Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An unusual [4 + 2] fusion strategy to forge meso-N/O-heteroarene-fused (quinoidal) porphyrins with intense near-infrared Q-bands†
Chengming
Li
a,
Lei
Zhu
b,
Wenbo
Liang
a,
Rongchuan
Su
a,
Jiangliang
Yin
a,
Yanmei
Hu
a,
Yu
Lan
 b,
Di
Wu
b,
Di
Wu
 *a and
Jingsong
You
*a and
Jingsong
You
 *a
*a
aKey Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu 610064, P. R. China. E-mail: jsyou@scu.edu.cn
bSchool of Chemistry and Chemical Engineering, Chongqing Key Laboratory of Theoretical and Computational Chemistry, Chongqing University, Chongqing 400030, P. R. China
First published on 17th June 2019
Abstract
Here we present a divergent synthesis of brand-new types of meso-N/O-heteroarene-fused (quinoidal) porphyrins through Rh-catalyzed β-C–H activation/annulation of 5,15-dioxoporphyrins and dioxime derivatives with alkynes, in which the synthetic disconnections are difficult to access through the commonly used intramolecular cyclization strategy. Using the O-methyl oxime as a traceless oxidizing directing group, the meso-N-embedded pyridine-fused anti-quinoidal porphyrin 3 and pyridinium-fused cation 4 are formed with controllable chemoselectivity and complete anti-selectivity. Replacing the exocyclic oxime with a carbonyl group delivers the pyran-fused porphyrin 5, achieving structural conversion from a quinoidal conformation to a stable porphyrin macrocycle. Further oxidation of the expanded dimer 5ea gives the oxonium 6, which exhibits intense near-infrared (NIR) Q-bands up to 1300 nm. Theoretical studies demonstrate that the incorporation of a heteroatom at the meso-position enables more effective π-extension, resulting in a 22π aromatic (vs. 18π aromatic) character of pyran-fused porphyrins (syn/anti-5aa). Compared with the commercially available methylene blue (MB), syn-5al exhibits a better ability (ΦΔ = 0.61) to sensitize singlet oxygen (1O2) when irradiated with a 680 nm laser beam, and has potential as a photodynamic therapy (PDT) photosensitizer in the body's therapeutic window (650–900 nm).
Introduction
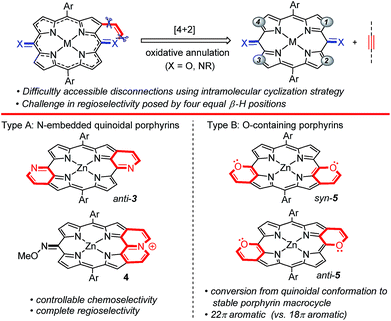
Much attention has been paid to structurally modified π-conjugated porphyrins with unique optical and electronic properties.1–4 Fusing an aromatic segment directly to the porphyrin peripheral framework is undoubtedly one of the most efficient methods to expand conjugated electronic systems,5–8 among which meso-heteroatom-containing heteroarene-fused porphyrins are an attractive research subject. The coplanarization of the lone pair of peripheral meso-heteroatoms with the π-electronic system of the porphyrin macrocycle enables more efficient π-extension.6,7 The commonly used synthetic strategies rely on the coupling reactions of meso/β-halide with arylamine, lithium diphenylphosphide, pyridine-2-thiol or 2-trimethylsilylphenyl zinc chloride, followed by intramolecular cyclization. Despite significant advances, these strategies generally involve the tedious multistep synthesis of porphyrin precursors, leading to a relatively low synthetic efficiency and limited structural diversity of fused porphyrins. Thus, developing straightforward access to these types of heteroarene-fused porphyrins is an appealing task in porphyrin chemistry.Transition metal-catalyzed annulations with alkynes through chelation-assisted C–H activation have