Open Access Article
Open Access ArticleAsymmetric Baeyer–Villiger oxidation: classical and parallel kinetic resolution of 3-substituted cyclohexanones and desymmetrization of meso-disubstituted cycloketones†
Wangbin
Wu
,
Weidi
Cao
,
Linfeng
Hu
,
Zhishan
Su
 ,
Xiaohua
Liu
,
Xiaohua
Liu
 * and
Xiaoming
Feng
* and
Xiaoming
Feng
 *
*
Key Laboratory of Green Chemistry and Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu 610064, China. E-mail: xmfeng@scu.edu.cn; liuxh@scu.edu.cn
First published on 10th June 2019
Abstract
Regioselectivity is a crucial issue in Baeyer–Villiger (BV) oxidation. To date, few reports have addressed asymmetric BV oxidation of 3-substituted cycloketones due to the high difficulty of controlling regio- and stereoselectivity. Herein, we report the asymmetric BV oxidation of 3-substituted and meso-disubstituted cycloketones with chiral N,N′-dioxide/Sc(III) catalysts performed in three ways: classical kinetic resolution, parallel kinetic resolution and desymmetrization. The methodology was applied in the total and formal synthesis of bioactive compounds and natural products. Control experiments and calculations demonstrated that flexible and adjustable catalysts played a significant role in the chiral recognition of substrates.
Introduction
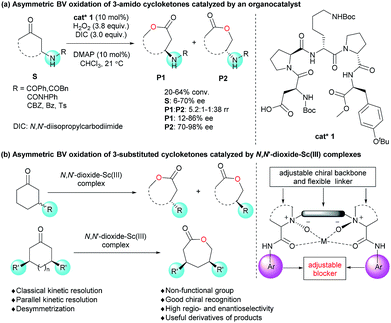
Asymmetric Baeyer–Villiger oxidation provides direct, efficient access to chiral lactones from cycloketones,1–3 including kinetic resolution of racemic cycloketones and desymmetrization of mesomeric cycloketones. Classical kinetic resolution (CKR) and parallel kinetic resolution (PKR) are two main sub-categories in kinetic resolution reactions. Impressive developments have been made in the CKR of highly ring-strained 2-substituted monocyclic ketones and bicyclic cyclobutanones via BV oxidation.4 PKR enabled the generation of two regioisomers of lactones. Several examples related to bicyclic cyclobutanones were realized, yet high ee values could not be obtained for both regioisomers simultaneously.5 Previously reported desymmetrization of cycloketones via BV oxidation mainly focused on 3-substituted cyclobutanones and 4-substituted cyclohexanones.6,7 Systematic studies on mesomeric disubstituted cycloketones are scarce; only 3,5-cis-dimethyl cyclohexanone was discussed in biocatalytic cases.7d,f To sum up, despite remarkable advancements in asymmetric BV oxidation, the scope of substrates is still limited. Meanwhile, to develop an efficient catalytic system that can promote all three above-mentioned types of Baeyer–Villiger oxidation reactions is also highly meaningful.4b,6b,7eOn the other hand, regioselectivity has long been a “camphor mystery” in BV oxidation.2c,8 In comparison with 2-substituted cyclic ketones, asymmetric BV oxidation of 3-substituted cyclic ketones (cyclopentanones and cyclohexanones) was less discussed owing to the high difficulty to control regio- and stereoselectivity.9 Several biocatalyst-promoted reactions have been reported with moderate stereoselectivity9c or regioselectivity.9a,b,d In 2014, Miller's group developed the asymmetric BV oxidation of 3-substituted cycloketones with a peptide-based organocatalyst, where hydrogen bonding between the catalyst and the functional groups of the substrates resulted in moderate to good regio- and stereoselectivities (Scheme 1a).9f
The induced-fit model of BV oxidation in biocatalysis provides such a sight of view for molecular catalysts that a conformationally flexible structure can streamline the adjustment of catalysts toward cycloketones with different configurations and conformations, leading to high regio- and stereo-selectivity.4d,4f,7c,10 The privileged chiral N,N′-dioxide, bearing a catenulate alkyl linker as well as two backbones and aniline groups bound to Lewis acids, is by nature a flexible structure,11 which forms an adjustable blocker for chiral recognition. Herein, we describe novel CKR, PKR and desymmetrization of 3-substituted cycloketones (non-functional group) with a single chiral N,N′-dioxide/Sc(III) catalytic system (Scheme 1b).
