Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Role of torsional strain in the ring-opening polymerisation of low strain [n]nickelocenophanes†
Rebecca A.
Musgrave‡
 a,
Rebekah L. N.
Hailes‡
a,
Rebekah L. N.
Hailes‡
 a,
Vincent T.
Annibale
a,
Vincent T.
Annibale
 a and
Ian
Manners
*ab
a and
Ian
Manners
*ab
aSchool of Chemistry, University of Bristol, Bristol BS8 1TS, UK. E-mail: imanners@uvic.ca
bDepartment of Chemistry, University of Victoria, Victoria, BC V8W 3V6, Canada
First published on 10th September 2019
Abstract
Ring-opening polymerisation (ROP) of strained [1]- and [2]metallocenophanes and related species is well-established, and the monomer ring-strain is manifest in a substantial tilting of the cyclopentadienyl ligands, giving α angles of ∼14–32°. Surprisingly, tetracarba[4]nickelocenophane [Ni(η5-C5H4)2(CH2)4] (2) undergoes ROP (pyridine, 20 °C, 5 days) to give primarily insoluble poly(nickelocenylbutylene) [Ni(η5-C5H4)2(CH2)4]n (12), despite the lack of significant ring-tilt. The exoenthalpic nature of the ROP was confirmed by DFT calculations involving the cyclic precursor and model oligomers (ΔH0ROP = −14 ± 2 kJ mol−1), and is proposed to be a consequence of torsional strain present in the ansa bridge of 2. The similarly untilted disila-2-oxa[3]nickelocenophanes [Ni(η5-C5H4)2(SiMe2)2O] (13) and [Ni(η5-C5H4)2(SiMePh)2O] (14) were found to lack similar torsional strain and to be resistant to ROP under the same conditions. In contrast, 1-methyltricarba[3]nickelocenophane {Ni(η5-C5H4)2(CH2)2[CH(CH3)]} (15) with a significant tilt angle (α ∼ 16°) was found to undergo ROP to give soluble polymer {Ni(η5-C5H4)2(CH2)2[CH(CH3)]}n (18). The reversibility of the process in this case allowed for the effects of temperature and reaction concentration on the monomer–polymer equilibrium to be explored and thereby thermodynamic data to be elucidated (ΔH0ROP = −8.9 kJ mol−1, ΔG0ROP = −3.1 kJ mol−1). Compared to the previously described ROP of the unsubstituted analogue [Ni(η5-C5H4)2(CH2)3] (1) (ΔH0ROP = −10 kJ mol−1, ΔG0ROP = −4.0 kJ mol−1), the presence of the additional methyl substituent in the ansa bridge appears to marginally disfavour ROP and leads to a corresponding decrease in the equilibrium polymer yield.
Introduction
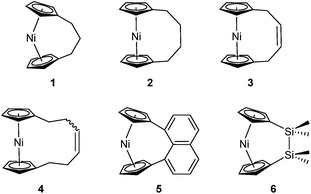
Metal-containing polymers (metallopolymers) are of widespread interest due to the large variety of properties resulting from incorporation of a diverse range of metal centres.1–17 The metals can be located either in the main-chain of the polymer or in side groups, and the interactions between the metal ions and ligands can be strong, leading to essentially static binding, or weak, which can allow for a reversible, dynamic nature.18 Metallopolymers have proven crucial in a variety of applications, including data storage,6 antibacterial activity,7 artificial metalloenzymes,8,9 emissive materials,10,11 nanopatterning,12–14 stimuli-responsive behaviour,15 and sensors.16,17Since the first report of the ring-opening polymerisation (ROP) of an [n]ferrocenophane in 1992,19 this method has become a well-established pathway to main-chain iron-containing polymers with a variety of bridging elements. The resulting polyferrocenes have attracted interest as a result of their redox responsivity,20–22 self-assembly behaviour,23–27 and preceramic properties28,29 amongst others.30 In contrast, polynickelocenes, synthesised via the ROP of [n]nickelocenophanes, are limited in number. For the 20 valence electron (VE) [n]nickelocenophanes, the two extra electrons (compared to 18 VE [n]ferrocenophanes) are accommodated in molecular orbitals with antibonding character, which results in a weaker and elongated Ni–Cp bond.31 This bond elongation causes a concomitant increase in the angle between the Cp ring planes, α, compared to analogous iron and cobalt species,32–34 and the low bond strength helps to explain the small number of reported [n]nickelocenophanes (all structurally characterised examples, 1–6, are displayed in Fig. 1).34–37
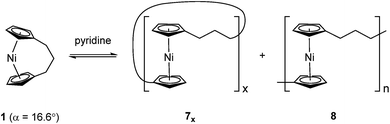
Recently the ROP of tricarba[3]nickelocenophane 1 to yield polynickelocene 7x/8 was reported.35,38 It was demonstrated that 7x/8 exists in a labile state in which a dynamic equilibrium with 1 is evident in polar organic solvents (Scheme 1). This was postulated to be a consequence of both the weak Ni–Cp bonds (the presumably homolytic M–Cp dissociation energy based in combustion experiments is 250 kJ mol−1 for nickelocene vs. 305 kJ mol−1 for ferrocene), and the reduced energy penalty upon tilting for nickelocene vs. ferrocene.38,39
