structure–activity analyses,27 structures of N-terminal fragments of IP3R25,28,29 and of complete IP3R,9b alongside mutagenesis studies have established that IP3 initiates IP3R activation by causing partial closure of the clam-like IBC. The 1- and 5-phosphates of IP3 interact primarily with residues in the α-domain of the IBC, while the 4-phosphate interacts with IBC-β (Fig. 3A). Adenophostin A (8) and adenophostin B (9) bind to IP3R with greater affinity than IP3 and they are ∼6–10-fold more potent than IP3 in evoking Ca2+ release.30,31 Hitherto, adenophostin A is the most potent known agonist of IP3R.31 Our model for the interaction of adenophostin A with the IBC,32 which is supported by mutagenesis33 and SAR analyses,34,35 suggests that the glucose 3′′,4′′-bisphosphate motif of adenophostin A mimics the 4,5-bisphosphate motif of IP3, while the 2′′-hydroxyl and 2′-phosphate of adenophostin A mimic the non-essential 6-hydroxyl and 1-phosphate groups of IP3.25,36 A recently published cryo-EM structure of IP3R1 with and without adenophostin A bound37 hints at substantially different binding modes for adenophostin A. However, the resolution of ligands in such structures is far from optimal and the ligand structure here is chemically incorrect.
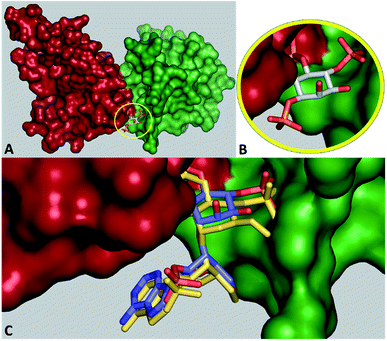 | ||
| Fig. 3 (A) IP3-binding core (IBC) of IP3 receptors with IP31 bound (PDB 1N4K). The IBC-α is shown in red, the IBC-β in green and IP31 in stick representation (carbon atoms in grey). (B) Close-up view of (A). (C) Model of D-chiro-inositol adenophostin 10 (carbons in light blue stick representation) with adenophostin A 8 (all atoms in yellow stick representation) binding to the IBC. | ||
We focused on developing a synthetic route to “D-chiro-inositol adenophostin” 10. The aim was to replace the α-glucopyranosyl unit of 8 with the most similarly-configured inositol unit (D-chiro-inositol, acting substantially as a pseudo-sugar). The resulting ligand would allow us to study any potentially novel effects of this modification on Ca2+ release. Since the phosphorylated inositol-nucleoside hybrid closely resembles adenophostin A, it would establish whether nature evolved a phosphorylated glucose moiety as the best mimic of the inositol bisphosphate motif or whether the activity of adenophostin A can be still further improved by replacing glucose with a motif more similar to the cyclitol in IP3. Finally, and importantly, if high adenophostin A-like potency were achieved in 10, the hybrid would offer an axial hydroxyl group at the D-chiro-inositol C6 position (C2 position in myo-inositol), not present in adenophostin A. This position could be modified (e.g. via extended substituents) to target the cleft between the IBC domains (Fig. 3B) and might facilitate the design of high-affinity ligands as potential antagonists by virtue of their ability to prevent closure of the IBC clam. Such a strategy cannot be envisaged for adenophostin A.
Examining all the bonds that need to be formed to assemble 10, it became clear that its total synthesis would be challenging, especially the formation of the sec–sec ether bond between the inositol ring and the ribose sugar. Linkages are usually made between a reactive sugar glycosyl acceptor at the 1-position and inositol, both in protected form, to give inositol-sugar conjugates after deprotection (Fig. 1). Non-glycosidic conjugates of this type are quite rare. However, some years ago in the synthesis of a pseudo-disaccharide a cyclitol derivative was used as the nucleophile and a triflate on a rigid sugar unit as the electrophile.20 This strategy would not easily work in our case because a D-chiro-inositol derivative would need to react as a nucleophile ideally with a triflate at the 3-position of a xylose derivative. SN2 reactions using 3-trifluoromethanesulfonyl- and 3-tosyl-xylose derivatives are described, but coupled products are only achieved when using non-alkaline nucleophiles like azide or thiophenolate.38,39 With the hydrogen at C4 in xylose placed almost anti-periplanar to the leaving group, the less reactive 3-tosyl derivative undergoes mainly elimination to form an olefin when reacted with alkoxides, but cleavage of the tosyl group is also observed.39
We report here a divergent strategy for the synthesis of 10 that includes the first sec–sec ether formation between a cyclitol and a ribose sugar as one key feature. Others are a selective mono-tosylation of a racemic inositol diol derivative and subsequent conversion of the product into diastereoisomeric camphanate esters separable by column chromatography. One diastereoisomer provides the chiral inositol triflate to be used as a building block, and the other provides a route to L-(+)-bornesitol in order to confirm absolute configurations. Coupling of the inositol triflate with the ribose alkoxide of a pent-4-ene orthoester derivative and subsequent reaction with a silylated nucleobase assembles the core structure for phosphorylation. Pan-phosphorylation of all hydroxyl and amino groups and removal of all protecting groups inventively in a single step affords the final product 10 without recourse to an adenine N6-protection strategy.
