Open Access Article
Open Access ArticleStereoselective total synthesis of parthenolides indicates target selectivity for tubulin carboxypeptidase activity†
Robert R. A.
Freund
 a,
Philipp
Gobrecht
b,
Zhigang
Rao
c,
Jana
Gerstmeier
c,
Robin
Schlosser
a,
Helmar
Görls
d,
Oliver
Werz
c,
Dietmar
Fischer
a,
Philipp
Gobrecht
b,
Zhigang
Rao
c,
Jana
Gerstmeier
c,
Robin
Schlosser
a,
Helmar
Görls
d,
Oliver
Werz
c,
Dietmar
Fischer
 b and
Hans-Dieter
Arndt
b and
Hans-Dieter
Arndt
 *a
*a
aInstitut für Organische Chemie und Makromolekulare Chemie, Friedrich-Schiller-Universität, Humboldtstr. 10, 07743 Jena, Germany. E-mail: hd.arndt@uni-jena.de
bLehrstuhl für Zellphysiologie, Ruhr-Universität Bochum, Universitätsstr. 150, ND/4, 44780 Bochum, Germany
cInstitut für Pharmazie, Friedrich-Schiller-Universität, Philosophenweg 14, 07743 Jena, Germany
dInstitut für Anorganische Chemie und Analytische Chemie, Friedrich-Schiller-Universität, Humboldtstr. 8, 07743 Jena, Germany
First published on 26th June 2019
Abstract
The 2-(silyloxymethyl)allylboration of aldehydes was established to enable stereoselective access to α-(exo)-methylene γ-butyrolactones under mild conditions. Acid-labile functionality and chiral carbonyl compounds are tolerated. Excellent asymmetric induction was observed for β,β′-disubstituted α,β-epoxy aldehydes. These findings led to the enantioselective total synthesis of the sesquiterpene natural product (−)-parthenolide, its unnatural (+)-enantiomer, and diastereoisomers. Among all the isomers tested in cell culture, only (−)-parthenolide showed potent inhibition of microtubule detyrosination in living cells, confirming its exquisite selectivity on tubulin carboxypeptidase activity. On the other hand, the anti-inflammatory activity of the parthenolides was weaker and less selective with regard to compound stereochemistry.
Introduction
α-(exo)-Methylene γ-butyrolactones (αMγBs) contribute to the biological activity of more than 5000 natural products.1 Many of these show interesting potential in chemical biology and drug development.1,2 A prominent αMγB natural product is the sesquiterpenoid (−)-parthenolide (1),3 an anti-inflammatory natural product from feverfew (Tanacetum parthenium) that induces apoptosis in cancer (stem) cells.4 Its mode of action is complex and features inhibition of NF-κB activation,5 alteration of DNA methylation,6 induction of oxidative stress,7 and inhibition of tubulin carboxypeptidase (TCP) activity.8 The latter has become a highly important focus for neuroregeneration8c,d,9 and heart failure treatment research.10 Importantly, TCP has only recently been identified on the genetic level8d,11 and its activation and regulation are still difficult to study. Tool compounds with specific activity on TCP would have significant importance for medical research and potentially drug development. In this regard, for enabling thorough studies of parthenolide, a fully stereocontrolled de novo synthesis of the natural product was sought that is based on exchangeable building blocks and provides liberal access to stereoisomers for studying target selectivity.Previously reported syntheses of parthenolide did not allow a comprehensive study (Scheme 1). Chen and co-workers employed the linear epoxy aldehyde 2a,b and an intramolecular, non-diastereoselective Nozaki–Hiyama–Kishi allylation (a), followed by (Z → E) isomerization of the internal alkene.12 The lactone was obtained after aminolysis of the nitrile and refunctionalization (d). A second synthesis featured an aldol addition of chiral camphorsultam derivative 4a,b to α,β-unsaturated aldehyde 3 (a), leading to a separable product mixture.13 Refunctionalization, intramolecular alkylation (b) hydroxyl-directed epoxidation and oxidative lactonization (d) gave 1. Recently, Liu and co-workers reported a racemic parthenolide synthesis14 based on Still's ring enlarging oxy-Cope rearrangement of 1,5-diene (±)-5 (b), followed by (Z → E) isomerization of an internal alkene.15 α-Alkylation of the resulting ketone with ethyl bromoacetate (6, c), reduction, epoxidation, oxidative lactonization (d) and α-methylenation (7) completed the synthesis. Additionally, syntheses of parthenolide's natural precursor costunolide (8) were described,16 from which parthenolide is available.17 Various syntheses leading to diastereomers of the natural product have been reported as well.12,18