Variable temperature 1H NMR spectroscopy allowed for the elucidation of the enthalpic and entropic parameters characterising this ROP process: a small, favorable value of ΔH (−10 kJ mol−1), and a very small, unfavorable value for ΔS (−20 J K−1 mol−1).38 In general, the thermodynamic driving force for the ROP of [n]metallocenophanes is ascribed to the strain in these precursors,40–44 which is generally quantified by the tilt angle, α, the Cpcent–Cpipso–E angle, β (Cpcent = Cp centroid, Cpipso = Cp ipso carbon, E = bridging element), and the angle δ, which is defined as the Cpcent–M–Cpcent angle (Fig. 2). Estimations of the value for the ROP enthalpy, ΔHROP, for [n]ferrocenophanes were previously made on the basis of differential scanning calorimetry (DSC) analyses of the exotherms associated with ROP. These values range from ∼−12 kJ mol−1 for carbaphospha[2]ferrocenophane 11 (α = 14.9°)47 to ∼−130 kJ mol−1 for thia[1]ferrocenophane 9 (α = 31.0°),48 with the ΔHROP value for the well-known dimethylsila[1]ferrocenophane 10 being −72 kJ mol−1 (α = 20.8°) (Fig. 2).32,43 Further variations in ROP exotherms have also been reported as a result of the alteration of sterics at the ansa bridge position.45,46 The value for the ROP of tricarba[3]nickelocenophane is comparable to that of 11, with which it has a comparable tilt angle (1: α = 16.6°).38,47
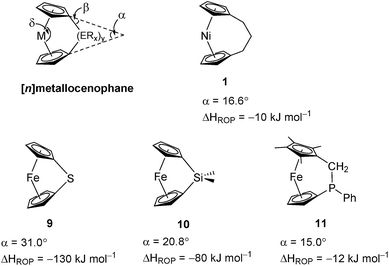 | ||
| Fig. 2 Geometric parameters characterising structural distortions in [n]metallocenophanes, and species 1, 9, 10, 11 with their respective tilt angles. | ||
As it has been shown that the tilting of ferrocene invokes a higher energy penalty than nickelocene, the observed ΔHROP value for 11 is smaller than expected based on tilt angle alone, but this is presumably due to the large phenyl substituent in the ansa bridge of 11 (steric bulk in the bridge has been previously shown to reduce ΔHROP).38,45 The ΔHROP value for 1 is similar to those for moderately strained rings: e.g. THF (ΔH0ROP = −19 kJ mol−1) and hexamethylcyclotrisiloxane (ΔH0ROP = −23 kJ mol−1).49
Whilst the polymerisation of 1 is a highly unusual example of [n]metallocenophane ROP due the presence of a dynamic equilibrium, these types of reversible ROP are more common for organic and inorganic cyclic species, e.g. THF in the presence of Lewis acids;50 cyclopentene in the presence of various transition metal alkylidene complexes;51,52 and substituted cyclic six-membered carbonates in the presence of DBU.53
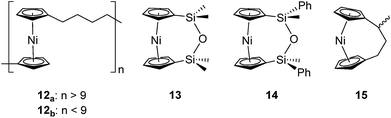
Based on the small value for the ΔH0ROP of 1,38 which has a tilt angle of 16.6°, we were recently surprised to find that tetracarba[4]nickelocenophane 2, which possesses a negligible tilt angle (α = 1.0(3)°; δ = 178.63(11)°), also undergoes ROP (pyridine, 0.74 M, 5 days, 20 °C).54 The resulting polynickelocene 12 (Fig. 3) was isolated as a predominantly insoluble material. Herein we examine the nature of the thermodynamic driving force for the ROP of 2 using DFT calculations. We also describe and attempt to explain the ROP behaviour of several new [3]nickelocenophanes 13, 14, and 15 (Fig. 3).
Results and discussion
Comparative structural data for tricarba[3]nickelophane 1 and tetracarba[4]nickelophane 2
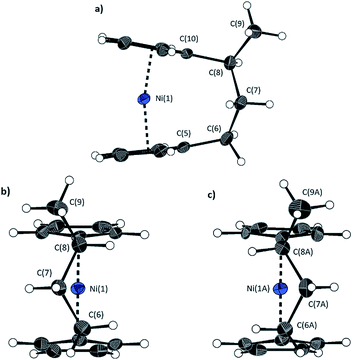
The lack of ring-tilt exhibited by 2 demonstrates that the thermodynamic driving force for this polymerisation is clearly not manifested in the reduction of tilt upon ring-opening. Whilst the ROP of 2 may still be exoenthalpic, it is not feasible to prove this experimentally as in the case of 1, due to the insolubility of polymeric 12 at 20 °C and the resulting lack of dynamic equilibrium.54 It should be noted that the ROP of 2 is reversible at higher temperatures (above 50 °C), presumably as heating is necessary to solubilise the high molar mass component of 12. Ring-strain energy in cyclic monomers is manifested in the enthalpic component of the free energy of ring-opening, which can also comprise other contributions to strain in addition to that resulting from ring tilt about the metal centre. These include: angle strain, caused by deviation of bond angles from the ideal; torsional strain, due to repulsion occurring when ring substituents separated by three bonds appear in an eclipsed conformation instead of the more stable staggered conformation; and transannular strain, generated by non-bonding interactions between ring substituents on non-adjacent atoms. Thus, these aspects of strain must also be considered when analysing the ROP of 2. The structures of 2 and 1,35,54 shown in Fig. 4 and 5, respectively, possess different degrees of these types of strain.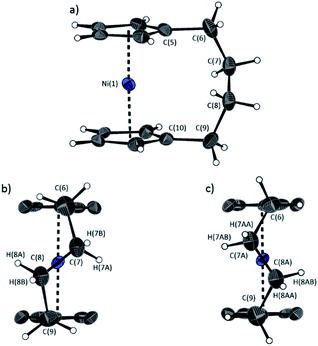 | ||
