Open Access Article
Open Access ArticleA new, substituted palladacycle for ppm level Pd-catalyzed Suzuki–Miyaura cross couplings in water†
Balaram S.
Takale
 a,
Ruchita R.
Thakore
a,
Sachin
Handa
a,
Ruchita R.
Thakore
a,
Sachin
Handa
 b,
Fabrice
Gallou
b,
Fabrice
Gallou
 c,
John
Reilly
d and
Bruce H.
Lipshutz
c,
John
Reilly
d and
Bruce H.
Lipshutz
 *a
*a
aDepartment of Chemistry and Biochemistry, University of California, Santa Barbara, California 93106, USA. E-mail: lipshutz@chem.ucsb.edu
bDepartment of Chemistry, University of Louisville, Louisville, KY 40292, USA
cNovartis Pharma AG, Basel, Switzerland
dNovartis Institute for Medical Research, Cambridge, MA 02139, USA
First published on 6th August 2019
Abstract
A newly engineered palladacycle that contains substituents on the biphenyl rings along with the ligand HandaPhos is especially well-matched to an aqueous micellar medium, enabling valued Suzuki–Miyaura couplings to be run not only in water under mild conditions, but at 300 ppm of Pd catalyst. This general methodology has been applied to several targets in the pharmaceutical area. Multiple recyclings of the aqueous reaction mixture involving both the same as well as different coupling partners is demonstrated. Low temperature microscopy (cryo-TEM) indicates the nature and size of the particles acting as nanoreactors. Importantly, given the low loadings of Pd invested per reaction, ICP-MS analyses of residual palladium in the products shows levels to be expected that are well within FDA allowable limits.
Introduction
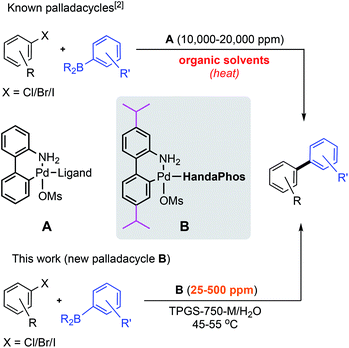
In a recent review by Alami and Messaoudi,1 palladacycles were characterized as among the “most powerful” pre-catalysts to highly reactive, mono-ligated forms of Pd(0).2 While they are stable, quite convenient, and broadly applicable, these features may not be sufficiently attractive for long term usage typically in the 1–5 mol% range. That is, not only is awareness of the endangered status of Pd gaining in appreciation, such reagents are also almost invariably used in environmentally egregious organic solvents and with limited levels of solvent and precious metal recycling.3 Put another way, and notwithstanding Nobel Prize-level recognition bestowed in 2010 on Pd-catalyzed Suzuki–Miyaura (SM) cross-coupling chemistry developed several decades earlier,4 such an approach to modern Pd-based catalysis as practiced today is both costly and not sustainable. Access to worldwide supplies, even under the best of geopolitical circumstances, is determined by current limits of technology that prevent access to metals that lie too deep within the Earth's surface.5One solution to this inevitable shortage calls for switching, in large measure, from a petroleum- to a water-based discipline,6 akin to the role of water as the reaction medium in nature, in general, and biocatalysis in particular.7 Along with this gradual transition comes myriad opportunities for developing new catalysts engineered to function both within an altered reaction medium and under newly unfolding rules for which analogies in organic solvent are non-existent.8 Palladacycles present one such opportunity where, under traditional conditions (i.e., use in organic solvents), the focus has been on modifications that include, e.g., the nature of the leaving group. Such design changes that feature steric and stereoelectronic effects, conformational biases, etc., have little to do with palladacycle solubility. In an aqueous medium containing micellar-based nanoreactors,9 however, solubilization becomes a crucial parameter. Thus, one key to successful couplings in water involves influencing the binding constant of a reagent to the micellar inner core: the greater the incentive to enter the site of reaction, the more catalytic activity is to be expected and the lower the catalyst loadings. Hence, the question arises: could the appropriate substitution pattern on the biaryl skeleton within a palladacycle pre-catalyst enhance its micellar entry, and thereby reduce the required level of otherwise precious metal, and associated (oftentimes equally precious) ligand, in a cross-coupling reaction?
