Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Consecutive HDDA and TDDA reactions of silicon-tethered tetraynes for the synthesis of dibenzosilole-fused polycyclic compounds and their unique reactivity†
Akihito
Mitake
a,
Rikako
Nagai
a,
Ayato
Sekine
a,
Hideaki
Takano
a,
Natsuhiko
Sugimura
b,
Kyalo Stephen
Kanyiva
 c and
Takanori
Shibata
c and
Takanori
Shibata
 *a
*a
aDepartment of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, Shinjuku, Tokyo 169-8555, Japan. E-mail: tshibata@waseda.jp; Fax: +81-3-5286-8098
bMaterials Characterization Central Laboratory, School of Advanced Science and Engineering, Waseda University, Shinjuku, Tokyo 169-8555, Japan
cGlobal Center for Science and Engineering, School of Advanced Science and Engineering, Waseda University, Shinjuku, Tokyo 169-8555, Japan
First published on 30th May 2019
Abstract
Silicon-tethered tetraynes possessing a 1,3-diyne moiety underwent consecutive hexadehydro- and tetradehydro-Diels–Alder reactions to give a series of fused polycyclic aromatic compounds containing a dibenzosilole skeleton. The benzene ring in the product acted as a 1,3-diene and reacted with the active alkyne as well as oxygen to provide [4 + 2] cycloadducts.
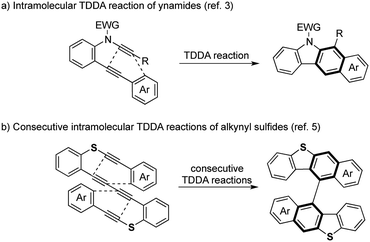
While the Diels–Alder reaction is a [4 + 2] cycloaddition of a 1,3-diene and an alkene, the dehydro-Diels–Alder (DDA) reaction involves an alkyne moiety(ies) in the substrate(s).1 For example, the tetradehydro-Diels–Alder (TDDA) reaction of a 1,3-enyne and an alkyne gives a substituted benzene ring with perfect atom-economy via a strained cyclic allene intermediate along with a 1,5-hydrogen shift. In particular, the intramolecular TDDA reaction of arylalkynes, where part of the arene acts as an ene moiety, is attractive, because fused polycyclic aromatic compounds can be prepared in one pot. Saá is a pioneer in the synthetic use of TDDA and comprehensively studied an intramolecular reaction of diarylacetylene and alkyne;2 the reaction of ynamides gave carbazole derivatives (Scheme 1a).3 Our group also reported an intramolecular TDDA reaction for the synthesis of binaphthyl compounds.4 Recently, we developed the consecutive intramolecular TDDA reaction of sulfur-tethered tetraynes for the preparation of axially chiral bis(benzothiophene) derivatives and further upgraded this transformation to an enantioselective synthesis by a chiral metal-catalyzed reaction (Scheme 1b).5
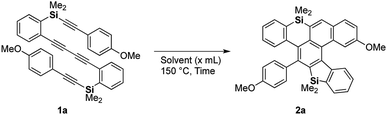
Against this background, we next examined the thermal reaction using silicon analogue 1a6 in hot toluene for the preparation of an axially chiral bis(benzosilole) derivative. Unexpectedly, we obtained dibenzosilole-fused heptacyclic compound 2a, the structure of which was finally decided upon based on X-ray analysis (Scheme 2). We considered that consecutive intramolecular DDA reactions gave polycyclic compounds. The first step is a hexadehydro-Diels–Alder (HDDA) reaction of 1,3-diyne and alkyne to give benzosilole-fused benzyne and the second step is a TDDA reaction with the remaining arylalkyne moiety. Since the first report of the HDDA reaction,7a Hoye reported various reagents for trapping of the reactive benzyne intermediates.7b–v Recently, consecutive HDDA reactions were developed for the one-pot synthesis of fused polycyclic compounds.7w In contrast, we demonstrate here the first example of consecutive HDDA and TDDA reactions as well as a HDDA reaction along with [2 + 2 + 2 + 2] cycloaddition. We further discuss the unique reactivity of the benzene moiety of the silicon-containing polycyclic compound.