Results and discussion
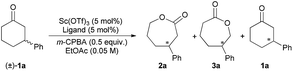
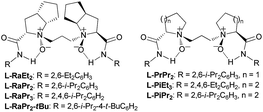
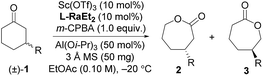
Our investigation began with the CKR of racemic 3-phenyl cyclohexanone (1a) by using m-chloro peroxobenzoic acid (m-CPBA, 0.5 equiv.) as an oxidant in the presence of 5 mol% L-PrPr2/Sc(OTf)3 complex in EtOAc at 30 °C (Table 1, entry 1). The corresponding mixture of lactones 2a and 3a was obtained in moderate yield with poor regio- and stereoselectivity, while racemic 1a was recovered. Next, the backbones of the chiral N,N′-dioxide ligands were evaluated and found to have an important effect on the regio- and stereoselectivity (Fig. 1). L-RaPr2 derived from L-ramipril was superior to L-proline-derived L-PrPr2 and L-pipecolic acid derived L-PiPr2 (Table 1, entry 3 vs. 1–2). Both regio- and stereoselectivity of the reaction were improved by introducing a bulky group into the para-position of the phenyl group in the ligand (Table 1, entries 4 and 5). For instance, the ligand L-RaPr2-tBu bearing tert-butyl groups, coordinated with Sc(OTf)3, catalyzed the reaction and gave a mixture of 2a and 3a in an 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 ratio with 81% ee of 2a (Table 1, entry 5). Upon lowering the temperature to 0 °C, the ratio of 2a to 3a could be improved to 85
17 ratio with 81% ee of 2a (Table 1, entry 5). Upon lowering the temperature to 0 °C, the ratio of 2a to 3a could be improved to 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 and the ee value of 2a was increased to 85% in 12 h (Table 1, entry 6). However, upon further decreasing the temperature to −20 °C, no better result was achieved (Table 1, entry 7). To our delight, the regio- and stereoselectivity had a significant improvement with the addition of Al(Oi-Pr)34e,6f (2a
15 and the ee value of 2a was increased to 85% in 12 h (Table 1, entry 6). However, upon further decreasing the temperature to −20 °C, no better result was achieved (Table 1, entry 7). To our delight, the regio- and stereoselectivity had a significant improvement with the addition of Al(Oi-Pr)34e,6f (2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 91
3a = 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 93% ee of 2a, and 81% ee of recovered 1a), albeit with prolonged reaction time (72 h) (Table 1, entry 8). It was found that when Al(Oi-Pr)3 and 3 Å MS were both used as additives, optimal reaction results could be obtained within 48 h (Table 1, entry 9, 48% yield of the mixture of 2a and 3a, 2a:3a = 92
9, 93% ee of 2a, and 81% ee of recovered 1a), albeit with prolonged reaction time (72 h) (Table 1, entry 8). It was found that when Al(Oi-Pr)3 and 3 Å MS were both used as additives, optimal reaction results could be obtained within 48 h (Table 1, entry 9, 48% yield of the mixture of 2a and 3a, 2a:3a = 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 93% ee of 2a, 48% yield of 1a, and 82% ee of 1a).
8, 93% ee of 2a, 48% yield of 1a, and 82% ee of 1a).
| Entrya | Ligand | T (°C) | Additives | Yieldb (%) | eec (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1a | 2a + 3a |
2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3ac 3ac |
1a | 2a | 3a | ||||
| a Unless otherwise specified, the reaction was performed with Sc(OTf)3 (5 mol%), ligand (5 mol%), 1a (0.20 mmol) and m-CPBA (0.5 equiv.) in EtOAc (0.05 M) at 30 °C for 12 h under an air atmosphere. b Yields of the isolated products. c Determined by HPLC analysis using a chiral stationary phase. d At 0 °C. e At −20 °C for 72 h. f Al(Oi-Pr)3 (50 mol%) was added. g At −20 °C for 48 h, Al(Oi-Pr)3 (50 mol%) and 3 Å MS (50 mg) were added. | |||||||||
| 1 | L-PrPr2 | 30 | — | 61 | 33 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
1 | 9 | 5 |
| 2 | L-PiPr2 | 30 | — | 49 | 50 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
2 | 23 | 17 |
| 3 | L-RaPr2 | 30 | — | 47 | 43 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
13 | 69 | 84 |
| 4 | L-RaPr3 | 30 | — | 49 | 51 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
42 | 77 | 72 |
| 5 | L-RaPr2-tBu | 30 | — | 48 | 48 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
54 | 81 | 78 |
| 6d | L-RaPr2-tBu | 0 | — | 53 | 44 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
62 | 85 | 68 |
| 7e | L-RaPr2-tBu | −20 | — | 73 | 25 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
24 | 73 | 89 |