Substituted biarylamine-based palladacycles are currently unknown,10 since in organic solvent there is no reason to pursue such derivatization. In water, however, where new rules are operating,8b the prospects for not only providing the convenience of palladacycles that lead to especially reactive catalysts as well as the potential for addressing the endangered nature of platinoids provides more than ample justification for investigating this non-traditional approach. In this report we describe such a newly adorned palladacycle pre-catalyst that, indeed, allows for a general and environmentally responsible process for SM couplings to be run, in most cases, in water under mild conditions and at the 300 ppm (0.03 mol%) level of Pd (Scheme 1).
Results and discussion
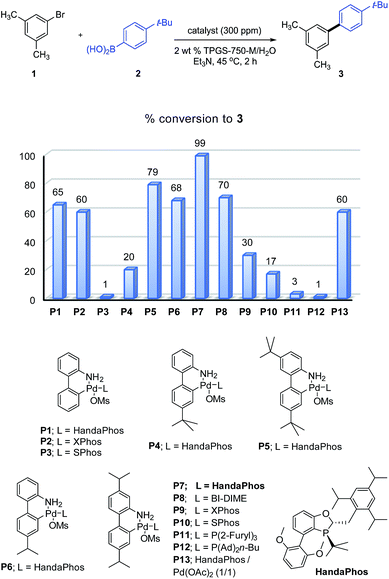
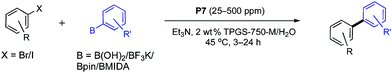
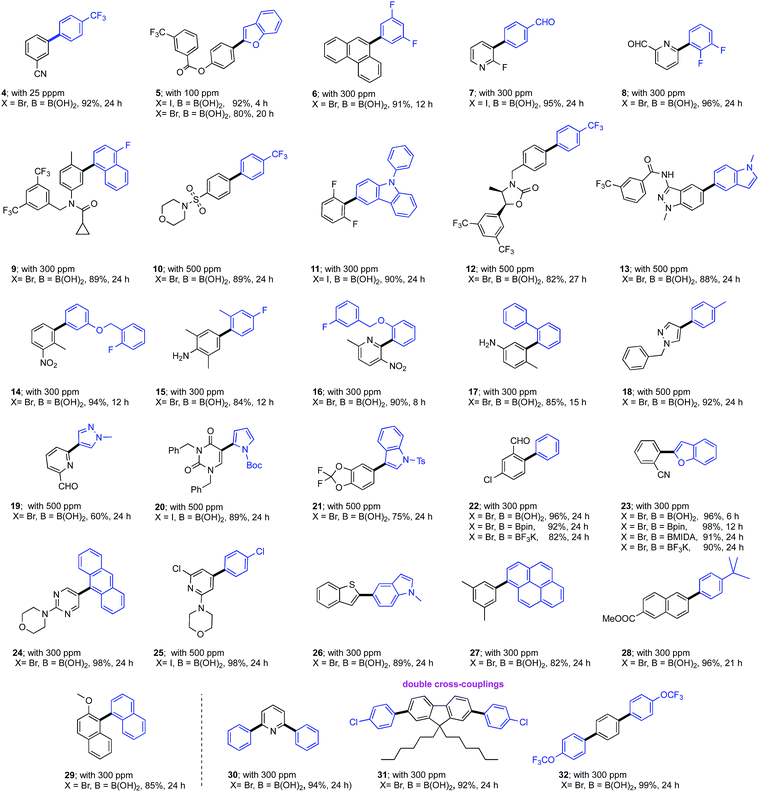
Several newly substituted palladacycles were prepared initially containing the ligand rac-HandaPhos11 (P1, P4, P5, P6 and P7), the structures of which are shown in Scheme 2. These included either one or two lipophilic t-butyl residues (as in P4 and P5, respectively), along with those bearing one (P6) or two isopropyl moieties (P7), as compared to the parent array (P1). Several additional ligands commonly used within palladacycles were also prepared and tested under identical conditions in the model reaction between bromide 1 and boronic acid 2 to arrive at biaryl 3. Clearly, the most effective catalyst, by far, is the diisopropyl-substituted HandaPhos palladacycle, P7. Surprisingly, even the di-t-butyl analog, P5, was not as effective. What may also be found striking at first, but is perfectly in harmony with the “new rules” associated with this chemistry in water,8 are the results observed for catalysts P9 and P10, where HandaPhos has been replaced by XPhos and SPhos, respectively. While both are excellent choices for SM couplings in traditional organic solvents (e.g., toluene or dioxane),12 they are non-functional at 300 ppm in water. Likewise, other well-known ligands that make up catalysts P11 and P12 were not competitive under these conditions. Additional optimization studies regarding the choice of surfactant, base, as well as results using organic solvents are described in the associated ESI.