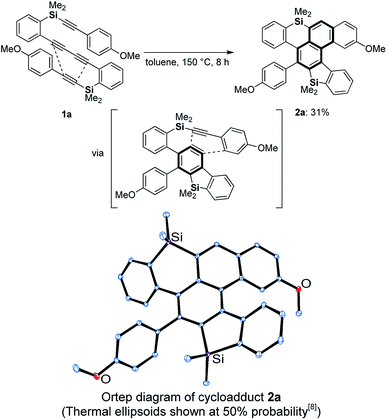 | ||
| Scheme 2 Consecutive HDDA and TDDA reactions of silicon-tethered tetrayne 1a possessing a 1,3-diyne moiety. | ||
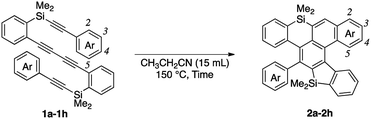
We chose 1,4-bis(2-(dimethyl-(2-(4-methoxyphenyl)ethynyl)-silyl)phenyl)buta-1,3-diyne (1a) as a model substrate and screened the thermal conditions for the consecutive HDDA and TDDA reactions (Table 1). When dibutyl ether was used, tetrayne 1a was completely consumed within 8 h and dibenzosilole-fused cycloadduct 2a was obtained in moderate NMR yield (entry 1). While benzonitrile realized a yield comparable to that with the etherate solvent, propionitrile gave the best yield of 72% with a longer reaction time (entries 2 and 3). After we investigated the concentration effect, the yield was improved to 83% under dilute conditions, but with a prolonged reaction time (entries 3–5). Tetrayne 1a was completely consumed within 1 h under microwave irradiation, but the reaction became messy, and the yield of 2a was low (entry 6). We determined that entry 5 represented the best conditions.
| Entry | Solvent (x mL) | Time (h) | NMR yieldb (%) |
|---|---|---|---|
| a The reaction was conducted on a 0.05 mmol scale. b Yields were determined by NMR using 1,1,2,2-tetrachloroethane as an internal standard. c The reaction was conducted on a 0.03 mmol scale under microwave irradiation. | |||
| 1 | Dibutyl ether (5) | 8 | 56 |
| 2 | PhCN (5) | 4 | 52 |
| 3 | CH3CH2CN (5) | 48 | 72 |
| 4 | CH3CH2CN (1.7) | 48 | 41 |
| 5 | CH3CH2CN (15) | 48 | 83 |
| 6c | CH3CH2CN (3) | 1 | 27 |
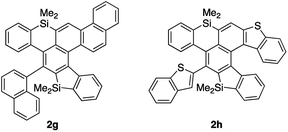
We next examined the substrate scope of aryl groups on the alkyne termini in propionitrile at 150 °C (Table 2). Compound 2a was obtained in 81% isolated yield. Phenyl- and 4-fluorophenyl-substituted tetraynes 1b and 1c were transformed into the corresponding heptacyclic compounds 2b and 2c in moderate yields. Electron-rich and ortho-substituted arenes could also be used and cycloadducts 2d and 2e9 were obtained in moderate yields. In the case of 4-biphenyl-substituted product 2f, the yield was low because it was difficult to isolate due to its high crystallinity. The reactions of 1-naphthyl and 2-benzothiophenyl-substituted tetraynes 1g and 1h also proceeded to give octacyclic cycloadduct 2g9 and dihetero[6]helicene 2h consisting of dibenzothiophene and dibenzosilole, respectively.
| Entry | Ar | Time (h) | Yield (%) |
|---|---|---|---|
| a Tetrayne (0.05 mmol) and propionitrile (15 ml) were used and the product was purified by preparative TLC. b Yield in parenthesis was determined by NMR using 1,1,2,2-tetrachloroethane as an internal standard. | |||
| 1 | 4-MeOC6H4 | 40 | 81 (2a) |
| 2 | C6H5 | 48 | 55 (2b) |
| 3 | 4-FC6H4 | 48 | 51 (2c) |
| 4 | 3,5-(MeO)2C6H3 | 24 | 56 (2d) |
| 5 | 2-MeOC6H4 | 40 | 52 (2e) |
| 6 | 4-PhC6H4 | 24 | 43 (53)b (2f) |
| 7 | 1-Naphthyl | 24 | 53 (2g) |
| 8 | 2-Benzothienyl | 24 | 51 (2h) |
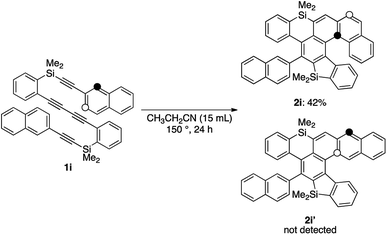
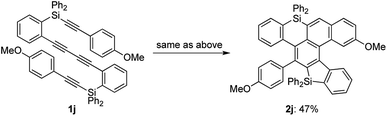
While the reaction of 2-naphthyl-substituted tetrayne 1i possibly affords two regioisomers 2i and 2i′, sila[6]helicene 2i was the only cycloadduct detected, probably due to the higher reactivity of the α-position of the naphthyl group (Scheme 3). Diphenylsilyl-tethered tetrayne 1j was also transformed into the corresponding cycloadduct 2j (Scheme 4).