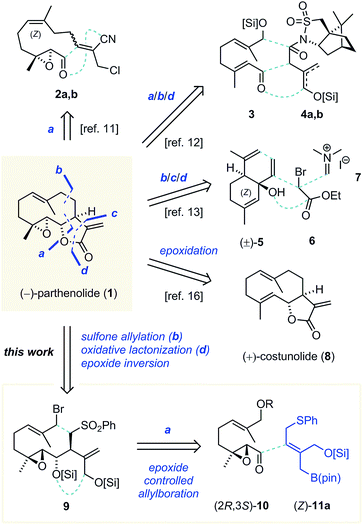 | ||
| Scheme 1 Previous targeted syntheses of parthenolides and stereodivergent synthesis planning of this work, revealing a reactive 2-(silyloxy-methyl)allylboronate. [Si] = SiR1R2R3. | ||
In order to realize a modular, enantioselective, and stereocontrolled synthesis, we envisioned to use an intramolecular sulfone allylation to close the ten-membered ring (Scheme 1, bottom).13,19 Precursor 9 already contains the epoxide motif and is geared for late stage oxidative lactonization to form the reactive αMγB.17,18b Several indirect as well as direct syntheses of αMγBs have been reported.1a,b Among them, allylboration methods offered predictable high stereoselectivity,1b,20 which has been a major challenge in previous parthenolide syntheses. Accordingly, diastereoselective allylboration directed by the epoxide's and the boronate's stereochemistry, simplifies sulfone 2 to the chiral α,β-epoxy aldehyde 10 and the novel 2-(silyloxymethyl)allylboronate (Z)-11a. The epoxide group was expected to provide 1,2-asymmetric induction,21 but its level and its sense (Felkin or anti-Felkin) were not clear for these unknown allylboronates at the onset of our studies. However, by using either enantiomer of the epoxy aldehyde, and additionally epoxide inversion, any desired stereoisomer should be obtained (see also Scheme 5 below).
Moreover, by design, the allylboronate 11a should operate as a “linchpin” reagent for αMγB synthesis in general. Being electron rich, it was expected to display intrinsically high allylation rates and to reliably install the trans configuration of the αMγB (→1).20 Hence, these allylboronates should allow allylboration-based αMγB synthesis from acid labile carbonyl compounds such as aldehyde 10. These substrates are incomepatible with 2-(alkoxycarbonyl)allylboration reagents that demand activation by either Lewis/Brønsted acids or elevated temperature.20,22 Hence, a significant scope and broader applicability were expected for these new allyl boronates.
Results and discussion
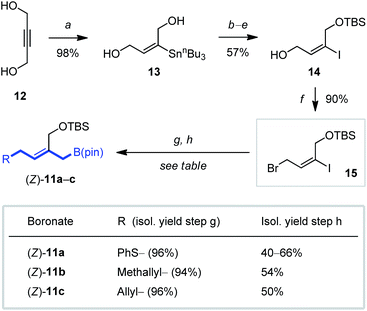
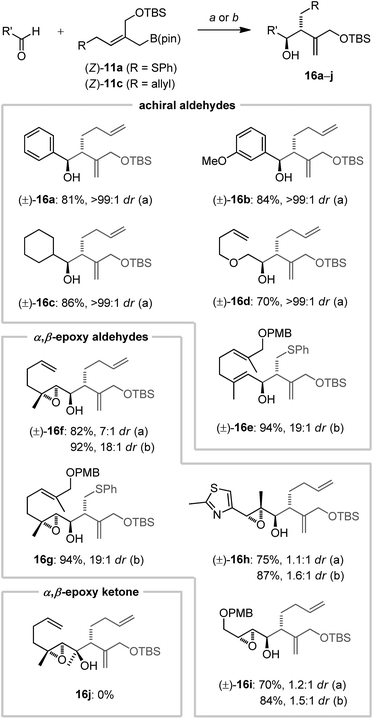
To explore the scope and feasibility of this approach and the utility of the reagents envisioned, we prepared the pseudo-trans configured (Z)-2-(silyloxymethyl)allylboronates (Z)-11a–c (Scheme 2) starting from 2-butyne-1,4-diol (12) by using a combination of syn-selective hydrostannylation23 and regioselective functionalization to obtain the versatile alkenyl halide 15 (50% yield, 6 steps). Introduction of a desired side chain by nucleophilic substitution, followed by a Negishi coupling with Knochel's IZnCH2B(pin) reagent24 allowed then the stereo- and regioselective preparation of the stable 2-(silyloxymethyl)allylboronates (Z)-11a–c in good yields (Scheme 2).Next, reagents (Z)-11a–c were investigated for allylboration of achiral aldehydes and chiral α,β-epoxy carbonyl compounds (for their preparation see the ESI†), where focus was placed on acid-labile features (Scheme 3). Gratifyingly, allylboration proceeded cleanly between 0 and 25 °C without the addition of external acidic activators. In fact, buffering by using solid NaHCO3 was found essential to obtain reproducible yields, especially for more labile aldehydes. Optimized conditions for the reaction of boronates (Z)-11a or (Z)-11c with aryl (→16a,b), alkyl (→16c) as well as a β-alkoxy aldehydes (→16d) gave the homoallylic alcohol products in very good yield and excellent diastereoselectivity (Scheme 3). An α,β-unsaturated aldehyde (→16e) was equally effective (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). α,β-Epoxy aldehyde substrates were investigated next. All aldehydes with α,β,β′ epoxide substitution patterns (→16f,g) were transformed with excellent yield and >18
1 dr). α,β-Epoxy aldehyde substrates were investigated next. All aldehydes with α,β,β′ epoxide substitution patterns (→16f,g) were transformed with excellent yield and >18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. In contrast, for the reactions of an α,α′,β trisubstituted (→16h) or an α,β disubstituted epoxy aldehyde (→16i) negligible 1,2-asymmetric induction was observed.