†Several examples of SM couplings were carried out using 300 ppm of catalyst P7, as summarized in Table 1. While most cases were amenable to this very low loading of Pd, some could be conducted at levels even down to 25 (4) to 100 (5) ppm of P7. On the other hand, some cases (10, 12, 13, 18–21,13 and 25) required up to 500 ppm, possibly reflecting competition by the product for palladium. Both electron-donating and -releasing groups in the educt are tolerated. Heterocycles present in either the halide or boronic acid partner could be used. Partners with protecting groups are easily coupled (20 and 21). Polyaromatics such as 27 and 29 are easily fashioned. Alternatives to boronic acids, including Bpin, Molanderate BF3K salts,14 and MIDA boronates15 (see 22 and 23) appear to be compatible partners as well. Double SM couplings using the corresponding precursor dibromides proceeded smoothly to give the anticipated products (30, 31, and 32), using what is, formally, only 150 ppm of this Pd catalyst per bond formed.
| a Reaction conditions unless otherwise noted: 0.5 mmol aryl halide, 0.6 mmol aryl boronic acid, 1.0 mmol Et3N, 25–500 ppm P7 stirred at 45 °C in TPGS-750-M/H2O (0.5 M); isolated yields are shown. Double SM couplings were carried out using 1.2 mmol of aryl boronic acid, 2.0 mmol of Et3N, and 10% THF as a co-solvent. |
|---|
|
The importance of organic co-solvents has also been addressed, as these additives can play a dramatic role in scaling up reactions under micellar conditions.16 The co-solvent effect is responsible not only for increasing solubility of highly crystalline educts, but also enlarges micellar size, thereby expanding the interior volume available for reaction. The observed impact of three organic solvents used as 10 vol% in this aqueous surfactant system is shown in Scheme 3 involving coupling partners 33 and 34. Each solvent (THF, toluene, and acetone) was found to increase the rate of conversion. Analysis of the medium for 2 wt% TPGS-750-M/H2O vs. that with 10% THF by cryo-TEM (Scheme 3, bottom) revealed the enlargement of the former (ca. 50 nm) to ca. 200 nm due to the presence of THF, suggesting that larger nanoreactors may be responsible for enhancing the overall rates of these cross-couplings.
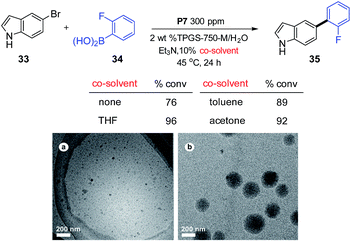 | ||
| Scheme 3 Effect of co-solvent. Image (a) cryo-TEM of TPGS-750-M/H2O; image (b) cryo-TEM of 10% THF in TPGS-750-M/H2O. | ||
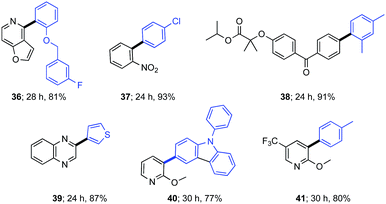
The potential use of less reactive aryl chlorides was briefly examined at the 500 ppm level of Pd catalysis (0.05 mol%). As the examples in Table 2 show, a variety of aromatic and heteroaromatic chlorides and boronic acids could be employed, arriving at the targeted biaryls in good isolated yields. Included in this study is the late stage derivatization of aryl chloride fenofibrate17 to analog 38.