We conducted mechanism study in order to figure out why silicon-tethered tetraynes underwent the HDDA reaction, not the TDDA reaction. As shown by the results of DFT calculations using triyne A as a model substrate, the activation energy of both HDDA and TDDA reactions via diradical intermediates7k was large (54.3 and 49.0 kcal mol−1, respectively). In contrast, the concerted pathway showed smaller activation energy; moreover, the concerted HDDA reaction (27.1 kcal mol−1) was clearly more favorable than the concerted TDDA reaction (35.6 kcal mol−1) (Fig. 1).10
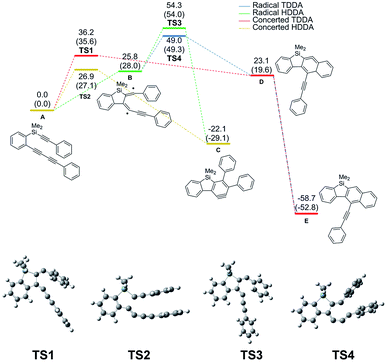 | ||
| Fig. 1 Relative Gibbs free energy (ΔG) diagram of Si-tethered triyne A at 423.15 K (kcal mol−1). Relative electronic energy (ΔE) is in parentheses. | ||
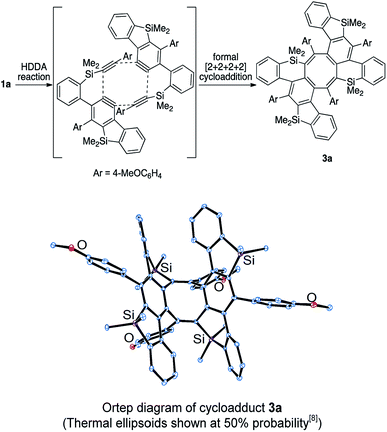
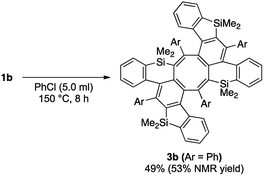
In all of the entries above, some by-products were always formed. Among them, the major isolated by-products were cyclooctatetraene derivatives. The structure of the by-product in the reaction of 1a was finally determined to be saddle-shaped compound 3a by X-ray crystallographic analysis (Scheme 5 below). We considered the mechanism to be dimerization of in situ-generated benzosilole-fused benzyne (Scheme 5 above). To the best of our knowledge, this is the first example of the thermal [2 + 2 + 2 + 2] cycloaddition of alkynes for the construction of an eight-membered ring system.11 Therefore, we further examined the reaction conditions.12 As a result, 3b was obtained in moderate yield as a major product under more concentrated conditions in chlorobenzene (Scheme 6).13
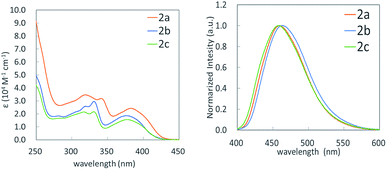
We measured UV-vis and fluorescence spectra of benzosilole-fused polycyclic compounds 2a–2c in dichloromethane as well as their quantum yields in both dichloromethane solution and the solid state (Fig. 2 and Table 3). These compounds are fluorescent and their quantum yields in the solid state were higher than those in solution. Substitution at the 6- and 15-positions did not affect their photophysical properties. The Stokes shifts were twice as much as those for simple sila[5]helicene,14 probably due to the fused siline ring. The torsion angle of the silahelicene moiety of 2a was 27.0°, which was significantly larger than that of the simple sila[5]helicene (17.6°).14
| Comp. | λ max(abs) [nm], (ε [×104 cm−1 M−1]) | λ max(emi) , [nm] | Φ , (solution, solid) |
|---|---|---|---|
| a UV-vis and fluorescence spectra of 2 were measured in CH2Cl2. b 2a 1.7 × 10−5 M; 2b 2.1 × 10−5 M; 2c 3.0 × 10−5 M. c 2a 1.7 × 10−6 M; 2b 2.1 × 10−6 M; 2c 3.0 × 10−6 M. d Excitation wavelength: 2a 383 nm; 2b 376 nm; 2c 377 nm. | |||
| 2a | 319 (3.5), 341 (3.2), 383 (2.4) | 459 | 0.15, 0.22 |
| 2b | 319 (2.6), 331 (3.0), 376 (1.9) | 463 | 0.14, 0.21 |
| 2c | 317 (2.1), 331 (2.2), 377 (1.6) | 458 | 0.14, 0.16 |
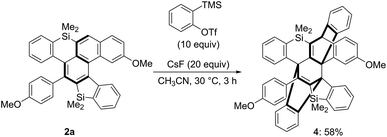