been proved to be one of the most efficient accesses to π-conjugated heterocycles over the last decade.9,10 However, peripheral modification to construct heteroarene-fused porphyrins through this strategy remains undeveloped. Considering that chelating heteroatoms such as nitrogen and oxygen are able to serve as both the directing group and the merging functionality,11 we envisaged that meso-heteroatom-containing six-membered heteroarene-fused (quinoidal) porphyrins could be rapidly constructed by direct [4 + 2] oxidative annulation of 5,15-dioxoporphyrins and their dioxime derivatives with alkynes through β-H cleavage, in which the synthetic disconnections are difficult to access without a C–H activation/annulation strategy (Scheme 1).12
 | ||
| Scheme 1 β-C–H activation/annulation of 5,15-dioxoporphyrins and their dioxime derivatives with alkynes for the synthesis of heteroarene-fused (quinoidal) porphyrins. | ||
The regioselective control of the above [4 + 2] oxidative annulation strategy could be a challenging task owing to the four equal β-H positions. In this work, using the internally oxidative ortho-C–H activation strategy, we have established a rhodium-catalyzed oxidative [4 + 2] annulation of readily available O-methyl dioximes of 5,15-dioxoporphyrins with alkynes, affording the doubly pyridine-fused anti-quinoidal porphyrins 3 and pyridinium-fused cations 4 with controllable chemoselectivity and complete regioselectivity (Scheme 1, type A). When 5,15-dioxoporphyrins are employed as the substrate, the doubly pyran-fused porphyrins 5 are obtained rather than quinoidal products (Scheme 1, type B).
Results and discussion
Synthesis and X-ray crystallographic analysis of pyridine/pyridinium-fused quinoidal porphyrins
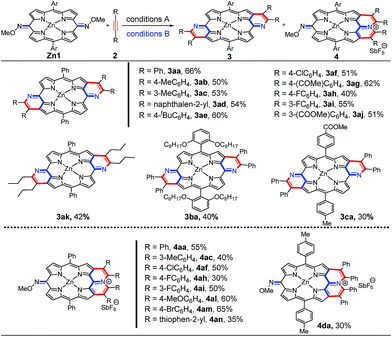
We initiated our work by using O-methyl dioxime of the 5,15-dioxoporphyrin Zn1a and diphenylacetylene as the substrates in the presence of [Cp*RhCl2]2 (5 mol%), AgSbF6 (20 mol%), and Ag2O (2 equiv.) in DCE (1.0 mL) at 120 °C for 24 h (Table S1†). To our delight, the doubly pyridine-fused anti-quinoidal porphyrin 3aa was afforded as a major product in 28% yield without the syn isomer (Table S1,† entry 1). Meanwhile, we isolated another product with a much larger polarity, the pyridinium-fused cation 4aa with the SbF6− counter anion, in 12% yield. Further optimization of reaction conditions improved the yield of 4aa to 55% along with less than 10% yield of 3aa (Table S1,† entry 9). The optimal catalytic system, comprising [Cp*RhCl2]2 (5 mol%) and AgSbF6 (20 mol%) in tetrahydrofuran (0.5 mL) at 100 °C for 24 h, afforded 3aa in 66% yield (Table S1,† entry 17).With the optimized conditions for 3 and 4, we explored the scope of internal alkynes and quinoidal porphyrins Zn1 (Scheme 2). The annulation has relatively wide substrate scopes. Diaryl alkynes with either an electron-donating or electron-withdrawing group at the phenyl ring gave the desired products 3ab–3aj in medium yields. The unsymmetrical Zn1c even gave the desired 3ca with complete anti-selectivity. In addition, a family of pyridinium-fused 4 was assembled in the presence of the oxidant and NaSbF6. This protocol was compatible with both the electron-donating groups and electron-withdrawing halo substituent, delivering the desired products in medium yields. 1,2-Di(thiophen-2-yl)ethyne gave 4an in 35% yield.