| Fig. 4 Three views of the molecular structure of 2.54 Thermal ellipsoids displayed at the 50% probability level. Hydrogen atoms are pictured as spheres of arbitrary radii (and some have been omitted for clarity). (a) The ansa bridge is disordered at two positions: C7/7A and C8/8A (those with highest relative occupancy (62%) are displayed). Alternate view of 2 displaying (b) major (62%) and (c) minor (38%) component of disordered bridge. Selected distances (Å) and angles (°) for major component (62%): Ni(1)–Cpcent 1.813(3)/1.817(3), α = 1.0(3), δ = 178.63(11). | ||
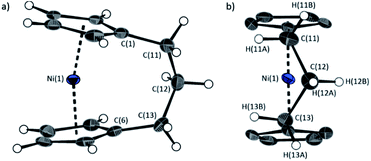 | ||
| Fig. 5 Two views (a and b) of the molecular structure of 1.35 Thermal ellipsoids displayed at the 50% probability level. Hydrogen atoms are pictured as spheres of arbitrary radii (and some have been omitted for clarity). Selected distances (Å) and angles (°): Ni(1)–Cpcent = 1.8039(14)/1.8035(14), α = 16.64(13), β = 4.2(3), δ = 166.33(5). | ||
Firstly, whilst both 1 and 2 appear to exhibit angle strain, as the bond angles within the ansa bridge deviate from the ideal tetrahedral geometry (109.5°), these deviations are smaller in 2 (C(5)–C(6)–C(7): 113.9(6)°, C(6)–C(7)–C(8): 112.4(7)°, C(7)–C(8)–C(9): 115.3(7)°, C(8)–C(9)–C(10): 114.6(6)°) than in 1 (C(1)–C(11)–C(12): 115.8(3)°, C(11)–C(12)–C(13): 115.0(2)°) (Table 1). While this does not eliminate angle strain in 2 as a contributor to its ROP propensity, there appears to be a far more significant factor: torsional strain, which appears to be appreciably greater in 2 than in 1. This is apparent in Fig. 4 and 5: protons at the bridging carbons of 1 adopt a fairly staggered conformation (H(11B)–C(11)–C(12)–H(12A): 47.6(3)°, H(12A)–C(12)–C(13)–H(13A): 41.3(4)°); whereas two protons flanking the central C–C bond in the ansa bridge of 2 are almost eclipsed in conformation (H(7B)–C(7)–C(8)–H(8B): 10.2(8)°, H(7AA)–C(7A)–C(8A)–H(8AA): 5.6(12)°). Torsional strain in 2 is expected to favour an exothermic ROP process, as for that of 1. Whilst it is also possible that ROP of 2 is entropically favoured, (i.e. driven by greater conformational freedom in the polymer vs. monomer), the ROP of 1 is known to be significantly endoentropic38 (as is typical of most ROP processes)49 and the ΔSROP value is unlikely to be so different as to become exoentropic in the case of 2.
| Distances (Å) | Angles (°) | |||
|---|---|---|---|---|
| Compound 2 | C(5)–C(6)–C(7) | 113.9(6) | ||
| C(6)–C(7) | 1.531(11) | C(6)–C(7)–C(8) | 112.4(7) | |
| C(7)–C(8) | 1.515(13) | C(7)–C(8)–C(9) | 115.3(7) | |
| C(8)–C(9) | 1.548(11) | C(8)–C(9)–C(10) | 114.6(6) | |
| H(7B)⋯H(8B) | 2.15906(8) | H(7B)–C(7)–C(8)–H(8B) | 10.2(8) | |
| C(6)–C(7A) | 1.556(12) | C(5)–C(6)–C(7A) | 112.3(8) | |
| C(7A)–C(8A) | 1.51(2) | C(6)–C(7A)–C(8A) | 116.2(10) | |
| C(8A)–C(9) | 1.543(15) | C(7A)–C(8A)–C(9) | 113.3(11) | |
| H(7AA)⋯H(8AA) | 2.1464(1) | C(8A)–C(9)–C(10) | 112.3(7) | |
| H(7AA)–C(7A)–C(8A)–H(8AA) | 5.6(12) | |||
| Compound 1 | C(1)–C(11)–C(12) | 115.8(3) | ||
| H(12A)⋯H(13A) | 2.26006(8) | C(11)–C(12)–C(13) | 115.0(2) | |
| H(12A)⋯H(11B) | 2.28442(8) | C(12)–C(13)–C(6) | 115.28(19) | |
| C(11)–C(12) | 1.529(5) | H(11B)–C(11)–C(12)–H(12A) | 47.6(3) | |
| C(12)–C(13) | 1.533(3) | H(12A)–C(12)–C(13)–H(13A) | 41.3(4) | |
DFT calculations of the enthalpy of ring-opening for 1 and 2
Due to the lack of feasibility of investigating the enthalpic contribution to ΔG0ROP of monomer 2 experimentally, we explored the ROP of both 2 and 1 using DFT. Calculations were performed by modelling cyclic monomers and a series of linear oligomers (Scheme 2). Species 1a is related to 1 by the addition of dihydrogen across the carbon backbone and serves as a model for polymer 8. Addition of one monomer (1) to yield the linear dimer (1b) provided the enthalpic change upon ring-opening of 1. Further addition of two successive monomers produced the linear trimer 1c, then the linear tetramer 1d. The enthalpy change upon ring-opening was estimated in each case and averaged over all linear oligomer models. As the calculated enthalpy of ring-opening, ΔHRO, for 1 (−11 ± 3 kJ mol−1) compares well to the experimental ΔHROP value (−10 kJ mol−1),38 the computational model was deemed appropriate. Thus, the model was applied in the same manner to ring-opened monomer 2a (Scheme 3). The ΔHRO value for 2 (−14 ± 2 kJ mol−1) is both negative and within error of that calculated for 1. This suggests that the ROP of 2 is also exoenthalpic, driven by the release of ring-strain. Clearly this ring-strain is manifest not in the ring-tilt, but instead presumably in the torsional strain present in the ansa bridge. All computational details regarding Schemes 2 and 3 are provided in the ESI.†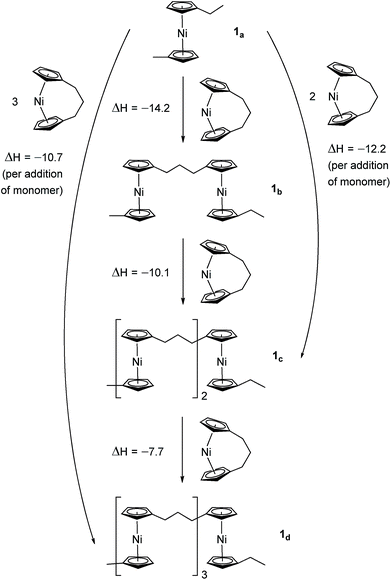 | ||