We further investigated the reactivity of the unique π-system containing silicon atoms. We first conducted the reaction of silahelicene 2a with excess amounts of in situ-generated benzyne as a reactive alkyne; consecutive [4 + 2] cycloaddition proceeded at 30 °C for 3 h to give polycyclic compound 4 possessing two bridged systems, the structure of which was confirmed by X-ray crystallographic analysis (Scheme 7).15 Even when the amount of the benzyne precursor was decreased, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cycloadduct could not be detected.
1 cycloadduct could not be detected.
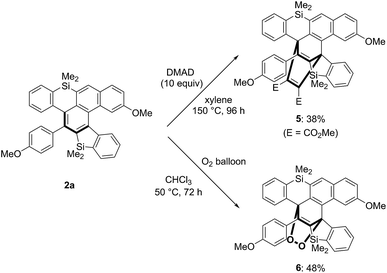
While the [4 + 2] cycloaddition of 2a with dimethyl acetylenedicarboxylate (DMAD) required a high reaction temperature as well as a long reaction time, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cycloadduct 5 was the only detectable product (Scheme 8 above).16 Interestingly, the reaction of 2a proceeded under an atmospheric pressure of oxygen at 50 °C to give cyclic peroxide 6 in moderate yield, the structure of which was also confirmed by X-ray crystallographic analysis (Scheme 8 below).17
1 cycloadduct 5 was the only detectable product (Scheme 8 above).16 Interestingly, the reaction of 2a proceeded under an atmospheric pressure of oxygen at 50 °C to give cyclic peroxide 6 in moderate yield, the structure of which was also confirmed by X-ray crystallographic analysis (Scheme 8 below).17
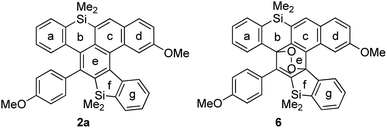
To explain the high reactivity with oxygen, we calculated the values of NICS(0) for cycloadduct 2a and cyclic peroxide 6 (Table 4).18 The aromaticities of rings c and e of 2a were relatively weak, because they are fused with the antiaromatic siline (ring b). Ring c gained aromaticity by [4 + 2] cycloaddition with oxygen (−3.30 to −7.22).
| Ring | 2a | 6 |
|---|---|---|
| a GIAO B3LYP/6-31+G(d,p). | ||
| a | −6.09 | −6.89 |
| b | 4.50 | 3.68 |
| c | −3.30 | −7.22 |
| d | −8.27 | −7.75 |
| e | −5.03 | 2.46 |
| f | 4.39 | 2.25 |
| g | −4.85 | −6.22 |
In conclusion, we have developed the first example of consecutive HDDA and TDDA reactions using silicon-tethered tetraynes possessing a 1,3-diyne moiety via benzosilole-fused benzynes. The obtained silicon-containing polycyclic aromatic compound acted as a diene and underwent [4 + 2] cycloaddition with active alkynes. Notably, it could react with non-activated oxygen to give a cyclic peroxide.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly executed under the cooperation of organization between Waseda University and JXTG Nippon Oil & Energy Corporation. This work was also supported by a Grant-in-Aid for Scientific Research (C) (No. 19K05485) from JSPS.Notes and references
- For selected reviews of DDA reactions, see; (a) P. Wessig and G. Müller, Chem. Rev., 2008, 108, 2051–2063 CrossRef CAS PubMed; (b) W. Li, L. Zhou and J. Zhang, Chem.–Eur. J., 2016, 22, 1558–1571 CrossRef CAS PubMed.