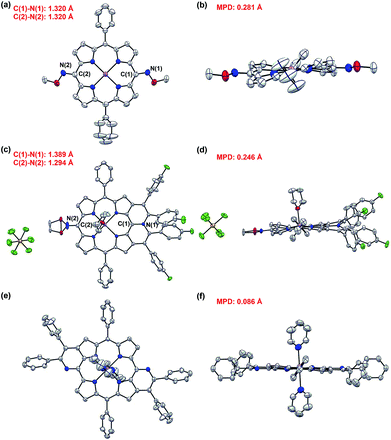
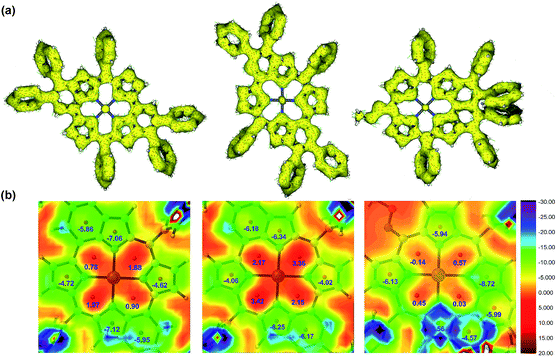
The exact structures have been confirmed by single crystal X-ray analysis of Zn1a, 3aa and 4ah.13Zn1a exhibits a distorted configuration owing to its non-aromatic quinoidal conformation (Fig. 1a and b). In comparison, the conformation of 4ah flattens slightly through π-conjugation between the pyridinium-fused moiety and quinoidal porphyrin framework (Fig. 1c and d). With the conjugative effect of two pyridine rings, 3aa appears to be fairly coplanar (Fig. 1e and f). As a result, the mean plane deviation (MPD) values of Zn1a, 4ah and 3aa exhibit a gradually flattening trend.14 The C1–N1 double bond length of 4ah is much longer than that of Zn1a, suggesting a weakened quinoidal character through the fusion of pyridinium rings.12
Synthesis and characterization of doubly pyran-fused porphyrins
Although a great number of peripherally fused porphyrins have been synthesized, meso-O-containing heteroarene-fused porphyrins have not been reported yet. In this work, we attempted the reaction of the 5,15-dioxoporphyrin Zn2a and diphenylacetylene at 120 °C in the presence of [Cp*RhCl2]2 (5 mol%), AgSbF6 (20 mol%), and Ag2O (2 equiv.) in DCE (1.0 mL) (Table S2†). The doubly pyran-fused porphyrin 5aa was isolated in 33% yield (Table S2,† entry 1). A mixture of syn- and anti-isomers was indicated by the 1H NMR spectrum, which clearly exhibits proportional signals for the three pairs of β protons. After extensive optimization of reaction conditions, the yield of 5aa was improved to 60% (syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti = ca. 2
anti = ca. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table S2,† entry 6). Unexpectedly, the single crystals of syn-5aa were obtained by recrystallization,13 which confirms the structural conversion from a quinoidal conformation to the stable porphyrin macrocycle.
1) (Table S2,† entry 6). Unexpectedly, the single crystals of syn-5aa were obtained by recrystallization,13 which confirms the structural conversion from a quinoidal conformation to the stable porphyrin macrocycle.