1 dr. In contrast, for the reactions of an α,α′,β trisubstituted (→16h) or an α,β disubstituted epoxy aldehyde (→16i) negligible 1,2-asymmetric induction was observed.
Substitution at the epoxide's β′ position seems to be key for diastereocontrol during allylboration. Notably, an α,β-epoxy ketone (16j) remained unreactive. The relative stereochemistry of alcohol 16f was assigned by NOESY NMR of a cyclized derivative (see the ESI†) which indicated strict Felkin control.21c Hence, the excellent stereocontrol of these novel allylboronates could be exploited for the synthesis of 4,5-dia-parthenolide (22). By using a late stage epoxide inversion, the natural parthenolide (1) could be synthesized.
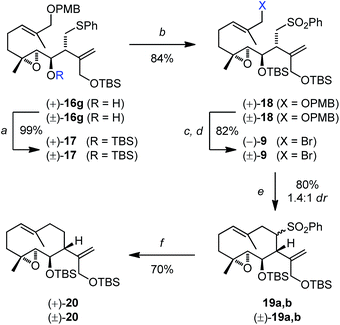
Consequently, the allylboration product (+)-16g (92% ee) was cleanly protected with a TBS group (→(+)-17, Scheme 4) at low temperature, to suppress (Lewis-) acid induced epimerization. Chemoselective oxidation of the thioether gave the sulfone (+)-18. The PMB ether of 18 was cleaved by treatment with DDQ in a quaternary aqueous solvent mixture, which drastically reduced the reaction time and reagent amount needed, compared to common biphasic mixtures. After work up with NaBH4 (to reduce some enal byproduct formed), the allylic alcohol obtained was transformed into the allylic bromide (−)-9 by mesylation and in situ substitution with LiBr (82% over 2 steps). Structure and relative configuration of (±)-9 were verified by single-crystal X-ray analysis (see the ESI†). Closure of the ten-membered ring was then achieved by intramolecular substitution. Optimized conditions featured addition of the sulfone 9 to excess KHMDS at 0 °C in THF at a final dilution of 4 mM, giving rise to the separable sulfone diastereomers 19a,b. The yield of this challenging transformation was somewhat scale dependent. Under optimized conditions the reaction could be performed on viable scale (100 mg) in high yield (80%). Next, the sulfone diastereomers 19a,b were reductively desulfonylated with sodium amalgam,25 providing epoxy germacrane (+)-20 in good yield (70%). Other reductive conditions, such as Mg/MeOH13 or [Na(15-c-5)]e/THF,26 led to the formation of inseparable side products or overreduction.
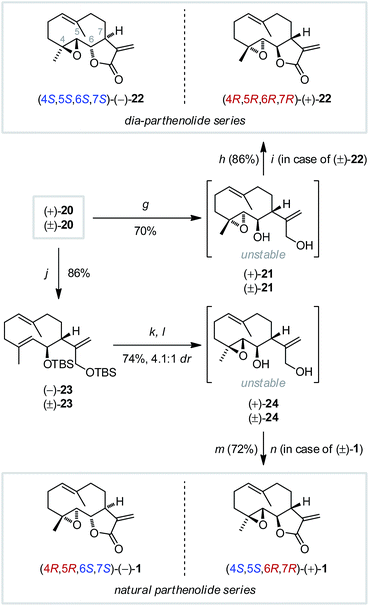
Twofold O-TBS deprotection (Scheme 5) of germacrane 20 then gave the labile epoxy diol (+)-21 that was immediately transformed into the αMγB by using PhI(OAc)2 and substoichiometric TEMPO,17 providing the (4R,5R,6R,7R)-parthenolide diastereomer (+)-22, also termed 4,5-dia-parthenolide,18b in good yield. The enantiomeric (4S,5S,6S,7S)-(−)-isomer was obtained from racemic (±)-22 by using chiral HPLC separation. The challenging deoxygenation of epoxide (+)-20 by low valent tungsten chlorides18a,27 was then investigated. Specific stoichiometry (WCl6/nBuLi 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and temperature control, both during the formation of the reagent (−78 to 15 °C) and for the transformation of the epoxide (0 to 10 °C), delivered the isomerically pure, acid sensitive germacrane (−)-23 in 86% yield. Interestingly, the more Lewis acidic WCl6/nBuLi 1
3) and temperature control, both during the formation of the reagent (−78 to 15 °C) and for the transformation of the epoxide (0 to 10 °C), delivered the isomerically pure, acid sensitive germacrane (−)-23 in 86% yield. Interestingly, the more Lewis acidic WCl6/nBuLi 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 reagent induced transannular cyclization,18a,28 whereas a reagent formed from WCl6/nBuLi in 1
2 reagent induced transannular cyclization,18a,28 whereas a reagent formed from WCl6/nBuLi in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 stoichiometry remained unreactive. Experiments employing a Rh-carbenoid-mediated deoxygenation method29 led to decomposition.