| a Reaction conditions unless otherwise mentioned: 0.5 mmol aryl chloride, 0.6 mmol arylboronic acid, 1.0 mmol Et3N, 500 ppm P7 stirred at 45 °C in TPGS-750-M/H2O (0.5 M) with 10% THF; isolated yields are shown. |
|---|
|
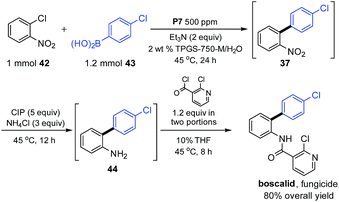
As an illustration of the opportunities to carry out multi-step processes given the commonality of reaction conditions (i.e., in aqueous nanoreactors at rt-45 °C), the commonly used fungicide boscalid18 could be prepared in three steps using a 1-pot protocol (Scheme 4). Initially, biaryl 37 was constructed that, without isolation, was subjected to nitro group reduction using our previously described carbonyl iron powder.19 The resulting aniline was then treated directly with 2-chloronicotynyl chloride. The final product, boscalid, was ultimately isolated in 80% overall yield. The seemingly incompatible addition of this acid chloride to this aqueous medium, while counter-intuitive at first, is yet another example of the “new rules” associated with chemistry in water. Reagents and/or reaction partners that are sensitive albeit insoluble in water simply do not hydrolyze or quench; rather, upon stirring they enter the hydrophobic inner micellar core where they react, usually as desired.
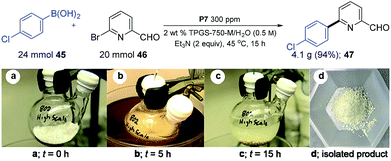
A multi-gram scale reaction between educts 45 and 46 was run in water using Pd catalyst P7 to document the prospects for scaling up these SM couplings (Scheme 5). Use of 24 mmol of 45 and 20 mmol of 46 in the presence of 40 mmol of Et3N were exposed to 300 ppm of P7. Stirring this heterogeneous mixture for 15 hours yielded 94% of the desired coupled product 47. In this case, the reaction was quite efficient in the absence of a co-solvent. That is, stirring was not an issue throughout the 15 h reaction period (see images (a–c)) The product 47 could be isolated as a white solid (image (d)), purified by simple filtration through silica gel.