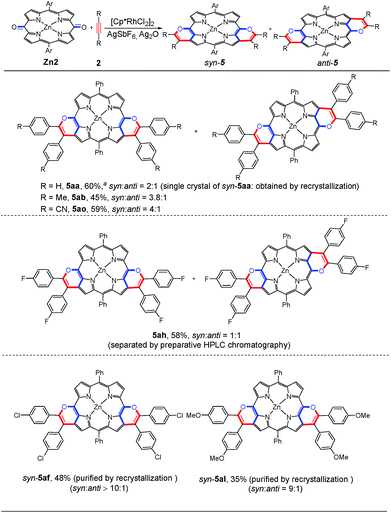
Subsequently, we investigated the scope of the substrates. As shown in Scheme 3, this annulation of Zn2 with a variety of alkynes typically gives a mixture of syn- and anti-isomers. 5aa, 5ab and 5ao were obtained with a relatively low regioselectivity ranging from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (syn
1 (syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti). Very interestingly, when the diaryl alkynes bear the chloro or methoxyl group, the target products 5af and 5al were formed with a large ratio of syn- and anti-isomers (up to >10/1). From these observations, we speculated that after the first annulation with the alkyne, the electron-rich character of the same side pyrrole ring could be further enhanced via the p–π conjugation effect between the chloro or methoxyl group and the phenyl ring. Thus, the second β-H cleavage tends to occur at the same side pyrrole ring. As a result, the syn-configuration is dominant in the [4 + 2] annulation process. Notably, a mixture of 5af could be further purified by recrystallization to give a pure syn-5af, which was confirmed by 1H NMR and single crystal X-ray analysis (Table S10†). Recrystallization of 5al could also afford pure syn-5al.
anti). Very interestingly, when the diaryl alkynes bear the chloro or methoxyl group, the target products 5af and 5al were formed with a large ratio of syn- and anti-isomers (up to >10/1). From these observations, we speculated that after the first annulation with the alkyne, the electron-rich character of the same side pyrrole ring could be further enhanced via the p–π conjugation effect between the chloro or methoxyl group and the phenyl ring. Thus, the second β-H cleavage tends to occur at the same side pyrrole ring. As a result, the syn-configuration is dominant in the [4 + 2] annulation process. Notably, a mixture of 5af could be further purified by recrystallization to give a pure syn-5af, which was confirmed by 1H NMR and single crystal X-ray analysis (Table S10†). Recrystallization of 5al could also afford pure syn-5al.
In consideration of the presence of syn- and anti-configurations, we were naturally interested in their different optical and electronic properties. The syn- and anti-isomers of 5ah were separated and purified by preparative HPLC chromatography (Fig. S1–S3†). Their characterization was then carried out by 1H NMR and IR spectroscopy. As shown in Fig. 2, the 1H NMR spectrum demonstrates that the mixture of 5ah contains three pairs of β-H signals in the downfield region. It is worth indicating that the 1H NMR spectra of syn-5ah and anti-5ah cannot be simply overlapped with that of the mixture of 5ah, which is probably due to the intermolecular π–π stacking interactions. The concentration-dependent 1H NMR spectral shifts of 5ah in CDCl3 solution display such interactions (Fig. S4†). The IR spectra of the syn- and anti-configurations are also different (Fig. S5 and S6†).
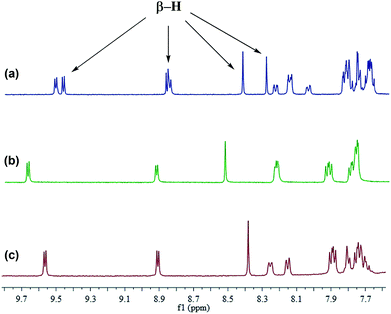 | ||
| Fig. 2 1H NMR spectra (400 MHz) of (a) the mixture of anti and syn isomers of 5ah, (b) anti-5ah, and (c) syn-5ah in CDCl3. | ||
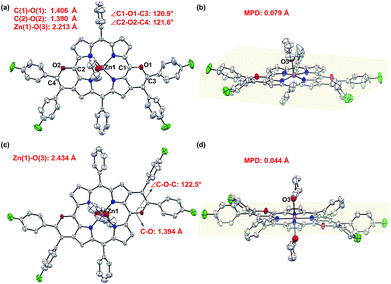
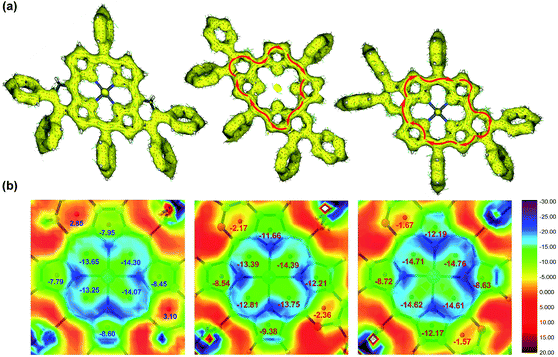
Single crystal X-ray diffractometry of syn-5ah or anti-5ah confirmed the spectroscopically derived connectivity of the two regioisomers and proved them both to be essentially planar, with only minor differences in the mean plane deviation of their C24N4O2Zn chromophores (Fig. 3).14 Slight differences in the metric parameters of the frameworks of both derivatives highlight the small structural effects of the two different orientations of two pyran rings, although they are, as shown above, electronically most distinct from each other.