| Scheme 2 DFT model of ring-opening of monomer 1 to linear oligomers and values of enthalpic ring-opening (kJ mol−1). | ||
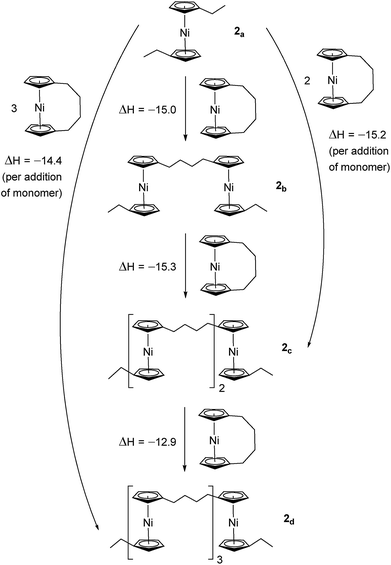 | ||
| Scheme 3 DFT model of ring-opening of monomer 2 to linear oligomers and values of enthalpic ring-opening (kJ mol−1). | ||
In addition to polynickelocenes mentioned above, we recently reported the formation of poly(tetramethyl-disilylnickelocene) [Ni(η5-C5H4)2(SiMe2)2]n16 from the disila-bridged [2]nickelocenophane 6 (Fig. 1).54 This monomer exhibits decreased tilt (α = 9.4(8)°)34vs.1 (α = 16.6(1)°). Despite this, ROP of 6 proceeds in pyridine to give predominantly insoluble polynickelocene, reminiscent of 12, with no reversibility at 20 °C. We therefore applied a similar DFT model to 6 and the corresponding linear oligomers to determine the enthalpy of ring opening (ΔHRO = −12 ± 3 kJ mol−1), which compares well to those determined for 1 and 2 (Scheme S1†).
As discussed, the insolubility of poly(nickelocenylbutylene) did not allow for the experimental determination of values of the entropy and enthalpy for ROP. Thus, other untilted monomers were targeted, that might undergo polymerisation to afford a soluble polymeric product. We considered the inclusion of a siloxane-based bridging element as it would be expected to significantly increase the solubility of a polynickelocene (syntheses of iron55 and titanium56 [n]metallocenophanes and [n]metalloarenophanes featuring siloxane-based bridges have been previously reported).
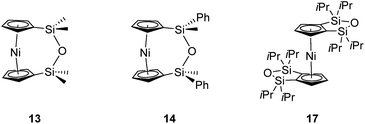
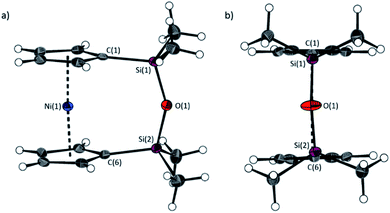
The synthesis of 1,1,3,3-tetramethyldisila-2-oxa[3]nickelocenophane 13 (Fig. 6) was conducted via a fly-trap procedure analogous to that previously employed for [n]nickelocenophanes 1,352,54 and 6,34 involving reaction of the lithiated ligand Li2[(C5H4)2(SiMe2)2O] and nickel(II) chloride.
The fly-trap reaction yielded 13 as a green crystalline solid in moderate yield (43%). 1H NMR spectroscopy (C6D6) revealed resonances at −239 and −248 ppm which were assigned to the α- and β-Cp proton environments respectively, and at 8.58 ppm which was assigned to the SiMe2 protons in the ansa bridge (Fig. S1†). Crystallisation from hexanes yielded green crystals that allowed for full characterisation by X-ray diffraction. As expected, 13 exhibits a very small tilt angle, α, of 3.76(10)° (Fig. 7).
A second siloxane-bridged [3]nickelocenophane with sterically demanding groups at silicon was also synthesised. 1,3-Dimethyl-1,3-diphenyldisila-2-oxa[3]nickelocenophane 14 (Fig. 6) was similarly prepared by the reaction of the lithiated fly-trap ligand Li2[(C5H4)2(SiMePh)2O] with nickel dichloride. Unlike other [n]nickelocenophanes, the green solid produced did not sublime, presumably due to its increased molar mass. Subsequent recrystallisations from hexanes and n-pentane yielded green single crystals suitable for X-ray diffraction. Two diastereomers of 14 are feasible given the diastereomeric nature of the ligand, but all isolated crystalline material proved to be the C2-symmetric (trans) isomer (Fig. 8). Compound 14 displays a small tilt angle of 4.18(8)°, marginally larger than that of 13. 1H NMR spectroscopy of the crystalline material revealed resonances at −237, −239, and −247 ppm assigned to the Cp proton environments, at 0.40 ppm assigned to the methyl protons in the ansa bridge, and between 7.58–8.54 ppm, attributed to the phenyl proton environments (Fig. S2†).
Interestingly, attempts to produce 1,1,3,3-tetraisopropyldisila-2-oxa[3]nickelocenophane were foiled due to an unforeseen ring closure reaction in the ligand synthesis (presumably due to the steric bulk of the isopropyl groups), leading to isolation of species 17 (Fig. 6) (further details regarding this species can be found in the ESI†).