| 8ef | L-RaPr2-tBu | −20 | Al(Oi-Pr)3 | 49 | 50 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
81 | 93 | 96 |
| 9g | L-RaPr2-tBu | −20 | 3 Å MS | 48 | 48 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
82 | 93 | 91 |
The substrate scope of CKR was then explored. A range of racemic 3-aryl cyclohexanones were transformed into the corresponding lactones smoothly under the optimized reaction conditions. Regardless of the presence of electron donating or withdrawing groups on the 3-phenyl group, excellent yields and moderate to good regioselectivities with good ee values of 2a–2g were obtained (Table 2, entries 1–7, 45–49% yields, 85–93% ee of 2, and up to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 rr). For the condensed-ring substrate 1h (Table 2, entry 8), the desired products were obtained in 50% yield and 87% ee of 2h with 95
5 rr). For the condensed-ring substrate 1h (Table 2, entry 8), the desired products were obtained in 50% yield and 87% ee of 2h with 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 rr. 3-Alkyl substituted cyclohexanones 1i (Bn) and 1j (Me) were also tolerated in this catalytic system, providing the corresponding lactones in 48% yield, 82% ee of 2i with 70
5 rr. 3-Alkyl substituted cyclohexanones 1i (Bn) and 1j (Me) were also tolerated in this catalytic system, providing the corresponding lactones in 48% yield, 82% ee of 2i with 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 rr and 44% yield, and 90% ee of 2j with 74
30 rr and 44% yield, and 90% ee of 2j with 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 rr, respectively (Table 2, entries 9 and 10). The ee values of the minor isomers 3i and 3j were excellent (97% ee and 95% ee). All the unreacted 3-aryl cyclohexanones 1 were recovered in excellent yields with moderate to good ee values.
26 rr, respectively (Table 2, entries 9 and 10). The ee values of the minor isomers 3i and 3j were excellent (97% ee and 95% ee). All the unreacted 3-aryl cyclohexanones 1 were recovered in excellent yields with moderate to good ee values.
| Entrya | R | Yieldb (%) | eec (b) | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 + 3 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3c 3c |
1 | 2 | 3 | ||
| a Unless otherwise specified, the reaction was performed with Sc(OTf)3 (5 mol%), L-RaPr2-tBu (5 mol%), 1a (0.20 mmol), m-CPBA (0.5 equiv.), Al(Oi-Pr)3 (50 mol%) and 3 Å MS (50 mg) in EtOAc (0.05 M) under an air atmosphere. b Yields of the isolated products. c Determined by HPLC or SFC analysis using a chiral stationary phase. For the absolute configuration of the products, see the ESI for more details. | |||||||
| 1 | Ph (1a) | 48 | 48 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
82 | 93 | 91 |
| 2 | 2-MeC6H4 (1b) | 48 | 48 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
72 | 88 | 73 |
| 3 | 3-MeC6H4 (1c) | 46 | 49 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
67 | 91 | 61 |
| 4 | 4-MeC6H4 (1d) | 49 | 47 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
67 | 85 | 90 |
| 5 | 4-n-BuC6H4 (1e) | 42 | 45 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
79 | 90 | 96 |
| 6 | 3-ClC6H4 (1f) | 49 | 49 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
74 | 90 | 75 |
| 7 | 4-F3CC6H4 (1g) | 43 | 45 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
88 | 91 | 73 |
| 8 | 2-Naphthyl (1h) | 49 | 50 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
62 | 87 | 98 |
| 9 | Bn (1i) | 43 | 48 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
40 | 82 | 97 |
| 10 | Me (1j) | 43 | 44 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
55 | 90 | 95 |
Then, we turned our attention to the PKR of racemic 3-substituted cyclohexanones. After a slight modification of the reaction conditions (see Table S1 in the ESI for details†), by altering the ligand L-RaPr2-tBu to L-RaEt2 as well as increasing the catalyst loading to 10 mol% and the reaction concentration to 0.10 M, 2a and 3a were obtained with 81% ee and 97% ee (Table 3, entry 1). The PKR of other 3-aryl substituted cyclohexanones proceeded well to give both lactone isomers with good to excellent enantioselectivities (Table 3, entries 2–7, 80–83% ee of 2 and 91–97% ee of 3). Substrate 1l bearing a n-butyl group was converted into the desired oxidation products in 84% mixed yield and 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 rr with 87% ee of 2l and 97% ee of 3l.