4 stoichiometry remained unreactive. Experiments employing a Rh-carbenoid-mediated deoxygenation method29 led to decomposition.
Epoxidation with opposite stereochemistry was achieved after twofold TBS group removal (TBAF) and directed Sharpless epoxidation (4.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, 74% yield), unfortunately without significant influence of the ligand's stereochemistry on the reaction stereoselectivity, in line with earlier reports for similar transformations.14,18b The labile epoxy diol (−)-24 thus obtained was oxidized to the αMγB to provide (+)-parthenolide [(4S,5S,6R,7R)-1] in 72% yield. Alternatively, chiral HPLC separation of racemic (±)-1 provided both enantiomers of natural parthenolide. The (−)-form [(4R,5R,6S,7S)-1] was identical with an authentic sample.
1 dr, 74% yield), unfortunately without significant influence of the ligand's stereochemistry on the reaction stereoselectivity, in line with earlier reports for similar transformations.14,18b The labile epoxy diol (−)-24 thus obtained was oxidized to the αMγB to provide (+)-parthenolide [(4S,5S,6R,7R)-1] in 72% yield. Alternatively, chiral HPLC separation of racemic (±)-1 provided both enantiomers of natural parthenolide. The (−)-form [(4R,5R,6S,7S)-1] was identical with an authentic sample.
Parthenolide has been associated with a variety of biological activities, which may be specific or unspecific, as αMγBs are fairly reactive electrophiles.2a,30 A hallmark of TCP activity, which was of special interest, is the modulation of tyrosinated tubulin in living cells.8d,31 As a relevant model, we primarily chose to test how parthenolide's stereochemistry influences microtubule detyrosination in axonal growth cones of primary sensory neurons. It was previously shown that inhibition of microtubules detyrosination by parthenolide in a low concentration range of 0.5–5 nM markedly promotes microtubule dynamics and axon growth of cultured sensory neurons, thereby accelerating axonal growth.8 It, therefore, mimics the beneficial effects of constitutively active glycogen synthase kinase 3 (GSK3) on nerve regeneration by modulating microtubule detyrosination via MAP1B activation.8b However, parthenolide loses its beneficial effect on axon growth in vitro again at higher concentrations and even reduces axon growth at very high concentrations.8 This is because a strong inhibition of detyrosination rather destabilizes microtubules and subsequently axon extension.8 Previous work with costunolide and parthenolide indicated that parthenolide's Michael acceptor and the epoxide group are both crucial for the activity observed.8a
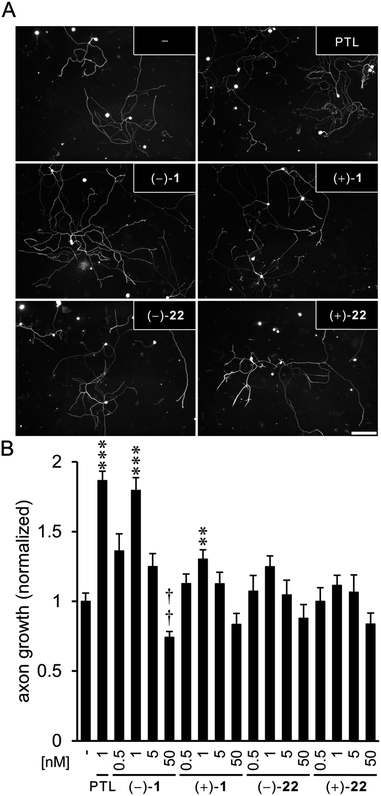
In order to gain a deeper understanding of the target selectivity, we compared natural parthenolide derived from feverfew to synthetic (−)-1, (+)-1, (−)-22, and (+)-22 in mouse primary cell culture (Fig. 1). Both natural parthenolide and synthetic (−)-1 inhibited detyrosination of microtubules in axonal growth cones at 1 nM to a similar degree, while (+)-1 showed a smaller but still considerable effect. Isomers (−)-22 and (+)-22 indicated no significant activity, also not at higher concentration (not shown). To corroborate this finding, we functionally tested the compounds regarding their concentration-dependent activity on axon growth (Fig. 2). Again, both natural and synthetic parthenolide [(−)-1] almost equally promoted axon growth of cultured sensory neurons at 0.5 nM and to a stronger extent at 1 nM, when the lengths of regenerated axons were evaluated after 48 hours in culture (Fig. 2A and B). At 5 nM, the beneficial effects of native and synthetic parthenolide [(−)-1] were almost abolished, and 50 nM even reduced axon growth, thereby demonstrating a very similar functional profile over different concentrations. In contrast to those compounds, the enantiomer (+)-1 showed a small, but statistically significant effect only at 1 nM. Interestingly, also at 50 nM, the enantiomer (+)-1 did not reduce axon regeneration. Isomers (−)-22 and (+)-22 showed no significant increase or decrease in axon growth in the tested concentration range, which is in line with the results from detyrosination experiments.