Theoretical calculations on aromatic delocalization
To gain insight into the changes of the aromatic contribution caused by the peripherally fused-heteroarenes, we calculated the anisotropy of the induced current density (ACID) and the nucleus-independent chemical shift (NICS) of porphyrins 3aa, 4aa, and syn- and anti-5aa. In the meantime, the anti-quinoidal porphyrin M3 and porphyrin syn-M4 were simulated and calculated as references (Scheme 4). M3 is assigned to be nonaromatic due to Clar's sextet of peripheral benzenes, which is similar to the reported quinoidal porphyrin.15 The calculated NICS(1) values of 3aa demonstrate the local aromaticity of two pyridine rings, resulting in its nonaromatic character being similar to that of M3 (Fig. 4). The approximate zero NICS(1) values of 4aa reveal the nonaromatic character.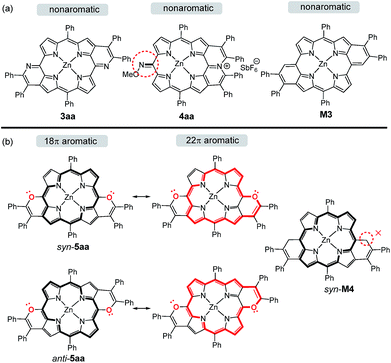 | ||
| Scheme 4 The aromaticity of 3aa, 4aa, simulated M3 and syn-M4, and the resonance structures of syn-5aa and anti-5aa. | ||
Differing by the appearance of a disrupted node at the exocyclic methene bonds of syn-M4, both syn- and anti-5aa exhibit a strong clockwise current density flow, which can be drawn as a 22π conjugation pathway (Fig. 5). The calculated NICS(1) values in the inner core region of anti-5aa are more negative than those of syn-5aa, suggesting the enhanced aromatic contribution by peripheral anti fusion. These results further account for the more downfield shifted β-H signals of anti-5ah than of syn-5ah in the 1H NMR spectra shown in Fig. 2.5b Notably, the two pyrrole rings adjacent to the fused pyran moieties show more negatively shifted NICS(1) values than the others. These results demonstrate that the weak aromatic contribution of fused pyran moieties is non-negligible and finally enhances the 22π-electron conjugated circuit through the p–π conjugation effect between the meso-oxygen atom and the porphyrin macrocycle (Scheme 4).