38 rr with 87% ee of 2l and 97% ee of 3l.
| Entrya | R | Yieldb (%) | eec (%) | ||
|---|---|---|---|---|---|
| 2 + 3 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3c 3c |
2 | 3 | ||
| a Unless otherwise specified, the reaction was performed with Sc(OTf)3 (10 mol%), L-RaEt2 (10 mol%), 1 (0.10 mmol), m-CPBA (1.0 equiv.), Al(Oi-Pr)3 (50 mol%) and 3 Å MS (50 mg) in EtOAc (0.10 M) at −20 °C under an air atmosphere. b Yields of the isolated products. c Determined by HPLC or SFC analysis using a chiral stationary phase. For the absolute configuration of the products, see the ESI for more details. | |||||
| 1 | Ph (1a) | 98 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
81 | 97 |
| 2 | 2-MeC6H4 (1b) | 94 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
80 | 93 |
| 3 | 3-MeC6H4 (1c) | 97 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 47 |
82 | 91 |
| 4 | 4-MeC6H4 (1d) | 97 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
80 | 96 |
| 5 | 4-n-BuC6H4 (1e) | 98 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
83 | 95 |
| 6 | 4-MeOC6H4 (1k) | 92 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48 |
83 | 97 |
| 7 | 2-Naphthyl (1h) | 94 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
81 | 96 |
| 8 | n-Bu (1l) | 84 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
87 | 97 |
Inspired by the CKR and PKR of 3-substituted cyclohexanones, we then focused on the desymmetrization of cis-3,5-diphenyl cyclohexanones. Upon further survey of the reaction parameters, the optimal conditions were found to be 4 (0.10 mmol), m-CPBA (0.10 mmol), L-RaPr2/Sc(OTf)3 complex (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10 mol%) and 3 Å MS (50 mg) in EtOAc at 0 °C for 48 h (see Table S4 in the ESI for details†). The substituents on the phenyl group of the cyclohexanones were proven to have little effect on this reaction, and a series of desymmetrization products 5a–5g were obtained in excellent yields and enantioselectivities (Table 4, entries 1–7, 96–99% yields, and 93–97% ee). The absolute configuration of 5a was determined to be (4R,6R) by X-ray crystallographic analysis.12a Dimethyl substituted 4h could undergo transformation as well and gave the target lactone 5h in 99% yield with 91% ee (Table 4, entry 8). In addition, 3,4-di-phenyl cyclopentanone 4i was also tolerated in this desymmetrization reaction (Table 4, entry 9, 99% yield, 96% ee).
1, 10 mol%) and 3 Å MS (50 mg) in EtOAc at 0 °C for 48 h (see Table S4 in the ESI for details†). The substituents on the phenyl group of the cyclohexanones were proven to have little effect on this reaction, and a series of desymmetrization products 5a–5g were obtained in excellent yields and enantioselectivities (Table 4, entries 1–7, 96–99% yields, and 93–97% ee). The absolute configuration of 5a was determined to be (4R,6R) by X-ray crystallographic analysis.12a Dimethyl substituted 4h could undergo transformation as well and gave the target lactone 5h in 99% yield with 91% ee (Table 4, entry 8). In addition, 3,4-di-phenyl cyclopentanone 4i was also tolerated in this desymmetrization reaction (Table 4, entry 9, 99% yield, 96% ee).
| Entrya | R | Yieldb (%) | eec (%) |
|---|---|---|---|
| a Unless otherwise specified, the reaction was performed with Sc(OTf)3 (10 mol%), L-RaPr2 (10 mol%), 4 (0.10 mmol), m-CPBA (1.0 equiv.), and 4 Å MS (50 mg) in EtOAc (0.05 M) at −20 °C for 48 h under an air atmosphere. b Yields of the isolated products. c Determined by HPLC or SFC analysis using a chiral stationary phase. For the absolute configuration of the products, see the ESI for more details. d For 4i, n = 0; L-PiEt3 was used instead of L-RaPr2 at 0 °C for 24 h. | |||
| 1 | Ph (4a) | 97 | 96 |
| 2 | 3-MeC6H4 (4b) | 98 | 97 |
| 3 | 4-OMeC6H4 (4c) | 96 | 93 |
| 4 | 4-FC6H4 (4d) | 98 | 94 |
| 5 | 3-ClC6H4 (4e) | 99 | 93 |
| 6 | 4-ClC6H4 (4f) | 98 | 94 |
| 7 | 4-BrC6H4 (4g) | 99 | 94 |
| 8 | Me (4h) | 99 | 91 |
| 9d | Ph (4i) | 99 | 96 |
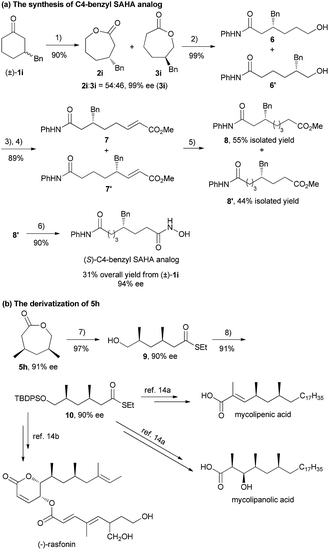
(S)-C4-benzyl suberoylanilide hydroxamic acid (SAHA) exhibits high selectivity for histone deacetylases (HDAC) 6 and 8, which can regulate gene expression via deacetylation of nucleosomal histones. Recently, Pflum's group realized the synthesis of the (S)-C4-benzyl SAHA analog in 9 steps (6.3% overall yield) from (R)-4-benzyloxazolidin-2-one.13 In contrast, as shown in Scheme 2a, the (S)-C4-benzyl SAHA analog could be obtained in 31% overall yield with 94% ee in 6 steps from racemic 1i, involving the key step of asymmetric BV oxidation of 1i to a mixture of 2i and 3i. The Syn-1,3-dimethyl moiety served as a core chiral skeleton in various natural products,14 such as mycolipenic acid, mycolipanolic acid and (−)-rasfonin. Manipulating the desymmetrization product 5h with a two-step transformation, syn-1,3-dimethyl thioester 10 was obtained, which could be easily transformed into the aforementioned natural products (Scheme 2b, see the ESI for details†).