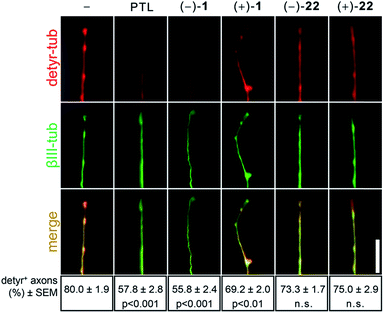 | ||
| Fig. 1 Detyrosination of tubulin in axonal growth. Top: Representative images of cultured sensory axon tips treated either with DMSO (−), plant-derived parthenolide (PTL, c = 1 nM), and synthetic parthenolides (−)-1, (+)-1, (−)-22, and (+)-22 (c = 1 nM each). Axons were immunostained for detyrosinated tubulin (detyr-tub, red) and βIII-tubulin (βIII-tub, green, see ESI†). Scale bar: 20 μm. Bottom: Quantification of axons tips from cultured sensory neuron positive for detyrosinated tubulin. Axon tips were determined to be positive with a gray value above 30 after background subtraction. Data represent means ± SEM of two independent experiments. Statistics: Significance very high: p ≤ 0.001; considerable: p ≤ 0.01; n.s.: not significant, SEM: standard error mean. | ||
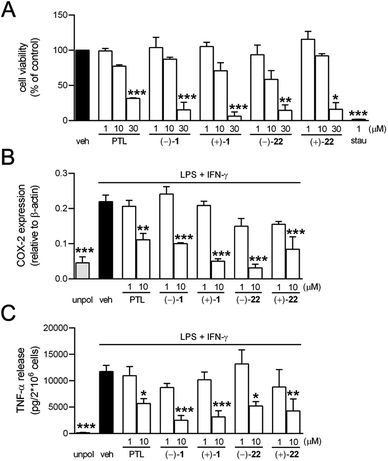
In order to study the selectivity of parthenolides against TCP activity as a putative target compared to its reported anti-inflammatory activity,32 we investigated the anti-inflammatory features of natural parthenolide along with the synthetic stereoisomers (−)-1, (+)-1, (−)-22, and (+)-22 in human monocyte-derived macrophages during lipopolysaccharide (LPS)/interferone (INF)-γ-induced 48 h polarization into the M1 phenotype. Parthenolide, at low micromolar concentrations, inhibits Iκ-B kinase5b and related expression of cyclooxygenase (COX)-2 and tumor necrosis factor (TNF)-α in LPS-activated macrophages.33 MTT assays revealed some but no significant loss of cell viability by the compounds at 10 μM, while at 30 μM all parthenolides displayed significant cytotoxicity (Fig. 3A). At 10 μM, PTL suppressed COX-2 expression and TNF-α release, which was more pronounced for synthetic (−)-1 and (+)-1, with (+)-1 being little superior over (−)-1 (Fig. 3B and C). Of interest, isomers (−)-22 and (+)-22 (10 μM, each) were equally bioactive, and (−)-22 turned out to be the most potent stereoisomer in suppressing COX-2 (Fig. 3B and C).
In combination, these data show that natural (−)-parthenolide as well as the stereoisomers are not significantly cytotoxic for macrophages up to 10 μM, but loss of cell viability is evident at 30 μM. These data compare favorably with data obtained earlier for the natural product on neuronal cells.8b Anti-inflammatory activity (i.e., suppression of COX-2 and TNF-α)33 could be confirmed, but somewhat unexpectedly, no marked differences seem to differentiate the stereoisomers. Furthermore, compared to dedicated anti-inflammatory agents such as glucocorticoids, the observed activity was generally mild and occurred at levels that are close to the level of cytotoxicity during macrophage (M1) activation. In contrast, bioactivity on neurons is observed at the nM level, three orders of magnitude lower than the anti-inflammatory activity against macrophages at non-cytotoxic concentrations (i.e., 10 μM).