Synthesis of the pyrylium-fused porphyrin dimer
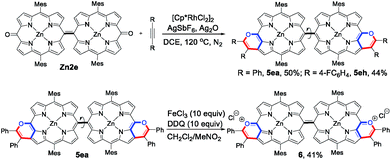
As an extension, the 5,15-dioxoporphyrin dimer Zn2e was prepared16 and could undergo annulation with alkynes, delivering the doubly pyran-fused porphyrin dimers 5ea and 5eh in 50% and 44% yields, respectively (Scheme 5). Notably, 5ea and 5eh displayed a single configuration due to the rotation of the exocyclic C–C linkage. Treatment of 5ea with FeCl3 and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) afforded a sole product, in which the two pyran rings were oxidized to deliver the doubly pyrylium-fused porphyrin dimer 6. The ESI mass spectrum of 6 exhibited its parent ion peak at m/z 799.2404 (calcd for C104H78N8O2Zn2, m/z 799.2410 [M]+), corresponding to its quinoidal oxonium dication.Optical and electronic properties
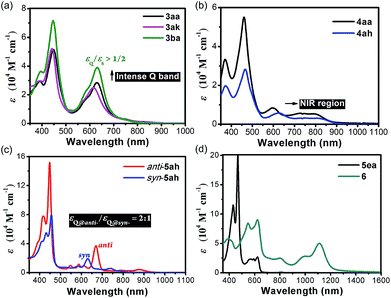
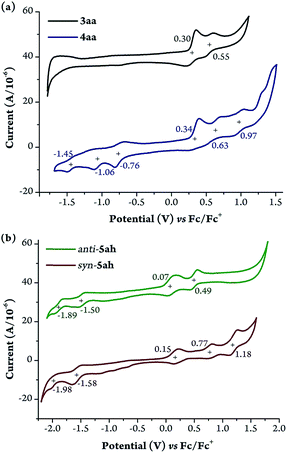
The absorption and emission spectra were measured in chromatographically pure CH2Cl2 (Fig. 6 and Tables S3 and S4†). 3 show quite intense Q-band absorption with a ratio of εQ/εS up to over 1/2. The cations 4 have significantly red-shifted Q bands expanding up to 900 nm. Through the fusion of pyran rings, syn-5ah and anti-5ah show red-shifted Q-band absorption spectra, with a weak absorption tail up to 900 nm (Fig. 6c), indicating that their conjugated π-electron systems are largely perturbed by peripheral moieties. The intensity of the Q-band absorption of anti-5ah is about twice that of syn-5ah. 5ea shows broader and enhanced Q bands (Fig. 6d). After further oxidation, the oxonium dication 6 exhibits Soret bands at 548 and 622 nm with an intense red-shifted Q band centered at 1113 nm, which demonstrates a great ability to absorb both the visible and near-infrared light up to approximately 1300 nm.Cyclic voltammetric studies were then performed under N2 (Fig. 7 and Table S5; for details, see the ESI†). 3aa showed two quasi-reversible oxidation waves without any reduction wave (Fig. 7a). In contrast, 4aa exhibited three positive oxidation potentials and three reduction waves. Both syn and anti-5ah displayed reversible oxidation waves and two quasi-reversible reduction waves (Fig. 7b). The smaller HOMO–LUMO gap for anti-5ah (1.57 eV vs. 1.73 eV) just accounts for the more red-shifted Q-band absorption of anti-5ah than of syn-5ah (Fig. 6).