- (a) D. Rodríguez, A. Navarro, L. Castedo, D. Domínguez and C. Saá, Org. Lett., 2000, 2, 1497–1500 CrossRef; (b) D. Rodríguez, A. Navarro-Vázquez, L. Castedo, D. Domínguez and C. Saá, J. Org. Chem., 2003, 68, 1938–1946 CrossRef PubMed; (c) D. Rodríguez, M. F. Martínez-Esperón, A. Navarro-Vázquez, L. Castedo, D. Domínguez and C. Saá, J. Org. Chem., 2004, 69, 3842–3848 CrossRef PubMed.
- (a) M. F. Martínez-Esperón, D. Rodríguez, L. Castedo and C. Saá, Org. Lett., 2005, 7, 2213–2216 CrossRef PubMed; (b) M. F. Martínez-Esperón, D. Rodríguez, L. Castedo and C. Saá, Tetrahedron, 2006, 62, 3843–3855 CrossRef; (c) M. F. Martínez-Esperón, D. Rodríguez, L. Castedo and C. Saá, Tetrahedron, 2008, 64, 3674–3686 CrossRef.
- T. Shibata, R. Fujiwara and D. Takano, Synlett, 2005, 2062–2066 CrossRef CAS.
- T. Shibata, A. Sekine, A. Mitake and K. S. Kanyiva, Angew. Chem., Int. Ed., 2018, 57, 15862–15865 CrossRef CAS PubMed.
- The silicon-tethered tetrayne 1a was subjected to the consecutive intramolecular [2 + 2 + 2] cycloaddition with an ortho-phenylene-tethered diyne for the preparation of a helically chiral disila[7]helicene: T. Shibata, T. Uchiyama, Y. Yoshinami, S. Takayasu, K. Tsuchikama and K. Endo, Chem. Commun., 2012, 48, 1311–1313 RSC.
- The first report of the HDDA reaction: (a) T. R. Hoye, B. Baire, D. Niu, P. H. Willoughby and B. P. Woods, Nature, 2012, 490, 208–212 CrossRef CAS. Examples of the reaction of benzyne generated by HDDA with various trapping reagents: (b) D. Niu, P. H. Willoughby, T. R. Hoye, B. P. Wood and B. Baire, Nature, 2013, 501, 531–534 CrossRef CAS PubMed; (c) D. Niu and T. R. Hoye, Nat. Chem., 2014, 6, 34–40 CrossRef CAS PubMed; (d) T. R. Hoye, B. Baire and T. Wang, Chem. Sci., 2014, 5, 545–550 RSC; (e) D. Niu, T. Wang, B. P. Woods and T. R. Hoye, Org. Lett., 2014, 16, 254–257 CrossRef CAS; (f) J. Chen, B. Baire and T. R. Hoye, Heterocycles, 2014, 88, 1191–1200 CrossRef CAS PubMed; (g) P. H. Willoughby, D. Niu, T. Wang, M. K. Haj, C. J. Cramer and T. R. Hoye, J. Am. Chem. Soc., 2014, 136, 13657–13665 CrossRef CAS PubMed; (h) V. D. Pogula, T. Wang and T. R. Hoye, Org. Lett., 2015, 17, 856–859 CrossRef CAS PubMed; (i) Q. L. Nguyen, B. Baire and T. R. Hoye, Tetrahedron Lett., 2015, 56, 3265–3267 CrossRef PubMed; (j) J. Chen, V. Palani and T. R. Hoye, J. Am. Chem. Soc., 2016, 138, 4318–4321 CrossRef CAS PubMed; (k) T. Wang, D. Niu and T. R. Hoye, J. Am. Chem. Soc., 2016, 138, 7832–7835 CrossRef CAS PubMed; (l) T. Wang and T. R. Hoye, J. Am. Chem. Soc., 2016, 138, 13870–13873 CrossRef CAS PubMed; (m) J. Zhang, D. Niu, V. A. Brinker and T. R. Hoye, Org. Lett., 2016, 18, 5596–5599 CrossRef CAS PubMed; (n) F. Xu, X. Xiao and T. R. Hoye, Org. Lett., 2016, 18, 5636–5639 CrossRef CAS PubMed; (o) V. Palani, J. Chen and T. R. Hoye, Org. Lett., 2016, 18, 6312–6315 CrossRef CAS PubMed; (p) S. P. Ross and T. R. Hoye, Nat. Chem., 2017, 9, 523–530 CrossRef CAS PubMed; (q) S. P. Ross, B. Baire and