Results and discussion
Chemistry
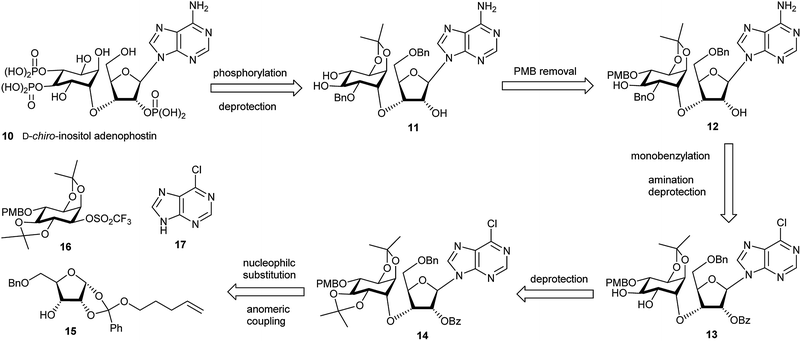
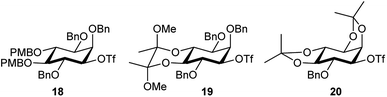
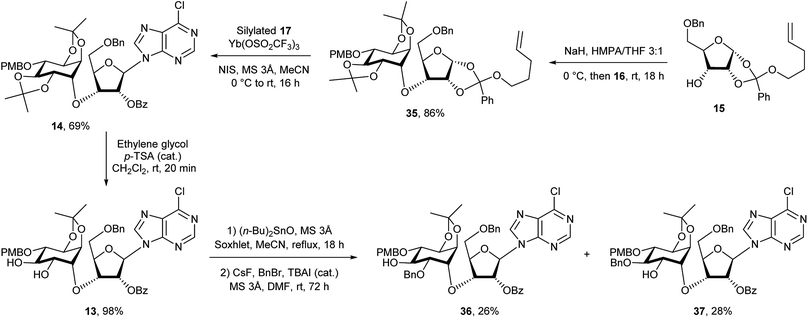
The retrosynthetic analysis of D-chiro-inositol adenophostin 10 (Scheme 1), leads to 14 as the first compound containing all three components, D-chiro-inositol, ribose and a purine base as derivatives. An initial analysis for the formation of the sec–sec ether suggested that myo-inositol triflates may be used. Three such potential triflates were synthesised in turn, with increasing rigidity of the inositol ring (18–20, Fig. 4).Initially, although triflate 18 in the chair conformation did not have an anti-periplanar H–Ins–OTf motif, the triflate eliminated to form a double bond in the presence of an alkoxide, demonstrating that the inositol framework needed to be more rigid. 13C NMR assignment indicated a quaternary carbon derived from proton abstraction located at δC = 154.1 and an olefinic C–H at δC = 97.0. The rigidity of the inositol ring was further increased to give derivative 19. However, elimination in the presence of base was still observed and similar olefinic fingerprint signals in the 13C NMR were seen as above. A still more constrained structure was an obvious further choice, so no elimination should occur. A third compound 20, provided a more rigid inositol triflate and the crude product from alkoxide treatment was purified by column chromatography. However, elimination still occurred and two products were observed in the 1H NMR spectrum. After a number of inositol triflates eliminated to form cyclic vinyl ethers, triflate 16 (and the most difficult to manipulate to the final product) was prepared (Scheme 5) and successfully used as a functionalised chiral myo-inositol intermediate for the preparation of the D-chiro-inositol derivative 14. An inositol triflate with a similar protecting group pattern has been previously reported in SN2 reactions. However, only non-basic nucleophiles such as fluoride, azide and thioacetate were used, but no alkoxides.40
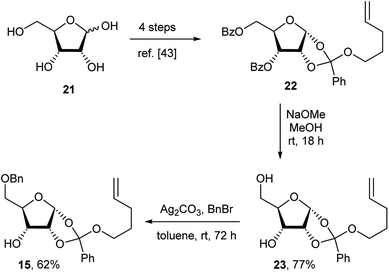
Although the formation of sec–sec ethers is well known, only a few examples lead to densely functionalised compounds.20,41,42 The main problem in the required SN2 type reaction is the use of highly substituted, sterically hindered starting materials. A limited number of electrophiles, such as a triflate within a rigid organic framework have been used successfully.20,41 Compound 14 could be assembled in two steps from the ribose orthoester derivative 15. Note that although 15 is depicted as one isomer at the orthoester moiety, which is indeed the case, in fact the exact stereochemistry at the quaternary carbon has not been proven. Diverse attempts to derivatise 15 and 23 to give a crystalline product for X-ray studies were made, but all of these failed. Compound 15 was coupled to the L-myo-inositol triflate derivative 16. Subsequent anomeric coupling of the orthoester group with silylated 6-chloropurine 17 gave compound 14.