For the first time, these data confirm that the interaction of (−)-parthenolide with the structurally yet uncharacterized tubulin carboxypeptidase target is fairly specific on the molecular level—despite its covalently reactive and potentially indiscriminate αMγB functional group.30b While further research is definitely necessary, our data may suggest that not only the presence of the epoxide in the parthenolide structure but also its positioning and hence its impact on conformation or reactivity are crucial for this mode of action. Furthermore, we found the activity mediated by TCP modulated at much lower levels of parthenolide (nM) in neuronal cells than anti-inflammatory and toxic side effects occurred in macrophages (μM). While these data cannot directly be taken as a measure for therapeutic index, they do indicate that parthenolide may indeed serve as a promising template for developing non-toxic agents for neuroregeneration.
Conclusions
In order to evaluate bioactivity of parthenolide stereoisomers, we have introduced electron-rich (Z)-allylboronates (11) for the stereocontrolled synthesis of α-(exo-)methylene γ-butyrolactones from acid labile precursors. For high levels of 1,2-asymmetric induction with α,β-epoxy aldehydes, β,β′ disubstitution was found to be crucial. For these substrates apparent Felkin–Anh control is operative during allylboration. These developments led to the first convergent stereoselective total synthesis of (+)- and (−)-parthenolide (1) in 16 steps from geranyl acetate (4.3% overall yield), as well as its stereoisomers. By using these compounds the specificity of the intervention of (−)-parthenolide with microtubule detyrosination and the importance of a specific molecular interaction event when targeting this activity were validated. The synthetic methodology presented herein should facilitate the efficient synthesis of dedicated tubulin carboxypeptidase inhibiting tool compounds, and, in extension, of many other bioactive target compounds containing αMγBs.Author contributions
H.-D. A., D. F., and O. W. designed and supervised research. R. R. A. F. synthesized and characterized compounds, assisted by R. S. Cell culture and biological profiling of compounds was conducted by P. G., Z. R., and J. G. X-ray single crystal structure analysis was executed by H. G. The manuscript was written by H.-D. A., R. R. A. F., D. F., and O. W., with contributions of all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported in part by the TMWWDG (grant no. 43-5572-321-12040-12) and the DFG (INST 275/331-1 FUGG). R. R. A. F. gratefully acknowledges a doctoral fellowship from the Fonds der Chemischen Industrie (FCI). H.-D. A. and O. W. collaborate as PIs in the CRC ChemBioSys of the DFG (SFB 1127). The work was also supported in part by the DFG grant FI 867/12 (to D. F.). Mr Frédéric Gaigne is thanked for providing a sample of alcohol SI-2.Notes and references
- (a) H. M. R. Hoffmann and J. Rabe, Angew. Chem., Int. Ed. Engl., 1985, 24, 94–110 CrossRef; (b) R. R. A. Kitson, A. Millemaggi and R. J. K. Taylor, Angew. Chem., Int. Ed., 2009, 48, 9426–9451 CrossRef CAS PubMed; (c) Ł. Albrecht, A. Albrecht and T. Janecki, in Natural Lactones and Lactams, ed. T. Janecki, John Wiley & Sons, 2013 Search PubMed.
- (a) A. Ghantous, H. Gali-Muhtasib, H. Vuorela, N. A. Saliba and N. Darwiche, Drug Discovery Today, 2010, 15, 668–678 CrossRef CAS PubMed; (b) A. Janecka, A. Wyrębska, K. Gach, J. Fichna and T. Janecki, Drug Discovery Today, 2012, 17, 561–572 CrossRef CAS PubMed.
- Variable drawings of the structure of parthenolide are reported in the literature, differing in their representation of the epoxide's stereochemistry. The molecular structure used here is stereochemically correct. A detailed description is given in the ESI.† For the X-ray structure determination see: A. Quick and D. Rogers, J. Chem. Soc., Perkin Trans. 2, 1976, 465–469 RSC.
- A. M. L. Seca, A. M. S. Silva and D. C. G. A. Pinto, in Studies in Natural Products Chemistry, ed. Atta-ur-Rahman, Elsevier, 2017, vol. 52, pp. 337–372 Search PubMed.
- (a) S. P. Hehner, M. Heinrich, P. M. Bork, M. Vogt, F. Ratter, V. Lehmann, K. Schulze-Osthoff, W. Dröge and M. L. Lienhard Schmitz, J. Biol. Chem., 1998, 273, 1288–1297 CrossRef CAS PubMed; (b) S. P. Hehner, T. G. Hofmann, W. Dröge and M. L. Schmitz, J. Immunol., 1999, 163, 5617–5623 CAS; (c) B. H. Kwok, B. Koh, M. I. Ndubuisi, M. Elofsson and C. M. Crews, Chem. Biol., 2001, 8, 759–766 CrossRef CAS PubMed.