T. R. Hoye, Org. Lett., 2017, 19, 5705–5708 CrossRef CAS PubMed; (r) Y. Wang and T. R. Hoye, Org. Lett., 2018, 20, 88–91 CrossRef CAS PubMed; (s) S. P. Ross and T. R. Hoye, Org. Lett., 2018, 20, 100–103 CrossRef CAS PubMed; (t) J. Zhang, A. C. S. Page, V. Palani, J. Chen and T. R. Hoye, Org. Lett., 2018, 20, 5550–5553 CrossRef CAS PubMed; (u) Y. Wang, L. Zheng and T. R. Hoye, Org. Lett., 2018, 20, 7145–7148 CrossRef CAS PubMed; (v) S. Arora, V. Palani and T. R. Hoye, Org. Lett., 2018, 20, 8082–8085 CrossRef CAS PubMed. Example of recent consecutive HDDA reactions: (w) X. Xiao and T. R. Hoye, Nat. Chem., 2018, 10, 838–844 CrossRef CAS PubMed.
- CCDC 1826048 for 2a, CCDC 1826049 for 3a, CCDC 1890968 for 4, CCDC 1830394 for 6 contain the supplementary crystallographic data for this paper.†.
- Compounds 2e and 2g have axial chirality as well as helical chirality, and they were obtained as a diastereomeric mixture.
- A tetrayne tethered by a methylene moiety in place of a dimethylsilyl one underwent consecutive intramolecular TDDA reactions to afford a bibenzo[b]fluorene derivative, which was shown in detail in the ESI.†.
- Example of thermal [2 + 2 + 2 + 2] cycloaddition of an alkene: (a) P. Camps, J. A. Fernández, S. Vázquez, M. Font-Bardia and X. Solans, Angew. Chem., Int. Ed., 2003, 42, 4049–4051 CrossRef CAS. Example of metal-catalyzed [2 + 2 + 2 + 2] cycloaddition of an alkyne: (b) P. A. Wender, M. P. Croatt and B. Kühn, Organometallics, 2009, 28, 5841–5844 CrossRef CAS; (c) D. J. Nasrallah and M. P. Croatt, Eur. J. Org. Chem., 2014, 18, 3767–3772 CrossRef. Example of photochemical [2 + 2 + 2 + 2] cycloaddition of furan to β-naphthonitrile: (d) T. Sugioka, C. Pac and H. Sakurai, Chem. Lett., 1972, 9, 791–792 CrossRef.
- The table for the reaction condition screening is listed in ESI.†.
- To improve the yield of formal [2 + 2 + 2 + 2] cycloadduct, we tried to suppress the TDDA reaction of the second step. But the reaction of n-butyl-substituted tetrayne did not proceed at all. In the reaction of 2,6-dimethoxy-substituted tetrayne, the benzyne intermediate from the HDDA reaction was probably formed, but the formation of the [2 + 2 + 2 + 2] cycloadduct could not be detected.
- M. Murai, R. Okada, A. Nishiyama and K. Takai, Org. Lett., 2016, 18, 4380–4383 CrossRef CAS PubMed.
- S. Toyota, A. Takau, Y. Hitaka, M. Oki and K. Wakamatsu, Bull. Chem. Soc. Jpn., 2005, 78, 2228–2234 CrossRef CAS.
- Y. Kim, Z. Zhu and T. M. Swager, J. Am. Chem. Soc., 2004, 126, 452 CrossRef CAS PubMed.
- H. Kotani, K. Ohkubo and S. Fukuzumi, J. Am. Chem. Soc., 2004, 126, 15999–16006 CrossRef CAS PubMed.
- P. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao and N. J. R. v. E. Hommes, J. Am. Chem. Soc., 1996, 118, 6317–6318 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1826048, 1826049, 1890968 and 1830394. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc00960d |
| This journal is © The Royal Society of Chemistry 2019 |