Singlet oxygen quantum yields (ΦΔ) in the red/NIR Q-band region
Photodynamic therapy (PDT), a combination of a photosensitizer, light, and molecular oxygen (3O2), has been proved to be an emerging and noninvasive therapeutic technique for cancers and other benign diseases.17,18 Considering that the pyridine/pyran-fused porphyrins exhibit enhanced Q-band absorption in the red/NIR region, we were interested in their potential as the photosensitizer in the body's therapeutic window (650–900 nm). After serial screening, syn-5af and syn-5al exhibited a great ability to sensitize 1O2 (for details, see ESI Fig. S7–S11†). While the ΦΔ value of methylene blue (MB, as the standard) was found to be 0.52 in DMF, the ΦΔ values of syn-5af and syn-5al were calculated to be 0.54 and 0.63, respectively. Furthermore, syn-5al exhibits a great ability to sensitize 1O2 with a ΦΔ value of 0.61 when irradiated with a 680 nm laser beam.Conclusions
In summary, we have established a rhodium-catalyzed [4 + 2] annulation strategy to rapidly construct pyridine-fused anti-quinoidal porphyrins 3, pyridinium-fused cations 4, and doubly pyran-fused porphyrins 5, which exhibit controllable chemoselectivity and regioselectivity. Further oxidation of the expanded porphyrin dimer 5ea delivers the oxonium dication 6 with intense near-infrared absorption up to 1300 nm. Theoretical investigation based on the ACID and NICS(1) values reveals the 22π aromatic (vs. 18π aromatic) character of pyran-fused porphyrin (syn/anti-5aa) caused by the π/p–π conjugated effect between the macrocyclic core and meso-heteroarene. Compared with the commercially available MB, syn-5al exhibits a better ability to sensitize 1O2 when irradiated with a 680 nm laser beam, and exhibits potential as a PDT photosensitizer in the body's therapeutic window. The straightforward access for discovering organic functional molecules developed herein has well exemplified the charm of C–H activation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the National NSF of China (no. 21432005, 21772128 and 21772133).Notes and references
- T. Tanaka and A. Osuka, Chem. Soc. Rev., 2015, 44, 943 RSC
.
-
(a) A. Tsuda and A. Osuka, Science, 2001, 293, 79 CrossRef CAS PubMed
; (b) T. K. Ahn, K. S. Kim, D. Y. Kim, S. B. Noh, N. Aratani, C. Ikeda, A. Osuka and D. Kim, J. Am. Chem. Soc., 2006, 128, 1700 CrossRef CAS PubMed
.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242 CrossRef CAS PubMed
.
- M. K. Kuimova, S. W. Botchway, A. W. Parker, M. Balaz, H. A. Collins, H. L. Anderson, K. Suhling and P. R. Ogilby, Nat. Chem., 2009, 1, 69 CrossRef CAS PubMed
.
-
(a) J. Akhigbe, M. Zeller and C. Brückner, Org. Lett., 2011, 13, 1322 CrossRef CAS PubMed
; (b) Y. Mitsushige, S. Yamaguchi, B. S. Lee, Y. M. Sung, S. Kuhri, C. A. Schierl, D. M. Guldi, D. Kim and Y. Matsuo, J. Am. Chem. Soc., 2012, 134, 16540 CrossRef CAS PubMed
; (c) M. Akita, S. Hiroto and H. Shinokubo, Angew. Chem., Int. Ed., 2012, 51, 2894 CrossRef CAS PubMed
; (d) K. Gao, N. Fukui, S. Jung, H. Yorimitsu, D. Kim and A. Osuka, Angew. Chem., Int. Ed., 2016, 55, 13038 CrossRef CAS PubMed
; (e) N. Fukui, S.-K. Lee, K. Kato, D. Shimizu, T. Tanaka, S. Lee, H. Yorimitsu, D. Kim and A. Osuka, Chem. Sci., 2016, 7, 4059 RSC
.
- N. Fukui, W.-Y. Cha, S. Lee, S. Tokuji, D. Kim, H. Yorimitsu and A. Osuka, Angew. Chem., Int. Ed., 2013, 52, 9728 CrossRef CAS PubMed
.
-
(a) K. Fujimoto, J. Oh, H. Yorimitsu, D. Kim and A. Osuka, Angew. Chem., Int. Ed., 2016, 55, 3196 CrossRef CAS PubMed
; (b) K. Fujimoto and A. Osuka, Chem. Sci., 2017, 8, 8231 RSC
.
- M. Berthelot, G. Hoffmann, A. Bousfiha, J. Echaubard, J. Roger, H. Cattey, A. Romieu, D. Lucas, P. Fleurat-Lessard and C. H. Devillers, Chem. Commun., 2018, 54, 5414 RSC
.
-
(a) M. Gulías and J. L. Mascareñas, Angew. Chem., Int. Ed., 2016, 55, 11000 CrossRef PubMed
; (b) Y. Yang, K. Li, Y. Cheng, D. Wan, M. Li and J. You, Chem. Commun., 2016, 52, 2872 RSC
.