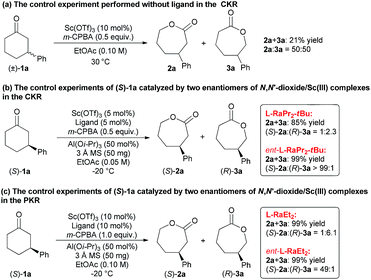
To elucidate the regioselectivity in both CKR and PKR, several control experiments were conducted. First, when Sc(OTf)3 was used to promote the CKR of 1a without a ligand, only 21% mixed yield of 2a and 3a with 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 rr was obtained (Scheme 3a). Furthermore, when the enantiopure substrate (S)-1a was tested under the standard conditions of CKR type of BV oxidation with the ligand ent-L-RaPr2-tBu derived from D-ramipril, a mixture of 2a and 3a was obtained in quantitative yield. However, when L-RaPr2-tBu was used, the reactivity was diminished and lower regioselectivity was obtained (Scheme 3b). These results suggest that (S)-1a matched with ent-L-RaPr2-tBu and gave the major product (S)-2a; however, (S)-1a mismatched with L-RaPr2-tBu and revealed poorer reactivity to give (R)-3a. A similar phenomenon was also observed in the PKR type of BV oxidation with L-RaEt2 and ent-L-RaEt2 as the ligands (Scheme 3c).
50 rr was obtained (Scheme 3a). Furthermore, when the enantiopure substrate (S)-1a was tested under the standard conditions of CKR type of BV oxidation with the ligand ent-L-RaPr2-tBu derived from D-ramipril, a mixture of 2a and 3a was obtained in quantitative yield. However, when L-RaPr2-tBu was used, the reactivity was diminished and lower regioselectivity was obtained (Scheme 3b). These results suggest that (S)-1a matched with ent-L-RaPr2-tBu and gave the major product (S)-2a; however, (S)-1a mismatched with L-RaPr2-tBu and revealed poorer reactivity to give (R)-3a. A similar phenomenon was also observed in the PKR type of BV oxidation with L-RaEt2 and ent-L-RaEt2 as the ligands (Scheme 3c).
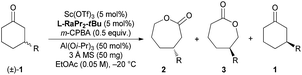
As discussed above, the migratory aptitude of 1a contributed little to the regioselectivity in the formation of lactones (Scheme 3a). We proposed that the stereoelectronic effect in the Criegee intermediate, a notion that the migrating group needs to be antiperiplanar to the leaving group in peroxide acids before the migration of the alkyl group in the Criegee intermediate, could be essential for the recognition of 1a (Fig. 2a).3d,e Since different aniline groups in L-RaPr2-tBu12b and L-RaEt2 resulted in different steric hindrances between 1a and catalysts, the energy difference in the alkyl migration step in the formation of 2a and 3a with different configurations would result in the difference of regioselectivity. To provide further evidence for the above conjecture, ONIOM (M06/6-31G*: HF/STO-3G) calculations were performed (see the ESI for details†). Based on previous theoretical studies of BV oxidation with chiral N,N′-dioxide/Sc(III) catalysts,15 the transition states in the alkyl group migration step in CKR and PKR were optimized and their Gibbs free energies were calculated (Fig. 2b, c). In L-RaPr2-tBu-TS-(R)-2a and L-RaEt2-TS-(R)-2a, 1a was placed away from the aniline groups, while in L-RaPr2-tBu-TS-(R)-3a and L-RaEt2-TS-(R)-3a, 1a was placed between the aniline group and the bicyclic ring backbone of the ligand. For L-RaPr2-tBu with bulky iso-propyl and tert-butyl groups on the aniline group, the larger steric hindrance between the ligand and 1a resulted in a larger energy difference between L-RaPr2-tBu-TS-(R)-3a and L-RaPr2-tBu-TS-(R)-2a (Fig. 2b, ΔG = 4.2 kcal mol−1), and so the former is the favored transition state while the latter is the disfavored one. Meanwhile, owing to a less bulky aniline group and the flexible catalyst structure, the energy difference between L-RaEt2-TS-(R)-3a and L-RaEt2-TS-(R)-2a was significantly smaller (Fig. 2c, ΔG = 0.7 kcal mol−1), and both transition states are favored. Such a revelation is consistent with the control experiments in Scheme 3. The theoretical study shows that the adjustable aniline groups and flexible catalyst structure proved to be powerful for the regioselectivity and enantioselectivity in the BV oxidation of 1a with N,N′-dioxide/Sc(III) catalysts through the recognition of the 3-position of the cyclohexanones in BV oxidation.