The ribose fragment of 10 was synthesised in four steps from D-(−)-ribose 21 to give the pent-4-ene orthoester 22.43 Reaction of 22 with sodium methoxide in methanol furnished diol 23 in high yield.43 Selective benzylation of the primary hydroxyl group using silver carbonate and benzyl bromide in toluene gave compound 15 in good yield (Scheme 2).44
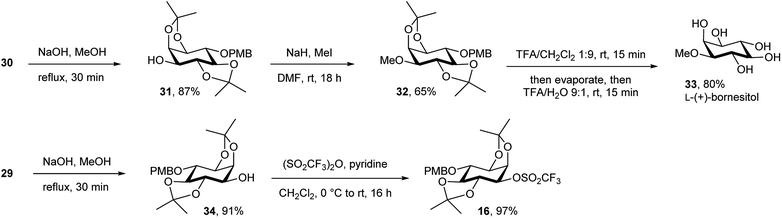
Synthesis of the chiral L-myo-inositol triflate derivative 16 started from racemic 1,2:4,5-di-O-isopropylidene-myo-inositol 2445 (Schemes 3 and 4). In order to convert an L-myo-inositol derivative into the D-chiro-inositol derivative via an SN2 reaction a triflate was required as a leaving group at C3. The hydroxyl group at C6 was protected as a p-methoxybenzyl (PMB) ether, but selective protection at the more reactive 3-hydroxyl group was required. Selective mono-tosylation of the hydroxyl group at C3 of 24 using tosyl chloride in pyridine lead to low yields of compound 25.46 However, a new method was devised for this conversion using tosyl imidazole47 in the presence of cesium fluoride in DMF at room temperature. Compound 25 was obtained in good yield along with a minor amount of the bis-tosylated product 26. Reaction of 25 with sodium hydride and p-methoxybenzyl chloride in DMF gave compound 27 in high yield. Subsequent removal of the tosyl group was achieved by dissolving 27 in dichloromethane followed by the addition of methanol and magnesium turnings.48 Compound 28 was treated with (1S)-(−)-camphanic chloride49 to afford diastereoisomers 29 and 30, which were separated by column chromatography and both were isolated in good yield (Scheme 3).
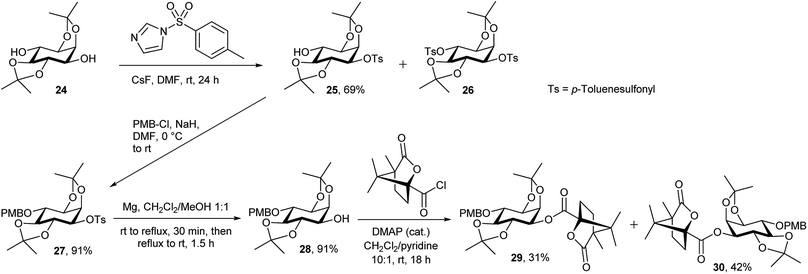 | ||
| Scheme 3 Synthesis of protected L-myo-inositol 3-O-camphanate 29 and D-myo-inositol 3-O-camphanate 30. | ||
The absolute configuration was determined using diastereoisomer 30, which was converted in several steps via ester hydrolysis, methylation and protecting group removal via31 and 32 into the known natural product L-(+)-bornesitol 33; the configuration of this product was confirmed by comparison to previously reported 1H NMR and optical rotation data.50 The chiral myo-inositol triflate 16 was then synthesised in good overall yield from 29 by ester hydrolysis and reaction of chiral alcohol 34 with triflic anhydride and pyridine in dichloromethane (Scheme 4).
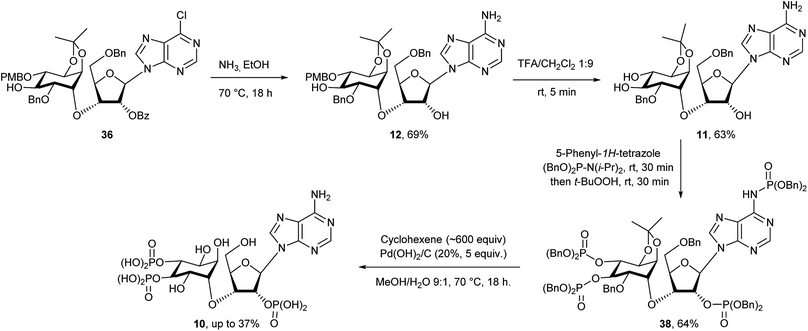
Compound 15 was first dissolved in anhydrous THF/HMPA before adding sodium hydride to generate the alkoxide. The chiral triflate 16 was then added to the alkoxide to give 35 in 86% yield, transforming the L-myo-inositol unit into the desired D-chiro-inositol derivative by inversion of configuration at C3. DMPU could also be used to replace HMPA as a co-solvent, but gave overall slightly lower yields (about 10% less). However, no reaction was observed when DMF alone was used as a solvent. Subsequent coupling of 35via its pent-4-ene orthoester group with silylated 6-chloropurine51 in the presence of an activator under anhydrous conditions gave compound 14 in good yield.43b The trans-isopropylidene group was then selectively removed by treatment with ethylene glycol and p-toluenesulfonic acid in dichloromethane for no longer than 20 minutes to give compound 13 in almost quantitative yield. After various attempts, we found that a one-pot reaction procedure strategy was unsuitable to mono-benzylate compound 13, since the harsh reaction conditions resulted in complex mixtures and the desired compound 36 was not observed.52 Selective mono-benzylation of 13 was best achieved in a two-stage procedure.53 First, 13 was reacted with n-Bu2SnO in refluxing acetonitrile using a Soxhlet condenser containing activated 3 Å MS. The resulting tin acetal was isolated and directly converted into the desired mono-benzylated compound 36 in 26% yield using benzyl bromide, cesium fluoride and tetra-n-butylammonium iodide in anhydrous DMF. However, no regioselectivity was observed and the other mono-benzylated compound 37 was isolated in a similar yield together with unconverted starting material 13 (Scheme 5).