- Z. Liu, S. Liu, Z. Xie, R. E. Pavlovicz, J. W. Wu, P. Chen, J. Aimiuwu, J. Pang, D. Bhasin, P. Neviani, J. R. Fuchs, C. Plass, P.-K. Li, C. Li, T. H.-M. Huang, L.-C. Wu, L. Rush, H. Wang, D. Perrotti, G. Marcucci and K. K. Chan, J. Pharmacol. Exp. Ther., 2009, 329, 505–514 CrossRef CAS PubMed.
- D. Carlisi, G. Buttitta, R. di Fiore, C. Scerri, R. Drago-Ferrante, R. Vento and G. Tesoriere, Cell Death Dis., 2016, 7, e2194 CrossRef CAS PubMed.
- (a) X. Fonrose, F. Ausseil, E. Soleilhac, V. Masson, B. David, I. Pouny, J.-C. Cintrat, B. Rousseau, C. Barette, G. Massiot and L. Lafanechère, Cancer Res., 2007, 67, 3371–3378 CrossRef CAS PubMed; (b) M. Barisic, R. Silva e Sousa, S. K. Tripathy, M. M. Magiera, A. V. Zaytsev, A. L. Pereira, C. Janke, E. L. Grishchuk and H. Maiato, Science, 2015, 348, 799–803 CrossRef CAS PubMed; (c) P. Gobrecht, A. Andreadaki, H. Diekmann, A. Heskamp, M. Leibinger and D. Fischer, J. Neurosci., 2016, 36, 3890–3902 CrossRef CAS PubMed; (d) C. Aillaud, C. Bosc, L. Peris, A. Bosson, P. Heemeryck, J. Van Dijk, J. Le Friec, B. Boulan, F. Vossier, L. E. Sanman, S. Syed, N. Amara, Y. Couté, L. Lafanechère, E. Denarier, C. Delphin, L. Pelletier, S. Humbert, M. Bogyo, A. Andrieux, K. Rogowski and M.-J. Moutin, Science, 2017, 358, 1448–1453 CrossRef CAS PubMed.
- P. Gobrecht, M. Leibinger, A. Andreadaki and D. Fischer, Nat. Commun., 2014, 5, 4561 CrossRef CAS PubMed.
- C. Y. Chen, M. A. Caporizzo, K. Bedi, A. Vite, A. I. Bogush, P. Robison, J. G. Heffler, A. K. Salomon, N. A. Kelly, A. Babu, M. P. Morley, K. B. Margulies and B. L. Prosser, Nat. Med., 2018, 24, 1225–1233 CrossRef CAS PubMed.
- J. Nieuwenhuis, A. Adamopoulos, O. B. Bleijerveld, A. Mazouzi, E. Stickel, P. Celie, M. Altelaar, P. Knipscheer, A. Perrakis, V. A. Blomen and T. R. Brummelkamp, Science, 2017, 358, 1453–1456 CrossRef CAS PubMed.
- J. Long, S.-F. Zhang, P.-P. Wang, X.-M. Zhang, Z.-J. Yang, Q. Zhang and Y. Chen, J. Med. Chem., 2014, 57, 7098–7112 CrossRef CAS PubMed.
- Z.-J. Yang, W.-Z. Ge, Q.-Y. Li, Y. Lu, J.-M. Gong, B.-J. Kuang, X. Xi, H. Wu, Q. Zhang and Y. Chen, J. Med. Chem., 2015, 58, 7007–7020 CrossRef CAS PubMed.
- L. Li, X. Pan, B. Guan and Z. Liu, Tetrahedron, 2016, 72, 4346–4354 CrossRef CAS.
- W. C. Still, J. Am. Chem. Soc., 1977, 99, 4186–4187 CrossRef CAS PubMed.
- (a) P. A. Grieco and M. Nishizawa, J. Org. Chem., 1977, 42, 1717–1720 CrossRef CAS; (b) H. Shibuya, K. Ohashi, K. Kawashima, K. Hori, N. Murakami and I. Kitagawa, Chem. Lett., 1986, 15, 85–86 CrossRef; (c) T. Takahashi, H. Nemoto, Y. Kanda, J. Tsuji, Y. Fukazawa, T. Okajima and Y. Fujise, Tetrahedron, 1987, 43, 5499–5520 CrossRef CAS; (d) S. Reddy and E. J. Corey, J. Am. Chem. Soc., 2018, 140, 16909–16913 CrossRef PubMed.
- J. Long, Y.-H. Ding, P.-P. Wang, Q. Zhang and Y. Chen, J. Org. Chem., 2013, 78, 10512–10518 CrossRef CAS PubMed.
- (a) K. Foo, I. Usui, D. C. G. Götz, E. W. Werner, D. Holte and P. S. Baran, Angew. Chem., Int. Ed., 2012, 51, 11491–11495 CrossRef CAS PubMed; (b) R. R. A. Freund and H.-D. Arndt, J. Org. Chem., 2016, 81, 11009–11016 CrossRef CAS PubMed.