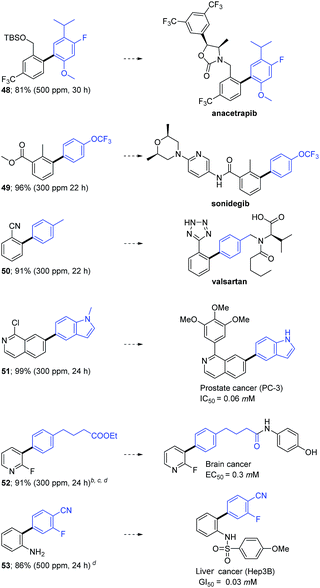
Biologically active targets, such as precursors to (a) Merck's anacetrapib (48),20a (b) sonidegib (49),20b and (c) Novartis' valsartan (50),20c could also be prepared efficiently under mild conditions using 300–500 ppm of catalyst P7 (Table 3). Additional representative examples of biaryls (51–53) en route to anticancer drugs are also to be found in this table.21
| a Reaction conditions unless otherwise noted: 0.5 mmol aryl halide, 0.6 mmol arylboronic acid, 1.0 mmol Et3N, cat P7 stirred at 45 °C in TPGS-750-M/H2O (0.5 M); isolated yields are shown. b The corresponding iodide was used as coupling partner. c The corresponding Bpin was used as the aryl boron compound. d Reaction temperature was 55 °C. |
|---|
|
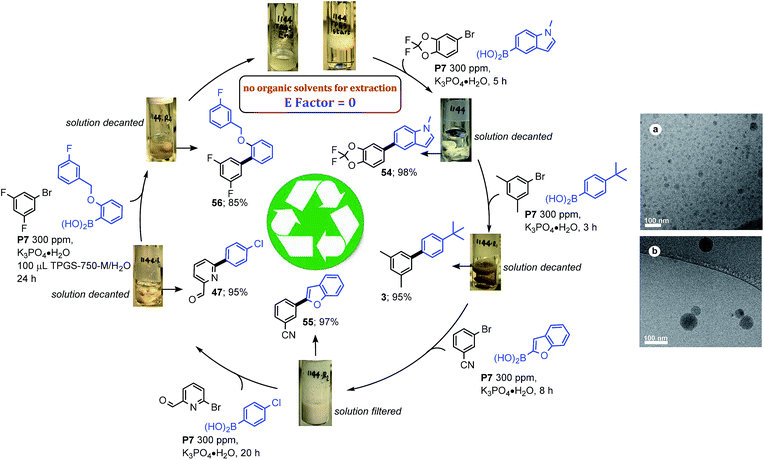
Facile recycling of the aqueous TPGS-750-M solution is an important aspect to this environmentally responsible technology, leading to very low levels of aqueous waste streams.22 By contrast, recycling of organic media typically requires fractional distillation to separate reaction and workup solvents for re-use. Scheme 6 illustrates just how effective a 2 wt% aqueous solution of TPGS-750-M can be, thereby dramatically minimizing aqueous waste streams. Recycling could be carried out using a different SM reaction with each of four recycles, following an initial coupling. Products were either separated via filtration or by decantation of the aqueous mixture; hence, individual extractions were not required prior to purification. cryo-TEM analysis of the aqueous mixture after five uses revealed that while the nanomicelles were of the same shape, they were unexpectedly larger (ca. 75 nm; Scheme 6).
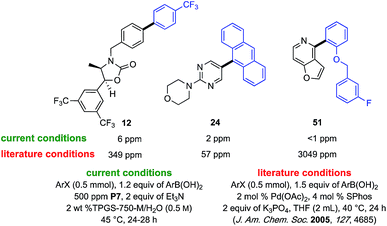
From the perspective of the pharmaceutical industry, it is commonly assumed that under traditional SM cross-coupling conditions the amount of residual Pd in the product is going to be outside of the acceptable 10 ppm limit imposed by the US FDA.23 Hence, additional processing is usually anticipated, potentially adding time and expense to the eventual API. But use of such low levels of Pd catalysts rarely exceed this limit. With catalyst P7 at the 300 and even 500 ppm loadings it was not surprising that, for the three cases randomly selected and examined by ICP-MS, no more than 6 ppm Pd was found for biaryls 12, 24, and 51 (Fig. 1). On the other hand, following traditional literature conditions used to make each of these biaryl products (e.g., 2 mol%, or 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm Pd), residual levels of Pd were found to be orders of magnitude greater.
000 ppm Pd), residual levels of Pd were found to be orders of magnitude greater.