Small samples of both chromatographically separable regioisomers 36 and 37 were treated with TFA/DCM to remove the p-methoxybenzyl group and the products then analysed by 1H NMR and COSY without and with the addition of D2O. All hydroxyl protons and the corresponding adjacent cyclitol ring protons were identified. The 1,2-diol relationship was indicated by a strong coupling between these two adjacent ring protons. For the 1,3-diol relationship that coupling was not found. Compound 36 led to a 1,2-diol, whereas 37 led to a 1,3-diol. Therefore, 36 was identified as the required 2-O-benzylated derivative that was then treated with ammonia in ethanol at 70 °C to give 12 in 69% yield with concomitant removal of the benzoyl ester (Scheme 6). After trying various methods we found that removal of the PMB protecting group was best achieved using TFA in anhydrous dichloromethane for no longer than 5 minutes.54 Longer reaction times and/or traces of moisture usually lead to increasing amounts of more polar by-products where the cis-isopropylidene protecting group had also been removed. For the final phosphorylation of triol 11 we had originally envisaged use of imidazolium triflate33,35d as a means of achieving O-selective phosphitylation over N-phosphitylation. While this approach has been used well in the past, we found it to be very cumbersome for the phosphorylation of low milligram quantities of triol. It was not possible to achieve complete selective phosphitylation and to completely exclude water, then titrate in the required amount of phosphitylating reagent without achieving substantial amounts of concomitant N-phosphitylation, although products from this could be easily separated. We therefore reasoned that since N-phosphates have been reported to be labile under acidic and/or hydrogenation reaction conditions55 such a contaminant should therefore convert into the free amino group during the final step of our synthesis. This idea thus argued for a pan-phosphitylation approach, whereby 11 was first phosphorylated with an excess of reagent that easily leads to the tris-O-phosphorylated mono-N-phosphorylated product that could be converted to the mixed tris-phosphate/mono-phosphoramidate 38 upon oxidation. To illustrate this further, the conversion of 11 to the fully deblocked 10 was carried out on a <10 mg scale. Compound 11 was treated with 5-phenyl-1H-tetrazole and dibenzyl diisopropylphosphoramidite56 in dichloromethane followed by oxidation of the product with t-butyl hydroperoxide to achieve the fully protected compound 38 in good overall yield (Scheme 6). The N-phosphate group was readily distinguished from the O-phosphate by its broad 31P NMR resonance peak at δ −0.9 in comparison to sharp peaks at δ −1.2 and −1.5 (two overlapping peaks) respectively. Subsequent treatment of 38 with hydrogen generated from cyclohexene in the presence of palladium hydroxide on carbon in methanol and water at 70 °C overnight led to an acidic reaction mixture (pH 3), as all O- and N-benzylated phosphate groups were converted to their free acids. Subsequently, this also lead to removal of the cis-isopropylidene group and degradation of the in situ generated acid-labile free N-phosphate by nucleophilic cleavage of its P–N bond.55 As a pleasing result, therefore, all different types of protecting group were removed in a single step to give D-chiro-inositol adenophostin 10 (Scheme 6). Yields of 10 were routinely quite variable and possibly complicated by retention of compound on the hydrogenation catalyst. The best yield achieved so far is 37%. We imagine that this mixed O- and N-phosphitylation methodology, avoiding a sometimes cumbersome extra N-protection strategy, will find application in many nucleotide synthesis situations where only small amounts of compound are available. For biological evaluation, 10 was purified by semi-prep HPLC (purity > 99%) and quantified by UV spectroscopy. Final structural characterisation was additionally confirmed by 1H–31P NMR correlation (see ESI†).