- (a) J. A. Marshall and D. G. Cleary, J. Org. Chem., 1986, 51, 858–863 CrossRef CAS; (b) I. R. Baldwin and R. J. Whitby, Chem. Commun., 2003, 2786–2787 RSC; (c) E. S. Burnell, A.-R. Irshad, J. Raftery and E. J. Thomas, Tetrahedron Lett., 2015, 56, 3255–3258 CrossRef CAS.
- T. G. Elford and D. G. Hall, Synthesis, 2010, 893–907 CAS.
- (a) W. R. Roush, J. A. Straub and M. S. VanNieuwenhze, J. Org. Chem., 1991, 56, 1636–1648 CrossRef CAS; (b) J. A. Hunt and W. R. Roush, J. Org. Chem., 1997, 62, 1112–1124 CrossRef CAS; (c) A. Mengel and O. Reiser, Chem. Rev., 1999, 99, 1191–1223 CrossRef CAS PubMed; (d) J. Malassis, N. Bartlett, K. Hands, M. D. Selby and B. Linclau, J. Org. Chem., 2016, 81, 3818–3837 CrossRef CAS PubMed.
- (a) V. Nyzam, C. Belaud and J. Villiéras, Tetrahedron Lett., 1993, 6899–6902 CrossRef CAS; (b) J. W. J. Kennedy and D. G. Hall, J. Am. Chem. Soc., 2002, 124, 11586–11587 CrossRef CAS PubMed; (c) T. G. Elford and D. G. Hall, J. Am. Chem. Soc., 2010, 132, 1488–1489 CrossRef CAS PubMed; (d) B. Wen, J. K. Hexum, J. C. Widen, D. A. Harki and K. M. Brummond, Org. Lett., 2013, 15, 2644–2647 CrossRef CAS PubMed; (e) J. Feng, X. Lei, R. Bao, Y. Li, C. Xiao, L. Hu and Y. Tang, Angew. Chem., Int. Ed., 2017, 56, 16323–16327 CrossRef CAS PubMed.
- K. Jiri, N. Zdenek, G. Mukund, N. Lucie, R. Ales, K. Jiri and P. Milan, Org. Lett., 2015, 17, 520–523 CrossRef PubMed.
- (a) P. Knochel, J. Am. Chem. Soc., 1990, 112, 7431–7433 CrossRef CAS; (b) R. W. Hoffmann, T. Sander and A. Hense, Liebigs Ann. Chem., 1993, 771–775 CrossRef CAS; (c) T. Watanabe, N. Miyaura and A. Suzuki, J. Organomet. Chem., 1993, 444, C1–C3 CrossRef CAS.
- M. A. Tius and A. Fauq, J. Am. Chem. Soc., 1986, 108, 6389–6391 CrossRef CAS.
- P. Lei, Y. Ding, X. Zhang, A. Adijiang, H. Li, Y. Ling and J. An, Org. Lett., 2018, 20, 3439–3442 CrossRef CAS PubMed.
- (a) K. B. Sharpless, M. A. Umbreit, M. T. Nieh and T. C. Flood, J. Am. Chem. Soc., 1972, 94, 6538–6540 CrossRef CAS; (b) K. C. Nicolaou, D. Rhoades, Y. Wang, R. Bai, E. Hamel, M. Aujay, J. Sandoval and J. Gavrilyuk, J. Am. Chem. Soc., 2017, 139, 7318–7334 CrossRef CAS PubMed.
- H. Neukirch, A. Guerriero and M. D'Ambrosio, Eur. J. Org. Chem., 2003, 3969–3975 CrossRef CAS.
- S. Raucher, K.-W. Chi, K.-J. Hwang and J. E. J. Burks, J. Org. Chem., 1986, 51, 5503–5505 CrossRef CAS.
- (a) I. Merfort, Curr. Drug Targets, 2011, 12, 1560–1573 CrossRef CAS PubMed; (b) P. A. Jackson, J. C. Widen, D. A. Harki and K. M. Brummond, J. Med. Chem., 2017, 60, 839–885 CrossRef CAS PubMed.
- C. Janke, J. Cell Biol., 2014, 206, 461–472 CrossRef CAS PubMed.
- A. Ghantous, A. Sinjab, Z. Herceg and N. Darwiche, Drug Discovery Today, 2013, 18, 894–905 CrossRef CAS PubMed.
- D. Hwang, N. H. Fischer, B. C. Jang, H. Tak, J. K. Kim and W. Lee, Biochem. Biophys. Res. Commun., 1996, 226, 810–818 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Syntheses of starting materials. Experimental procedures, analytical data for all new compounds. Cell and macrophage experiment methods and data. NMR based structure elucidation of compound 16f (PDF). X-ray of compound (±)-9 (CIF). CCDC 1902988. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc01473j |
| This journal is © The Royal Society of Chemistry 2019 |