Conclusions
In summary, a new palladacycle has been uncovered that mediates Suzuki–Miyaura couplings in water at the 300 ppm level of precious metal. Key to this methodology is placement of an isopropyl group on each aromatic ring of the biaryl skeleton making up the palladacycle, a substitution pattern that could not have been predicted given the lack of precedent for such pre-catalysts. Likewise, screening of several monophosphines, including some of the most commonly used for such Pd-catalyzed cross-couplings, ultimately identifying HandaPhos as the preferred ligand (i.e., P7), requires further study to rationalize the effectiveness of this novel ligand/palladacycle precursor combination. Applications to various targets within the pharma, agro, and materials domains have been demonstrated, along with the potential for large scale use, recycling of the aqueous reaction medium, and tandem 1-pot processes. The nature of the nanomicelles involved has been determined via cryo-TEM measurements, both initially as well as after use in the presence of added co-solvent. Residual levels of Pd in the products formed have been shown to be well within governmental limits for safety, further enhancing the attractiveness of this technology. The prognosis for use of the same pre-catalyst for other types of Pd-catalyzed cross-couplings (e.g., Stille, Sonogashira, and Heck couplings) looks encouraging, with the results from these ongoing studies to be reported in due course.Conflicts of interest
There are no conflicts to declare.Note added after first publication
This article replaces the version published on 13th August 2019, which contained errors in Scheme 3 and Table 2.Acknowledgements
Financial support provided by Novartis and PHT International (postdoctoral fellowship to B. S. T.) is warmly acknowledged with thanks.Notes and references
- A. Bruneau, M. Roche, M. Alami and S. Messaoudi, ACS Catal., 2015, 5, 1386 CrossRef CAS.
- (a) T. Kinzel, Y. Zhang and S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 1407 CrossRef PubMed; (b) N. C. Bruno, M. T. Tudge and S. L. Buchwald, Chem. Sci., 2013, 4, 916 RSC; (c) N. C. Bruno and S. L. Buchwald, Org. Lett., 2013, 15, 2876 CrossRef CAS; (d) N. C. Bruno, N. Niljianskul and S. L. Buchwald, J. Org. Chem., 2014, 79, 4161 CrossRef CAS.
- J. T. Bien, G. C. Lane and M. R. Oberholzer, Top. Organomet. Chem., 2004, 6, 263 CrossRef CAS.
- (a) C. C. C. Johansson Seechurn, A. Deangelis and T. J. Colacot, Introduction to New Trends in Cross-Coupling, in New Trends in Cross-Coupling: Theory and Applications, RSC Catalysis Series No. 21, ed. T. J. Colacot, The Royal Society of Chemistry, London, 2015, pp. 1–19 Search PubMed; (b) P. G. Gildner and T. J. Colacot, Organometallics, 2015, 34, 5497 CrossRef CAS; (c) J. Magano and J. R. Dunetz, Chem. Rev., 2011, 111, 2177 CrossRef CAS; (d) A. Biffis, P. Centomo, A. Del Zotto and M. Zecca, Chem. Rev., 2018, 118, 2249 CrossRef CAS PubMed.
- (a) D. Roy and Y. Uozumi, Adv. Synth. Catal., 2018, 360, 602 CrossRef CAS; (b) Endangered Elements, https://www.acs.org/content/acs/en/greenchemistry/research-innovation/endangered-elements.html, accessed 31 January 2019.
- (a) B. H. Lipshutz, J. Org. Chem., 2017, 82, 2806 CrossRef CAS; (b) T. Kitanosono, K. Masuda, P. Xu and S. Kobayashi, Chem. Rev., 2018, 118, 679 CrossRef CAS.
- (a) D. K. Romney, F. H. Arnold, B. H. Lipshutz and C. J. Li, J. Org. Chem., 2018, 83, 7319 CrossRef CAS; (b) F. H. Arnold, Angew. Chem., Int. Ed., 2018, 57, 4143 CrossRef CAS.
- (a) B. H. Lipshutz, F. Gallou and S. Handa, ACS Sustainable Chem. Eng., 2016, 4, 5838 CrossRef CAS; (b) B. H. Lipshutz, Curr. Opin. Green Sustain. Chem., 2018, 11, 1 CrossRef; (c) B. H. Lipshutz, Johnson Matthey Technol. Rev., 2017, 61, 196 CrossRef CAS.