Biology
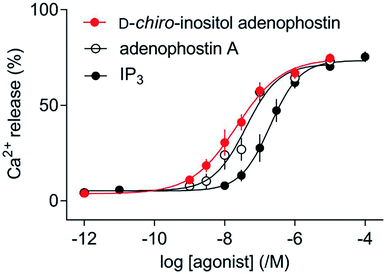
D-chiro-Inositol adenophostin 10 was investigated as an agonist at the IP3R using an intracellular Ca2+ mobilisation assay in comparison to adenophostin A 8 and IP31. We used a low-affinity Ca2+ indicator within the intracellular stores of permeabilized HEK cells expressing IP3R1 to record Ca2+ release evoked by synthetic ligands. Maximally effective concentrations of IP3, adenophostin A 8 or D-chiro-inositol adenophostin 10 caused release of a similar fraction (∼70%) of the Ca2+ sequestered by intracellular stores. However, adenophostin A 8 and 10 were 5.3 and 8.9-fold more potent than IP3, respectively. Moreover, D-chiro-inositol adenophostin 10 was 1.7-fold more potent than adenophostin A 8 (Fig. 5 and Table 1).| Compound | Ca2+ release | |||
|---|---|---|---|---|
| pEC50 | EC50 (nM) | (%) | h | |
| a Results are means ± SEM (Ca2+ release (%), Hill coefficient (h) and pEC50) or means (EC50, half maximal effective concentration) from six independent experiments each performed in duplicate. There were no significant differences between the ligands in the values for Ca2+ release (%) or h. The pEC50 values were significantly different (P < 0.05), for IP3vs. adenophostin A, IP3vs.D-chiro-inositol adenophostin and D-chiro-inositol adenophostin vs. adenophostin A. | ||||
| IP3 (1) | 6.73 ± 0.13 | 186 | 73 ± 2 | 1.2 ± 0.1 |
| Adenophostin A (8) | 7.45 ± 0.16 | 35 | 71 ± 3 | 1.5 ± 0.4 |
| D-chiro-Inositol adenophostin (10) | 7.67 ± 0.14 | 21 | 72 ± 3 | 1.4 ± 0.6 |
Conclusion
In summary, a concise synthesis of D-chiro-inositol adenophostin has been achieved. Key features of the strategy include a new selective mono-tosylation reaction of a racemic myo-inositol diol derivative and subsequent elaboration of the product into separable camphanate derivatives of a fully protected intermediate. One such diastereoisomer is converted to L-(+)-bornesitol to confirm the absolute configuration and the other leads to the required chiral myo-inositol triflate that is used as a synthetic building block. Critical formation of a sec–sec ether used a rigidly structured chiral inositol triflate with an alkoxide of a suitable protected ribose derivative; subsequent reaction of the fully protected coupled product with a silylated nucleobase assembled the core structure and base amination and protecting group manipulations finally afforded a triol for phosphorylation. After phosphitylation of both the adenine amino group and inositol hydroxyl groups, removal of all protecting groups was accomplished in a single step to afford the final product. Thus, replacement of the α-glucopyranosyl unit in adenophostin A with the equivalent D-chiro-inositol motif leads to the trisphosphate D-chiro-inositol adenophostin 10. 10 Was evaluated as an agonist for intracellular Ca2+ release through the IP3R and its EC50 of 21 nM was lower than that of 8 (35 nM). It is the most potent agonist of IP3Rs so far identified and may offer new opportunities to develop high-affinity antagonists of IP3R.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Wellcome Trust. B. V. L. P. and C. W. T. are Wellcome Trust Senior Investigators (grants no. 101010 and 101844). A. M. R. is a Fellow at Queens' College, Cambridge. We thank Dr J. M. Watt for HPLC support and Dr A. M. Riley for discussions and help with graphics.Notes and references
- (a) M. P. Thomas, S. J. Mills and B. V. L. Potter, Angew. Chem., Int. Ed., 2016, 55, 1614 CrossRef CAS PubMed; (b) B. V. L. Potter and D. Lampe, Angew. Chem., Int. Ed., 1995, 34, 1933 CrossRef CAS; (c) R. F. Irvine and M. J. Schell, Nat. Rev. Mol. Cell Biol., 2001, 2, 327 CrossRef CAS PubMed; (d) M. D. Best, H. Zhang and G. D. Prestwich, Nat. Prod. Rep., 2010, 27, 1403 RSC; (e) A. J. Hatch and J. D. York, Cell, 2010, 143, 1030 CrossRef CAS PubMed; (f) A. Chakraborty, S. Kim and S. H. Snyder, Sci. Signaling, 2011, 4, re1 CrossRef PubMed; (g) G. E. Gillaspy, New Phytol., 2011, 192, 823 CrossRef CAS PubMed; (h) J.-Y. Lee, Y.-r. Kim, J. Park and S. Kim, Ann. N. Y. Acad. Sci., 2012, 1271, 68 CrossRef CAS PubMed; (i) M. L. Croze and C. O. Soulage, Biochimie, 2013, 95, 1811 CrossRef CAS PubMed.