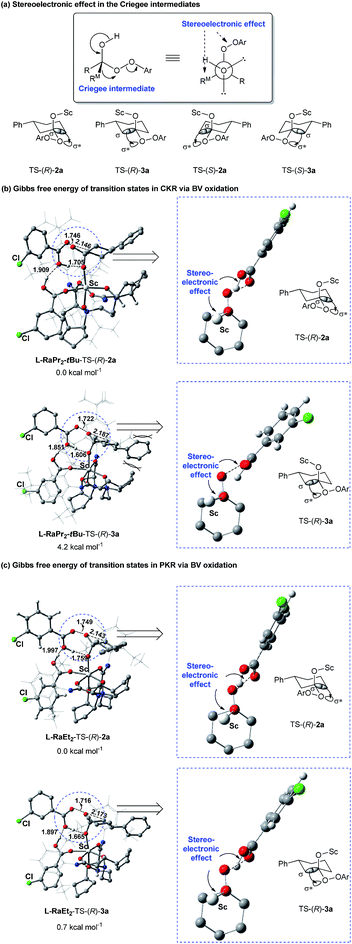 | ||
| Fig. 2 Gibbs free energy diagram of the optimized transition states of (±)-1a and m-CPBA catalyzed by N,N′-dioxide/Sc(III) complexes. | ||
Conclusions
We realized the catalytic asymmetric CKR and PKR of 3-substituted cyclohexanones and desymmetrization of meso-disubstituted cycloketones through BV oxidation with a single catalytic system. The pending problem of regio- and stereoselectivity in BV oxidation was solved by the modulation of the structure of chiral N,N′-dioxide/Sc(III) complexes. The experimental studies and theoretical calculations showed that flexible and adjustable catalysts can influence the migratory aptitude of the substrate via stereoelectronic control and chiral recognition. Besides, this methodology has been proven to be efficient in synthesizing useful bioactive compounds and natural products.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the National Natural Science Foundation of China (No. 21890723 and 21625205) and the Fundamental Research Funds for the Central Universities (2012017yjsy001) for the financial support.Notes and references
- A. Baeyer and V. Villiger, Ber. Dtsch. Chem. Ges., 1899, 32, 3625 CrossRef.
- For selected reviews, see: (a) G. R. Krow, Org. React., 1993, 43, 251 CAS; (b) G. Strukul, Angew. Chem., Int. Ed., 1998, 37, 1198 CrossRef; (c) M. Renz and B. Meunier, Eur. J. Org. Chem., 1999, 737 CrossRef CAS; (d) G.-J. ten Brink, I. W. C. E. Arends and R. A. Sheldon, Chem. Rev., 2004, 104, 4105 CrossRef PubMed.
- (a) C. Bolm, in Advances in Catalytic Processes, ed.M. P. Doyle, JAI Press, Greenwich, CT, 1997, vol. 2, p. 43 Search PubMed; (b) C. Bolm and O. Beckmann, in Comprehensive Asymmetric Catalysis, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer, Berlin, 1999, vol. 2, p. 803 Search PubMed; (c) C. Bolm, Baeyer–Villiger Reaction, in Asymmetric Synthesis: The Essentials, ed. M. Christmann and S. Bräse, Wiley-VCH, Weinheim, Germany, 2006, p. 57 Search PubMed; (d) L. Zhou, L. L. Lin, X. H. Liu and X. M. Feng, Baeyer–Villiger (BV) Oxidation/Rearrangement in Organic Synthesis, in Molecular Rearrangements in Organic Synthesis, ed. C. M. Rojas, Wiley-VCH, Weinheim, Germany, 2015, p. 35 Search PubMed; (e) C. M. Crudden, A. C. Chen and L. A. Calhoun, Angew. Chem., Int. Ed., 2000, 39, 2851 CrossRef CAS.