ROP conditions (d5-pyridine, 0.79 M, 48 h, 20 °C) employed successfully for 1, 2, and 6 were then applied to siloxane-bridged 13, but in contrast to tetracarba[4]nickelocenophane 2 (which also features a small tilt angle), exposure to pyridine did not induce polymerisation. This was demonstrated by 1H NMR spectroscopy, which showed only the presence of the unreacted monomer 13. There was no evidence of reactivity even after attempting ROP at higher concentration (1.31 M) for an extended period of time (4 weeks). Several further attempts were made to induce polymerisation; firstly, by reaction of Na[C5H5] (both as an equimolar reagent and a substoichiometric initiator) with 13 under photolytic conditions (THF, 5 °C), but again no reaction occurred as evidenced by 1H NMR spectroscopy. Additionally, 13 was treated with equimolar 1,2-bis(diphenylphosphino)ethane (dppe), but this resulted in complete expulsion of the ligand framework and formation of [Ni(dppe)2] (observed by 31P NMR spectroscopy at 44.8 ppm). Whilst similar reactivity has been observed for 1,35 the driving force was suggested to be the release of ring strain derived from ring tilt in 1, which cannot be the case for 13, because α is close to zero. To determine whether 13 would undergo thermal ROP, preliminary differential scanning calorimetry (DSC) experiments were performed. These indicated melt and crystallisation processes for 13, in addition to a small exotherm feature at ∼184 °C (Fig. S4†). No new transitions were observed even after repeated heating cycles, and thus we concluded that thermal ring-opening would also prove unsuccessful.
In a similar manner to 13, exposure of 14 to ROP conditions (d5-pyridine, 0.79 M, 48 h, 20 °C) did not induce polymerisation. The reaction was monitored by 1H NMR spectroscopy, which displayed only the presence of the monomer after one week. Unreacted starting material was obtained quantitatively via removal of the solvent in vacuo.
The cause for the stability of 13 and 14 to ROP, where the similarly untilted 2 undergoes facile polymerisation, may be deduced from the structural parameters. The longer Si–O bonds in the ansa bridge of 13 and 14 (1.630(3)/1.640(3) and 1.6356(14)/1.6366(14) Å, respectively) compared to the C–C bonds in that of 2 (ranging from 1.51(2)–1.556(12) Å), and the lack of substituents at the central bridge atom (oxygen), ensure that the torsional strain present in 2 does not occur in the siloxane-bridged species. Without this strain, and the ring-tilt that occurs in [n]nickelocenophanes 1 and 6, there is no thermodynamic propensity for polymerisation. In addition, in [3]nickelocenophane 14 there do not appear to be any interactions between the phenyl groups in the ansa bridge, which could have introduced torsional strain.
We tested our computational model for the ring-opening of [n]nickelocenophanes to form linear oligomers with the siloxane-bridged [3]nickelocenophane 13, as this species was resistant to ROP, and thus the calculated ΔHRO should prove unfavorable. As expected, the computational model predicted the enthalpy of ring-opening to be small and positive (ΔHRO = 5 ± 1 kJ mol−1) (Scheme S2†).
Synthesis and ROP of methyltricarba[3]nickelocenophane 15
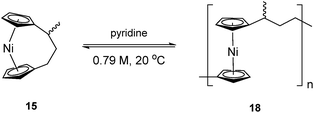
To accompany our study on the ROP behaviour of [n]nickelocenophanes, we investigated the ROP of a methylated derivative of 1, namely methyltricarba[3]nickelocenophane 15 (Fig. 3). The incorporation of bulky substituents in [n]metallocenophanes has been shown to significantly reduce their propensity to ROP,45 and therefore the formal replacement of H in 1 by the larger substituent, Me, in the bridge would generally be expected to make ROP less favourable.[n]Nickelocenophane 15, the first unsymmetrically substituted example, was synthesised as a racemate using the general method previously reported (reaction of lithiated ligand Li2{(C5H4)2(CH2)2[CH(CH3)]} with NiCl2).35,54 Sublimation and subsequent recrystallisation in hexanes afforded dark green crystals of 15 in 30% yield. 1H NMR spectroscopy of the crystalline material revealed overlapping resonances at −240, −243, and −247 ppm which were assigned to the four independent α-Cp protons, and overlapping resonances at −267 and −271 ppm which were assigned to the four independent β-Cp protons (see Fig. S5†), in line with previous assignments of Cp proton resonances in [n]nickelocenophanes where β-Cp protons appear at lower field.34,38 Resonances at −20.8 and −34.2 ppm were assigned to the β-H protons of the bridging carbons, the resonance at 14.1 ppm to protons in the ansa bridge methyl group, and as for species 1, α-H protons could not be identified.38 X-ray crystallographic data confirmed the structure, but due to co-crystallisation of the two enantiomers (Fig. 9), the application of significant conformational restraints was necessary, so the following data should be treated with prudence: α was determined as 16.3(2)°, very similar to that of 1 (16.6(13)°).