- (a) R. H. Michell, Biochem. Soc. Symp., 2007, 74, 223 CrossRef CAS; (b) R. H. Michell, Nat. Rev. Mol. Cell Biol., 2008, 9, 151 CrossRef CAS PubMed; (c) R. H. Michell, Adv. Enzyme Regul., 2011, 51, 84 CrossRef CAS PubMed.
- (a) J. B. Parys and H. De Smedt, Adv. Exp. Med. Biol., 2012, 740, 255 CrossRef CAS PubMed; (b) S. B. Shears, S. B. Ganapathi, N. A. Gokhale, T. M. H. Schenk, H. Wang, J. D. Weaver, A. Zaremba and Y. Zhou, Subcell. Biochem., 2012, 59, 389 CAS.
- A. Saiardi, Adv. Biol. Regul., 2012, 52, 351 CrossRef CAS PubMed.
- W. F. Boss and Y. J. Im, Annu. Rev. Plant Biol., 2012, 63, 409 CrossRef CAS PubMed.
- A. R. Alcázar-Román and S. R. Wente, Chromosoma, 2008, 117, 1 CrossRef.
- M. M. Tsui and J. D. York, Adv. Enzyme Regul., 2010, 50, 324 CrossRef.
- (a) R. Valluru and W. Van den Ende, Plant Sci., 2011, 181, 387 CrossRef CAS PubMed; (b) R. Valluru and W. Van den Ende, Plant Sci., 2012, 185–186, 340 CrossRef CAS.
- (a) M. J. Berridge, P. Lipp and M. D. Bootman, Nat. Rev. Mol. Cell Biol., 2000, 1, 11 CrossRef CAS PubMed; (b) N. Paknejad and R. K. Hite, Nat. Struct. Mol. Biol., 2018, 25, 660 CrossRef CAS PubMed.
- (a) M. P. Thomas and B. V. L. Potter, FEBS J., 2014, 281, 14 CrossRef CAS; (b) M. S. Wilson, T. M. Livermore and A. Saiardi, Biochem. J., 2013, 452, 369 CrossRef CAS.
- M. J. Berridge, Physiol. Rev., 2016, 96, 1261 CrossRef CAS.
- B. Antonsson, Biochim. Biophys. Acta, Lipids Lipid Metab., 1997, 1348, 179 CrossRef CAS.
- T. Kinoshita, J. Lipid Res., 2016, 57, 4 CrossRef CAS.
- (a) B. L. Turner, M. J. Papházy, P. M. Haygarth and I. D. McKelvie, Philos. Trans. R. Soc., B, 2002, 357, 449 CrossRef CAS PubMed; (b) B. L. Turner, A. W. Cheesman, H. Y. Godage, A. M. Riley and B. V. L. Potter, Environ. Sci. Technol., 2012, 46, 4994 CrossRef CAS.
- (a) M. Horbowicz, P. Brenac and R. L. Obendorf, Planta, 1998, 205, 1 CrossRef CAS PubMed; (b) R. L. Obendorf, K. J. Steadman, D. J. Fuller, M. Horbowicz and B. A. Lewis, Carbohydr. Res., 2000, 328, 623 CrossRef CAS; (c) K. J. Steadman, M. S. Burgoon, R. L. Schuster, B. A. Lewis, S. E. Edwardson and R. L. Obendorf, J. Agric. Food Chem., 2000, 48, 2843 CrossRef CAS; (d) K. J. Steadman, D. J. Fuller and R. L. Obendorf, Carbohydr. Res., 2001, 331, 19 CrossRef CAS; (e) M. B. Cid, F. Alfonso and M. Martín-Lomas, Carbohydr. Res., 2004, 339, 2303 CrossRef CAS; (f) N. Yang and G. Ren, J. Agric. Food Chem., 2008, 56, 757 CrossRef CAS PubMed; (g) N. Yang and G. Ren, J. Agric. Food Chem., 2008, 56, 761 CrossRef CAS PubMed; (h) W. Gui, B. A. Lemley, I. Keresztes, A. M. Condo Jr, K. J. Steadman and R. L. Obendorf, Carbohydr. Res., 2013, 380, 130 CrossRef CAS; (i) D. Donati, L. R. Lampariello, R. Pagani, R. Guerranti, G. Cinci and E. Marinello, Phytother. Res., 2005, 19, 1057 CrossRef CAS PubMed; (j) L. B. Lahuta and T. Dzik, J. Plant Physiol., 2011, 168, 352 CrossRef CAS.
- (a) Z. Guo, Curr. Org. Synth., 2013, 10, 366 CrossRef CAS PubMed; (b) M. G. Paulick and C. R. Bertozzi, Biochemistry, 2008, 47, 6991 CrossRef CAS PubMed.
- Z. Guo and L. Bishop, Eur. J. Org. Chem., 2004, 3585 CrossRef CAS.
- A. V. Nikolaev and N. Al-Maharik, Nat. Prod. Rep., 2011, 28, 970 RSC.
- (a) J. Xue and Z. Guo, J. Am. Chem. Soc., 2003, 125, 16334 CrossRef CAS PubMed; (b) X. Wu and Z. Guo, Org. Lett., 2007, 9, 4311 CrossRef CAS.