- (a) M. Andersson, F. Gallou, P. Klumphu, B. S. Takale and B. H. Lipshutz, Chem.–Eur. J., 2018, 24, 6778 CrossRef CAS PubMed; (b) M. T. De Martino, K. E. A. Abdelmohsen Loai, F. P. J. T. Rutjes and J. C. M. van Hest, Beilstein J. Org. Chem., 2018, 14, 716 CrossRef CAS.
- D. A. Albisson, R. B. Bedford, P. N. Scully and S. E. Lawrence, Chem. Commun., 1998, 2095 RSC.
- S. Handa, M. P. Andersson, F. Gallou, J. Reilly and B. H. Lipshutz, Angew. Chem., Int. Ed., 2016, 55, 4914 CrossRef CAS.
- (a) T. E. Barder, S. D. Walker, J. R. Martinelli and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 4685 CrossRef CAS PubMed; (b) M. A. Düfert, K. L. Billingsley and S. L. Buchwald, J. Am. Chem. Soc., 2013, 135, 12877 CrossRef.
- The lower yield in the reaction leading to product 19, isolated in 60% yield, was due to incomplete conversion. This may be due to some complexation of the Pd by the bi-heteroaromatic being formed. Alternatively, an increase in temperature to 55 °C might help further drive it towards completion.
- (a) G. A. Molander, J. Org. Chem., 2015, 80, 7837 CrossRef CAS PubMed; (b) S. Darses and J.-P. Genet, Chem. Rev., 2008, 108, 288 CrossRef CAS PubMed.
- N. A. Isley, Y. Wang, F. Gallou, S. Handa, D. H. Aue and B. H. Lipshutz, ACS Catal., 2017, 7, 8331 CrossRef CAS.
- C. M. Gabriel, N. R. Lee, F. Bigorne, P. Klumphu, M. Parmentier, F. Gallou and B. H. Lipshutz, Org. Lett., 2017, 19, 194 CrossRef CAS PubMed.
- J. Najib, Clin. Ther., 2002, 24, 2022 CrossRef CAS.
- E.-H. Pommer, N. Goetz, B. Zeeh and B. Giergensohn, U.S. Pat. 4001416, January 4, 1977.
- N. R. Lee, A. A. Bikovtseva, M. Cortes-Clerget, F. Gallou and B. H. Lipshutz, Org. Lett., 2017, 19, 6518 CrossRef CAS PubMed.
- The biaryl portions of these drugs are prepared using high catalyst loadings and harsh reaction conditions in organic solvents, see: (a) C. Chung, G. Humphrey, P. E. Maligres and T. J. Wright, WO 2013/066768, May 10, 2013; (b) J. Bradner, M. Erba and J. Qi, WO 2016/196879 A1, December 8, 2016; (c) I. Rukhman, B.-Z. Dolitzky and E. Flyaks, U.S. Pat. 7199144 B2, April 3, 2007.
- (a) D. J. Hwang, J. Wang, W. Li and D. D. Miller, ACS Med. Chem. Lett., 2015, 6, 993 CrossRef CAS; (b) M.-J. Lai, H.-Y. Lee, L.-H. Chuang, A.-C. Tsai, M.-C. Chen, H. L. Huang, Y.-W. Wu, C.-M. Teng, S. L. Pan, Y.-M. Liu, S. Mehndiratta and J.-P. Liou, J. Med. Chem., 2015, 58, 6549 CrossRef CAS; (c) A. Döbber, A. F. Phoa, R. H. Abbassi, B. W. Stringer, B. W. Day, T. G. Johns, M. Abadleh, C. Peifer and L. Munoz, ACS Med. Chem. Lett., 2017, 8, 395 CrossRef PubMed.
- B. H. Lipshutz, N. A. Isley, J. C. Fennewalda and E. D. Slack, Angew. Chem., Int. Ed., 2013, 52, 10911 CrossRef CAS.
- A. M. Thayer, Chem. Eng. News, 2013, 91, 10 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc02528f |
| This journal is © The Royal Society of Chemistry 2019 |