As the ROP of 1 produces the soluble products 7x/8, it was imagined that a polymeric product resulting from the ROP of 15 would exhibit similar solubility. The ROP of substituted [3]nickelocenophane 15 was investigated in d5-pyridine under the same conditions (0.79 M, 20 °C) as used for 1 (Scheme 4), and a gradual colour change from dark blue to green was observed. The reaction was followed by 1H NMR spectroscopy: after 24 h, alongside shifts assigned to Cp-ring protons in 15, a broad singlet was observed at −251 ppm, which was assigned to Cp-ring protons in polymer 18. Integration of Cp proton resonances in 15 and 18 indicated the presence of 41% polynickelocene 18. After a further 24 h, a negligible increase in conversion to polymer (42%) was detected, which did not change significantly over 7 days. This suggests the presence of an equilibrium between monomer and polymer that is reached after 24 h. Attempted isolation of the resulting polymer via precipitation of the reaction solution (pyridine, 0.79 M, 48 h, 20 °C) into rapidly stirring hexanes at 20 °C was unsuccessful. However, when the reaction solution was precipitated into cold hexanes (−78 °C), a green solid was isolated. 1H NMR spectroscopy of polymeric 18 revealed resonances at 185, 180, and 147 ppm which were assigned to the α-protons in the carbon bridge, 22.1 ppm which was assigned to the methyl protons, and a broad resonance at −246 ppm which was assigned to the Cp protons (Fig. S6†). Analysis by MALDI-TOF mass spectrometry (of the reaction mixture prior to precipitation) revealed assignable peaks up to ∼8000 g mol−1, which corresponds to a degree of polymerisation (DPn) of ∼33 (Fig. S7†), but similar analysis of the isolated polymer was unsuccessful, presumably due to difficulties in ionising the higher molar mass fraction.
In order to prove the presence of a dynamic equilibrium, polynickelocene 18 was stirred in d5-pyridine (0.44 M) for 48 h at 20 °C. Resonances assigned to protons in both monomer 15 and polymer 18 were observable by 1H NMR spectroscopy, consistent with the reversible nature of the polymerisation, and the presence of a dynamic equilibrium. The percentage of polymer as a function of time was further investigated at a range of ROP concentrations (0.11–1.31 M, samples 18a–18e, Table 2). 1H NMR spectroscopic analysis revealed an increase in the fraction of 18 with increased reaction concentration. Additionally, the yield of isolated polymer likely increases with concentration (isolation of polymer was only possible at 0.79 and 1.31 M). The discrepancy between the isolated polymer yield and that determined by integration of 1H NMR resonances is likely due to oligomeric species, which did not precipitate upon workup of the reaction solution.
| Sample | Conc. (M) | % of 18 (1H NMR spectroscopy) | % Yield of 18 (20 °C)a | % Yield of 18 (−78 °C)a | ||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 7 days | ||||
| a Temperature at which the reaction work-up was performed. b No polymer was isolated in this case. | ||||||
| 18a | 1.31 | 59.8 | 60.8 | 59.5 | 23 | 65 |
| 18b | 0.79 | 41.1 | 41.8 | 42.2 | 29 | |
| 18c | 0.44 | 16.9 | 25.0 | 24.3 | ||
| 18d | 0.22 | 3.17 | 14.3 | 14.3 | ||
| 18e | 0.11 | 10.7 | 11.6 | 11.5 | ||
The effect of reaction concentration on polymer molecular weight was investigated by dynamic light scattering (DLS). DLS experiments were performed in toluene, a marginal solvent for 18, but one in which monomer/polymer equilibration is particularly slow.38 A small amount of insoluble material (presumably higher molar mass polymer) was observed during the preparation of DLS samples, which was greater in proportion for 18a than for 18b. As this material was removed via filtration before analysis by DLS, the molecular weights (estimated relative to a poly(ferrocenyldimethylsilane) calibrant)57 are likely lower than the real values (Table 3).
| Sample | Conc. (M) | % Yield of 18 | T /(°C) | R h | Sigma | M W |
|---|---|---|---|---|---|---|
| a Molecular weights are relative to calibration with poly(ferrocenyldimethylsilane) in toluene.57 b Temperature at which the reaction work-up was performed. c No polymer was isolated in this case. | ||||||
| 18a | 1.31 | 23 | 20 | 7.42 | 1.69 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
| 65 | −78 | 9.35 | 3.04 | 126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
||
| 18b | 0.79 | 20 | ||||
| 29 | −78 | 6.90 | 1.65 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
||
In comparison to the unsubstituted analogues 7x/8, both isolated yields for polymer 18 and conversion determined by 1H NMR spectroscopy are significantly lower (Table 4). As these polymerisations exist as dynamic equilibria with oligomers and monomer, this suggests that the introduction of the methyl substituent to the ansa bridge causes the reaction equilibrium to favor [n]nickelocenophane relative to the case of 1. Generally, ROP processes are sensitive to the size of any side groups and become thermodynamically unfavorable with sterically demanding substituents.58 However, studies of the ROP of sila[1]ferrocenophanes have shown that even those with bulky side groups form high molar mass polymer in very good yield.45,59 This is likely due to the high intrinsic strain present in the [1]ferrocenophanes (72 kJ mol−1 for dimethylsila[1]ferrocenophane).19,43 Due to the lower strain present in [n]nickelocenophanes the introduction of a substituent may cause a lower propensity to ROP due to non-bonding interactions with hydrogens in the polymer bridge. Interestingly, DLS results suggest that the isolated polynickelocene 18 is of higher molecular weight than the unsubstituted polynickelocene 7x/8 isolated in analogous conditions. This result, however, should be treated with caution as the presence of a methyl substituent may lead to improved polymer–solvent interactions which would give a concomitant increase in hydrodynamic radius.
Equilibrated monomer concentrations of 15 were fitted to eqn (1), an adaption of the Van't Hoff equation, to allow for determination of ΔH0ROP and ΔS0ROP (Fig. 10).