- H. Paulsen and B. Mielke, Liebigs Ann. Chem., 1990, 169 CrossRef CAS.
- (a) T. Ogita, N. Otake, Y. Miyazaki and H. Yonehara, Tetrahedron Lett., 1980, 21, 3203 CrossRef CAS; (b) K. Isano, J. Antibiot., 1988, 41, 1711 CrossRef.
- M. Takahashi, T. Kagasaki, T. Hosoya and S. Takahashi, J. Antibiot., 1993, 46, 1643 CrossRef CAS.
- M. J. Berridge, Nature, 1993, 361, 315 CrossRef CAS PubMed.
- K. Mikoshiba, Adv. Biol. Regul., 2015, 57, 217 CrossRef CAS PubMed.
- I. Bosanac, J.-R. Alattia, T. K. Mal, J. Chan, S. Talarico, F. K. Tong, K. I. Tong, F. Yoshikawa, T. Furuichi, M. Iwai, T. Michikawa, K. Mikoshiba and M. Ikura, Nature, 2002, 420, 696 CrossRef CAS PubMed.
- K. J. Alzayady, L. Wang, R. Chandrasekhar, L. E. Wagner II, F. Van Petegem and D. I. Yule, Sci. Signaling, 2016, 9, ra35 CrossRef PubMed.
- A. M. Rossi and C. W. Taylor, J. Cell Sci., 2019, 132, jcs222463 CrossRef PubMed.
- M.-D. Seo, S. Velamakanni, N. Ishiyama, P. B. Stathopulos, A. M. Rossi, S. A. Khan, P. Dale, C. Li, J. B. Ames, M. Ikura and C. W. Taylor, Nature, 2012, 483, 108 CrossRef CAS PubMed.
- K. Hamada, H. Miyatake, A. Terauchi and K. Mikoshiba, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 4661 CrossRef CAS PubMed.
- M. Takahashi, K. Tanzawa and S. Takahashi, J. Biol. Chem., 1994, 269, 369 CAS.
- A. M. Rossi, A. M. Riley, B. V. L. Potter and C. W. Taylor, Current Topics in Membranes Series Elsevier, ed. I. Serysheva, Academic Press, Burlington, 2010, vol. 66, pp. 209–233 Search PubMed.
- H. J. Rosenberg, A. M. Riley, A. J. Laude, C. W. Taylor and B. V. L. Potter, J. Med. Chem., 2003, 46, 4860 CrossRef CAS PubMed.
- K. M. Sureshan, A. M. Riley, A. M. Rossi, S. C. Tovey, S. G. Dedos, C. W. Taylor and B. V. L. Potter, Chem. Commun., 2009, 45, 1204 RSC.
- E. P. Nerou, A. M. Riley, B. V. L. Potter and C. W. Taylor, Biochem. J., 2001, 355, 59 CrossRef CAS PubMed.
- (a) F. Chrètien, N. Moitessier, F. Roussel, J.-P. Mauger and Y. Chapleur, Curr. Org. Chem., 2000, 4, 513 CrossRef; (b) H. Hotoda, K. Murayama, S. Miyamoto, Y. Iwata, M. Takahashi, Y. Kawase, K. Tanzawa and M. Kaneko, Biochemistry, 1999, 38, 9234 CrossRef CAS PubMed; (c) V. A. Correa, A. M. Riley, S. Shuto, G. Horne, E. P. Nerou, R. D. Marwood, B. V. L. Potter and C. W. Taylor, Mol. Pharmacol., 2001, 59, 1206 CrossRef CAS PubMed; (d) K. M. Sureshan, A. M. Riley, M. P. Thomas, S. C. Tovey, C. W. Taylor and B. V. L. Potter, J. Med. Chem., 2012, 55, 1706 CrossRef CAS PubMed.
- S. T. Safrany, R. J. H. Wojcikiewicz, J. Strupish, S. R. Nahorski, D. Dubreuil, J. Chleophax, S. D. Gero and B. V. L. Potter, FEBS Lett., 1991, 278, 252 CrossRef CAS PubMed.
- G. Fan, M. R. Baker, Z. Wang, A. B. Seryshev, S. J. Ludtke, M. L. Baker and I. I. Serysheva, Cell Res., 2018, 28, 1158 CrossRef CAS PubMed.
- L. R. Comstock and J. M. Denu, Org. Biomol. Chem., 2007, 5, 3087 RSC.
- (a) A. K. Sanki and T. Pathak, Tetrahedron, 2003, 59, 7203 CrossRef CAS; (b) A. K. Sanki and T. Pathak, Synlett, 2002, 1241 CAS.
- F. Tagliaferri, S.-N. Wang, W. K. Berlin, R. A. Outten and T. Y. Shen, Tetrahedron, 1991, 47, 1 CrossRef.
- I. Cumpstey, Carbohydr. Res., 2009, 344, 2285 CrossRef CAS PubMed.