- For selected examples of the asymmetric CKR of racemic cycloketones via BV oxidation, see: (a) C. Bolm, G. Schlingloff and K. Weickhardt, Angew. Chem., Int. Ed., 1994, 33, 1848 CrossRef; (b) A. Gusso, C. Baccin, F. Pinna and G. Strukul, Organometallics, 1994, 13, 3442 CrossRef CAS; (c) Y. G. Peng, X. M. Feng, K. B. Yu, Z. Li, Y. Z. Jiang and C.-H. Yeung, J. Organomet. Chem., 2001, 619, 204 CrossRef CAS; (d) M. T. Reetz and S. Wu, J. Am. Chem. Soc., 2009, 131, 15424 CrossRef CAS PubMed; (e) L. Zhou, X. H. Liu, J. Ji, Y. H. Zhang, W. B. Wu, Y. B. Liu, L. L. Lin and X. M. Feng, Org. Lett., 2014, 16, 3938 CrossRef CAS PubMed; (f) N. C. Abascal and S. J. Miller, Org. Lett., 2016, 18, 4646 CrossRef CAS PubMed.
- For selected examples of the asymmetric PKR of racemic cycloketones via BV oxidation, see: (a) V. Alphand and R. Furstoss, J. Org. Chem., 1992, 57, 1306 CrossRef CAS; (b) C. Bolm and G. Schlingloff, J. Chem. Soc., Chem. Commun., 1995, 1247 RSC; (c) A. Watanabe, T. Uchida, R. Irie and T. Katsuki, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5737 CrossRef CAS PubMed; (d) F. Hollmann, A. Taglieber, F. Schulz and M. T. Reetz, Angew. Chem., Int. Ed., 2007, 46, 2903 CrossRef CAS PubMed; (e) S. Xu, Z. Wang, X. Zhang and K. Ding, Eur. J. Org. Chem., 2011, 110 CrossRef CAS.
- For selected examples of the desymmetrization of cyclobutanones via BV oxidation, see: (a) C. Bolm, T. K. K. Luong and G. Schlingloff, Synlett, 1997, 1151 CrossRef CAS; (b) C. Paneghetti, R. Gavagnin, F. Pinna and G. Strukul, Organometallics, 1999, 18, 5057 CrossRef CAS; (c) T. Uchida and T. Katsuki, Tetrahedron Lett., 2001, 42, 6911 CrossRef CAS; (d) S.-I. Murahashi, S. Ono and Y. Imada, Angew. Chem., Int. Ed., 2002, 41, 2366 CrossRef CAS; (e) S. Xu, Z. Wang, X. Zhang, X. Zhang and K. Ding, Angew. Chem., Int. Ed., 2008, 47, 2840 CrossRef CAS PubMed; (f) L. Zhou, X. H. Liu, J. Ji, Y. H. Zhang, X. L. Hu, L. L. Lin and X. M. Feng, J. Am. Chem. Soc., 2012, 134, 17023 CrossRef CAS PubMed.
- For selected examples of the desymmetrization of cyclohexanones via BV oxidation, see: (a) M. J. Taschner and D. J. Black, J. Am. Chem. Soc., 1988, 110, 6892 CrossRef CAS; (b) J. D. Stewart, K. W. Reed, C. A. Martinez, J. Zhu, G. Chen and M. M. Kayser, J. Am. Chem. Soc., 1998, 120, 3541 CrossRef CAS; (c) M. T. Reetz, B. Brunner, T. Schneider, F. Schulz, C. M. Clouthier and M. M. Kayser, Angew. Chem., Int. Ed., 2004, 43, 4075 CrossRef CAS PubMed; (d) D. V. Rial, D. A. Bianchi, P. Kapitanova, A. Lengar, J. B. van Beilen and M. D. Mihovilovic, Eur. J. Org. Chem., 2008, 1203 CrossRef CAS; (e) A. Cavarzan, G. Bianchini, P. Sgarbossa, L. Lefort, S. Gladiali, A. Scarso and G. Strukul, Chem.–Eur. J., 2009, 15, 7930 CrossRef CAS PubMed; (f) G. Li, M. J. L. J. Fürst, H. R. Mansouri, A. K. Ressmann, A. Ilie, F. Rudroff, M. D. Mihovilovic, M. W. Fraaije and M. T. Reetz, Org. Biomol. Chem., 2017, 15, 9824 RSC.