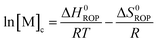 | (1) |
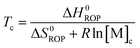 | (2) |
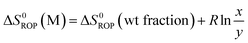 | (3) |
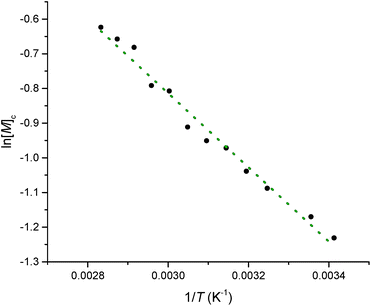 | ||
| Fig. 10 A Van't Hoff plot showing the relationship between loge of the equilibrium monomer concentration and reciprocal temperature for the ROP of 15. | ||
Conclusions
A series of low strain [n]nickelocenophanes have been studied with respect to their polymerisation behaviour. The recently reported, highly unusual ROP of untilted [Ni(η5-C5H4)2(CH2)4] (2) was investigated via DFT calculations. The exoenthalpic nature of the ROP is proposed to arise from the torsional strain present in the ansa bridge of 2. Disiloxa-bridged [3]nickelocenophanes (13 and 14), which also exhibit negligible ring-tilt, were synthesised and found to be stable with respect to ROP under similar conditions. In these cases, DFT calculations revealed that ROP is unfavorable and no analogous torsional strain was evident from the molecular structures determined by single-crystal X-ray diffraction. Unsymmetrically substituted methyltricarba[3]nickelocenophane 15 was also synthesised and in this case the reversible ROP of this species to give 18 was studied in order to provide insight into the propensity of the monomer to undergo ROP. The addition of a methyl substituent to the ansa bridge appears to make only a small difference to enthalpy of polymerisation compared to that of the unsubstituted analogue (15: ΔG0ROP = −3.1 kJ mol−1vs.1: ΔG0ROP = −4.0 kJ mol−1), but this is sufficient to significantly decrease the yields of 18 compared to 7x/8 under analogous conditions.This study provides insight into a series of recent observations that have revealed that, although only low strain [n]nickelocenophanes are synthetically accessible due to the lability of the Ni–Cp bonds,35,38,54 the low kinetic barrier to ring-opening reactions can be used productively to allow ROP. The demonstration that torsional strain can drive ROP of metallocycles offers a useful design principle to exploit in future work on functional polynickelocenes and related materials.
Experimental
Materials and equipment
All reactions and product manipulations of molecular species were carried out under an inert atmosphere of dinitrogen or argon using standard Schlenk line or glovebox techniques (MBraun glovebox MB150G-B maintained at <0.1 ppm H2O and <0.1 ppm O2), unless otherwise stated. Dry hexanes, dichloromethane and toluene were obtained from a Grubbs-type solvent system employing alumina and supported copper columns.60 THF was distilled under dinitrogen from Na/benzophenone. Pyridine and d5-pyridine were purchased from Fluka and Sigma-Aldrich respectively and distilled from CaH2 prior to use. d6-Benzene was purchased from Sigma-Aldrich and stored over molecular sieves.61 Silica gel (for flash chromatography) was purchased from VWR and used as received. Celite 521 was obtained from Sigma-Aldrich and heated to 200 °C for 16 h prior to use. Sodium metal, dicyclopentadiene, anhydrous nickel(II) chloride, 1,3-dichloro-1,1,3,3-tetramethyldisiloxane, 1,3-dichloro-1,3-dimethyl-1,3-diphenyldisiloxane, 1,3-dichloro-1,1,3,3-tetraisopropyldisiloxane, and 1,3-dibromobutane were used as supplied by Sigma-Aldrich. Anhydrous 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone (DMPU) was purchased from Sigma-Aldrich and degassed via three freeze–pump–thaw cycles. Na[C5H5]62 was prepared as described in the literature.Electrospray ionisation (ESI) mass spectra were recorded using a cone potential of +150 V in a THF/toluene mixture on a Bruker Daltonics Apex IV Fourier transform ion cyclotron mass spectrometer.
Matrix assisted laser desorption/ionisation time of flight (MALDI-TOF) mass spectra were collected on a 4700 Proteomics Analyser (Applied Biosystems) equipped with a Nd:YAG laser, operating at 335 nm. Positive ion mass spectra were obtained in linear mode over a range of m/z values, and laser intensity was varied. Samples for analysis were prepared from a THF solution of the analyte (1 mg mL−1) and a THF solution of the matrix (10 mg mL−1), trans-2-[3-(4-tertbutylphenyl)-2-methyl-2-propenylidene]malononitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 volume ratio) and drop-cast by micropipette into sample wells.
10 volume ratio) and drop-cast by micropipette into sample wells.
1H NMR spectra were recorded at ambient temperature on a VARIAN NMR 500 MHz spectrometer, and at variable temperature on a Bruker Advance III HD 500 Cryo spectrometer. All spectra are reported relative to external TMS and are referenced to the most downfield residual solvent resonance (d5-pyridine: δH 8.74 ppm, C6D6: δH 7.16 ppm). In all 1H NMR spectra of paramagnetic [n]nickelocenophane and polynickelocene species, backward linear prediction from 0 to 15 data points was employed to remove baseline distortion, phase correction was addressed manually, and a Bernstein polynomial fit was applied (polynomial order = 10). 1H NMR spectra were collected between +310 and −310 ppm to observe signals at both low and high field (number of scans = 2048, receiver gain = 50, relaxation delay = 0.10 s and acquisition time = 0.84 s).
Dynamic Light Scattering (DLS) experiments were performed to determine hydrodynamic radii of polymer solutions. Samples (2 mL) of different polymer concentrations (1 and 2 mg mL−1) in toluene were filtered through a 0.45 μm membrane filter into an optical glass cuvette (10.0 mm path length). The measurements were performed on a Malvern Instruments Zetasizer Nano S using a 5 mW He–Ne laser (633 nm) at 20 °C. The correlation function was acquired in real time and analysed with a function capable of modelling multiple exponentials. This process enabled the diffusion coefficients for the component particles to be extracted, and these were subsequently expressed as effective hydrodynamic radius, by volume, using the Stokes–Einstein relationship.
Photoirradiation experiments were carried out using Pyrex-glass-filtered emission (λ > 310 nm) from a 125 W high pressure Hg vapour lamp (Photochemical Reactors Ltd.).