-
(a) S. Rakshit, F. W. Patureau and F. Glorius, J. Am. Chem. Soc., 2010, 132, 9585 CrossRef CAS PubMed
; (b) S. Mochida, M. Shimizu, K. Hirano, T. Satoh and M. Miura, Chem.–Asian J., 2010, 5, 847 CrossRef CAS PubMed
; (c) X. Tan, B. Liu, X. Li, B. Li, S. Xu, H. Song and B. Wang, J. Am. Chem. Soc., 2012, 134, 16163 CrossRef CAS PubMed
; (d) J. Jayakumar, K. Parthasarathy and C.-H. Cheng, Angew. Chem., Int. Ed., 2012, 51, 197 CrossRef CAS PubMed
.
-
(a) Y. Zhao, S. Li, X. Zheng, J. Tang, Z. She, G. Gao and J. You, Angew. Chem., Int. Ed., 2017, 56, 4286 CrossRef CAS PubMed
; (b) J. Yin, M. Tan, D. Wu, R. Jiang, C. Li and J. You, Angew. Chem., Int. Ed., 2017, 56, 13094 CrossRef CAS PubMed
.
- The existing aromatic-fused quinoidal porphyrins feature the exocyclic C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds, whereas the aromatic-fused quinoidal porphyrins with the nitrogen atom at the meso-position instead of the carbon atom have rarely been presented. See: W. Zeng, B. S. Lee, Y. M. Sung, K.-W. Huang, Y. Li, D. Kim and J. Wu, Chem. Commun., 2012, 48, 7684 RSC
C double bonds, whereas the aromatic-fused quinoidal porphyrins with the nitrogen atom at the meso-position instead of the carbon atom have rarely been presented. See: W. Zeng, B. S. Lee, Y. M. Sung, K.-W. Huang, Y. Li, D. Kim and J. Wu, Chem. Commun., 2012, 48, 7684 RSC .
- CCDC 1857684 (Zn1a), 1852362 (3aa), 1852363 (4ah), 1865973 (syn-5aa), 1852372 (syn-5af), 1857685 (syn-5ah), and 1852366 (anti-5ah) contain the ESI crystallographic data for this paper.†.
- The MPD values of Zn1a, 3aa, 4ah, and syn- and anti-5ah (25, 31, 30, 31 and 31 atoms, respectively) are defined by the core atoms consisting of meso-carbons, pyrrole units, zinc atoms and heteroarene-fused units.
- M. Umetani, K. Naoda, T. Tanaka, S.-K. Lee, J. Oh, D. Kim and A. Osuka, Angew. Chem., Int. Ed., 2016, 55, 6305 CrossRef CAS PubMed
.
- Y. Jun-i, N. Fukui, K. Furukawa and A. Osuka, Chem.–Eur. J., 2018, 24, 1528 CrossRef CAS PubMed
.
- For selected reviews, see:
(a) J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Chem. Rev., 2010, 110, 2795 CrossRef CAS PubMed
; (b) M. Ethirajan, Y. Chen, P. Joshi and R. K. Pandey, Chem. Soc. Rev., 2011, 40, 340 RSC
.
- J. Tian, L. Ding, H.-J. Xu, Z. Shen, H. Ju, L. Jia, L. Bao and J.-S. Yu, J. Am. Chem. Soc., 2013, 135, 18850 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed information on experimental procedures, characterization data, computational calculations, crystallographic and spectroscopic data, and X-ray crystal structures (CIF). CCDC 1857684 (Zn1a), 1852362 (3aa), 1852363 (4ah), 1865973 (syn-5aa), 1852372 (syn-5af), 1857685 (syn-5ah) and 1852366 (anti-5ah). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc01596e |
| This journal is © The Royal Society of Chemistry 2019 |