- (a) B. M. Trost, P. Buhlmayer and M. Mao, Tetrahedron Lett., 1982, 23, 1443 CrossRef CAS; (b) V. Alphand, A. Archelas and R. Furstoss, Tetrahedron Lett., 1989, 30, 3663 CrossRef CAS; (c) V. Alphand and R. Furstoss, Tetrahedron: Asymmetry, 1992, 3, 379 CrossRef CAS.
- (a) M. M. Kayser, G. Chen and J. D. Stewart, J. Org. Chem., 1998, 63, 7103 CrossRef CAS; (b) S. Wang, M. M. Kayser and V. Jurkauskas, J. Org. Chem., 2003, 68, 6222 CrossRef CAS PubMed; (c) H. Iwaki, S. Wang, S. Grosse, H. Bergeron, A. Nagahashi, J. Lertvorachon, J. Yang, Y. Konishi, Y. Hasegawa and P. C. K. Lau, Appl. Environ. Microbiol., 2006, 72, 2707 CrossRef CAS PubMed; (d) M. J. Fink, T. C. Fischer, F. Rudroff, H. Dudek, M. W. Fraaije and M. D. Mihovilovic, J. Mol. Catal. B: Enzym., 2011, 73, 9 CrossRef CAS; (e) T. Reignier, V. de Berardinis, J.-L. Petit, A. Mariage, K. Hamzé, K. Duquesnea and V. Alphand, Chem. Commun., 2014, 50, 7793 RSC; (f) D. K. Romney, S. M. Colvin and S. J. Miller, J. Am. Chem. Soc., 2014, 136, 14019 CrossRef CAS PubMed.
- G. Ottolina, G. Carrea, S. Colonna and A. Rückemann, Tetrahedron: Asymmetry, 1996, 7, 1123 CrossRef CAS.
- (a) X. H. Liu, L. L. Lin and X. M. Feng, Acc. Chem. Res., 2011, 44, 574 CrossRef CAS PubMed; (b) X. H. Liu, L. L. Lin and X. M. Feng, Org. Chem. Front., 2014, 1, 298 RSC; (c) X. H. Liu, H. F. Zheng, Y. Xia, L. L. Lin and X. M. Feng, Acc. Chem. Res., 2017, 50, 2621 CrossRef CAS PubMed; (d) X. H. Liu, S. X. Dong, L. L. Lin and X. M. Feng, Chin. J. Chem., 2018, 36, 791 CrossRef CAS; (e) J. Wang, Y. Zuo, C. Hu and Z. Su, Catal. Sci. Technol., 2017, 7, 2183 RSC.
- (a) 5a (CCDC 1848335).†; (b) CCDC 1856535 (L-RaPr2-tBu/Sc(OTf)3).†.
- A. T. Negmeldin, J. R. Knoff and M. K. H. Pflum, Eur. J. Med. Chem., 2018, 143, 1790 CrossRef CAS PubMed.
- (a) B. ter Horst, J. van Wermeskerken, B. L. Feringa and A. J. Minnaard, Eur. J. Org. Chem., 2010, 38 CrossRef CAS; (b) Y. Huang, A. J. Minnaard and B. L. Feringa, Org. Biomol. Chem., 2012, 10, 29 RSC; (c) S. Chow, M. T. Fletcher, L. K. Lambert, O. P. Gallagher, C. J. Moore, B. W. Cribb, P. G. Allsopp and W. Kitching, J. Org. Chem., 2005, 70, 1808 CrossRef CAS PubMed; (d) B. ter Horst, B. L. Feringa and A. J. Minnaard, Org. Lett., 2007, 9, 3013 CrossRef CAS PubMed; (e) Y. Zhu, A. Loudet and K. Burgess, Org. Lett., 2010, 12, 4392 CrossRef CAS PubMed; (f) K. Matcha, A. V. R. Madduri, S. Roy, S. Ziegler, H. Waldmann, A. K. H. Hirsch and A. J. Minnaard, ChemBioChem, 2012, 13, 2537 CrossRef CAS PubMed; (g) D. Geerdink, J. Buter, T. A. van Beek and A. J. Minnaard, Beilstein J. Org. Chem., 2014, 10, 761 CrossRef PubMed; (h) J.-H. Tay, A. J. Argüelles, M. D. DeMars II, P. M. Zimmerman, D. H. Sherman and P. Nagorny, J. Am. Chem. Soc., 2017, 139, 8570 CrossRef CAS PubMed.
- N. Yang, Z. S. Su, X. M. Feng and C. W. Hu, Chem.–Eur. J., 2015, 21, 7264 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C, 19F NMR, and HPLC spectra (PDF). X-ray crystallographic data for 5a, L-RaPr2-tBu/Sc(OTf)3 (CIF). CCDC 1848335 and 1856535. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc01563a |
| This journal is © The Royal Society of Chemistry 2019 |