- (a) H. Paulsen and W. von Deyn, Liebigs Ann. Chem., 1987, 141 CrossRef CAS; (b) T. Akhtar and I. Cumpstey, Tetrahedron Lett., 2007, 48, 8673 CrossRef CAS; (c) T. Akhtar, L. Eriksson and I. Cumpstey, Carbohydr. Res., 2008, 343, 2094 CrossRef CAS PubMed.
- (a) C. V. S. Ramamurty, P. Ganney, C. S. Rao and B. Fraser-Reid, J. Org. Chem., 2011, 76, 2245 CrossRef CAS PubMed; (b) B. Fraser-Reid, P. Ganney, C. V. S. Ramamurty, A. M. Gómez and J. C. López, Chem. Commun., 2013, 49, 3251 RSC.
- (a) W. Koenigs and E. Knorr, Chem. Ber., 1901, 34, 957 CrossRef; (b) X. Zhu and R. R. Schmit, Angew. Chem., Int. Ed., 2009, 48, 1900 CrossRef CAS PubMed; (c) D. Crich, J. Org. Chem., 2011, 76, 9193 CrossRef CAS PubMed; (d) Z. Wimmer, L. Pechova and D. Saman, Molecules, 2004, 9, 902 CrossRef CAS PubMed.
- (a) J. Gigg, R. Gigg, S. Payne and R. Conant, Carbohydr. Res., 1985, 142, 132 CrossRef CAS; (b) S.-K. Chung and Y. Ryu, Carbohydr. Res., 1994, 258, 145 CrossRef CAS.
- D. R. Gauthier Jr and S. L. Bender, Tetrahedron Lett., 1996, 37, 13 CrossRef.
- G. Reyes-Rangel, J. Vargas-Caporali and E. Juaristi, Tetrahedron, 2016, 72, 379 CrossRef CAS.
- M. Sridhar, B. A. Kumar and R. Narender, Tetrahedron Lett., 1998, 39, 2847 CrossRef CAS.
- H. Gerlach, Helv. Chim. Acta, 1985, 68, 1815 CrossRef CAS.
- (a) J. Gigg, R. Gigg, S. Payne and R. Conant, J. Chem. Soc., Perkin Trans. 1, 1987, 1757 RSC; (b) A. M. Riley, H. Wang, S. B. Shears and B. V. L. Potter, Chem. Commun., 2015, 51, 12605 RSC.
- L. D. Nord, N. K. Dalley, P. A. McKernan and R. K. Robins, J. Med. Chem., 1987, 30, 1044 CrossRef CAS.
- (a) E. A. Mash, L. T. A. Kantor and S. C. Waller, Synth. Commun., 1997, 27, 507 CrossRef CAS; (b) V. Saikam, S. Dara, M. Yadav, P. P. Singh and R. A. Vishwakarma, J. Org. Chem., 2015, 80, 11916 CrossRef CAS PubMed; (c) M. Giordano and A. Iadonisi, J. Org. Chem., 2014, 79, 213 CrossRef CAS PubMed.
- (a) S. David and S. Hanessian, Tetrahedron, 1985, 41, 643 CrossRef CAS; (b) D. Wagner, J. Verheyden and J. G. Moffatt, J. Org. Chem., 1974, 39, 24 CrossRef CAS; (c) C. Augé, S. David and A. Veyrières, Chem. Commun., 1976, 375 RSC; (d) H. Takaku, K. Kamaike and H. Tsuchiya, J. Org. Chem., 1984, 49, 51 CrossRef CAS; (e) A. Nashed and L. Anderson, Tetrahedron Lett., 1976, 17, 3503 CrossRef; (f) M. A. Nashed and L. Anderson, Carbohydr. Res., 1977, 56, 419 CrossRef CAS; (g) M. A. Nashed, Carbohydr. Res., 1978, 60, 200 CrossRef CAS.
- L. Yan and D. Kahne, Synlett, 1995, 523 CrossRef CAS.
- (a) T. Wada, T. Moriguchi and M. Sekine, J. Am. Chem. Soc., 1994, 116, 9901 CrossRef CAS; (b) M. L. Wolfrom, P. J. Conigliaro and E. J. Soltes, J. Org. Chem., 1967, 32, 653 CrossRef CAS PubMed.
- (a) A. H. Fauq, A. P. Kozikowski, V. I. Ognyanov, R. A. Wilcox and S. R. Nahorski, Chem. Commun., 1994, 1301 RSC; (b) P. B. Brady, E. M. Morris, O. S. Fenton and B. R. Sculimbrene, Tetrahedron Lett., 2009, 50, 975 CrossRef CAS; (c) C. Moreau, T. Kirchberger, J. M. Swarbrick, S. J. Bartlett, R. Fliegert, T. Yorgan, A. Bauche, A. Harneit, A. H. Guse and B. V. L. Potter, J. Med. Chem., 2013, 56, 10079 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental protocols and NMR spectra. See DOI: 10.1039/c9sc00445a |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2019 |