Elemental analyses were carried out by Elemental Microanalysis Ltd using the Dumas combustion method.
Single crystal X-ray diffraction experiments were carried out at 100 K on a Bruker APEX II diffractometer using Mo Kα radiation (λ = 0.71073 Å). Data collections were performed using a CCD area detector from a single crystal mounted on a glass fibre. Intensities were integrated,63 from several series of exposures measuring 0.5° in ω or φ. Absorption corrections were based on equivalent reflections using SADABS.64 The structures were solved using SHELXS and refined against all Fo2 data with hydrogen atoms located geometrically and refined using a riding model in SHELXL.65 Crystallographic details are provided in the ESI.†
For detailed computational methods and calculated molecular coordinates see the ESI.†
The fly-trap ligand Li2[(C5H4)2(SiMe2)2O] (3.00 g, 10.9 mmol) and NiCl2 (1.55 g, 12.0 mmol) were thoroughly mixed in the absence of solvent and cooled to −78 °C. Dry and degassed THF (200 mL) pre-cooled to −78 °C was then added rapidly via cannula. The reaction mixture was stirred and allowed to warm to room temperature over a period of 16 h. After evaporation of the solvent under reduced pressure, the green residue was extracted with hexanes to give a dark green solution which was filtered through Celite (1′′ × 4′′). Again, all volatiles were removed in vacuo and the resulting green solid was purified by sublimation (40 °C/−78 °C, 5.0 × 10−2 mbar) and subsequent recrystallisation from hexanes at −40 °C to afford dark green crystals of 13 suitable for X-ray crystallographic analysis. Yield: 1.50 g (4.70 mmol, 43%). 1H NMR (500 MHz, C6D6): δ [peak width at half height] (ppm) 8.58 [18 Hz] (br s, C5H4–Si(CH3)2), −239.4 [906 Hz] (br s, C5H4), −248.1 [969 Hz] (br s, C5H4). ESI-MS (positive ion mode, 1,2-difluorobenzene): m/z 318.0401 [Ni(η5-C5H4)2(SiMe2)2O]+. Anal. calcd for C14H20NiOSi2: C 52.68%, H 6.32% found: C 53.35%, H 6.35%.
The fly-trap ligand Li2[(C5H4)2(SiMePh)2O] (1.00 g, 2.51 mmol) and NiCl2 (0.340 g, 2.63 mmol) were thoroughly mixed in the absence of solvent and cooled to −78 °C. Dry and degassed THF (100 mL) pre-cooled to −78 °C was then added rapidly via cannula. The reaction mixture was stirred and allowed to warm to room temperature over a period of 16 h. After evaporation of the solvent under reduced pressure, the green residue was extracted with hexanes to give a dark green solution which was filtered through Celite (1′′ × 4′′). Again, all volatiles were removed in vacuo and the resulting green solid was recrystallised in hexanes at −40 °C to afford a green solid and a dark green solution. The solid was separated from the solution and then recrystallised in n-pentane at −40 °C to give green crystals of 14 suitable for X-ray crystallographic analysis. Yield: 0.18 g (0.41 mmol, 16%). 1H NMR (500 MHz, C6D6): δ [peak width at half height] (ppm) 8.54–7.58 (m, C5H4–Si(C6H5)2), 0.40 [3 Hz] (s, C5H4–Si(CH3)), −237.2 [887 Hz] (br s, C5H4), −239.3 [891 Hz] (br s, C5H4), and −247.2 [1008 Hz] (br s, C5H4). ESI-MS (positive ion mode, toluene): m/z 442.0718 [Ni(η5-C5H4)2(SiMePh)2O]+. Anal. calcd for C24H24NiOSi2: C 65.02%, H 5.46% found: C 64.09%, H 5.67%.
The fly-trap ligand Li2{(C5H4)2(CH2)2[CH(CH3)]} (2.00 g, 10.1 mmol) and NiCl2 (1.37 g, 10.6 mmol) were thoroughly mixed in the absence of solvent and cooled to −78 °C. Dry and degassed THF (250 mL) pre-cooled to −78 °C was then added rapidly via cannula. The reaction mixture was stirred and allowed to warm up to room temperature over a period of 16 h. After evaporation of the solvent under reduced pressure, the green residue was extracted with hexanes to give a dark green solution which was filtered through Celite (1′′ × 4′′). Again, all volatiles were removed in vacuo and the resulting green solid was purified by sublimation (40 °C/−78 °C, 1.0 × 10−2 mbar) and subsequent recrystallisation from hexanes at −40 °C to afford dark green crystals of 15. Yield: 0.73 g (3.0 mmol, 30%). 1H NMR (500 MHz, C6D6): δ [peak width at half height] (ppm) 14.14 [95 Hz] (s, –CH3), −20.8 [232 Hz] (s, C5H4–CH2–CH2), −34.2 [194 Hz] (s, C5H4–CH2–CH2), −240.4 [900 Hz] (br s, α-C5H4), −242.9 [887 Hz] (br s, α-C5H4), −246.5 [1012 Hz] (br s, α-C5H4), −266.9 [1125 Hz] (br s, β-C5H4), −270.8 [1579 Hz] (br s, β-C5H4). The lack of a signal corresponding to the C5H4–CH2–CH2 protons is consistent with similar ansa [n]nickelocenophanes. ESI-MS (positive ion mode, toluene): m/z 242.0605 [Ni(η5-C5H4)2(CH2)2(CH(CH3))]+. Anal. calcd for C14H16Ni: C, 69.21; H, 6.64; found: C, 70.04; H, 6.56.
A green solid was isolated in the cases of 18a and 18bvia precipitation of the polymerisation solution into rapidly stirred −78 °C hexanes (30 mL). The precipitate was separated, dissolved in a minimum volume of THF (0.5 mL) and precipitated into −78 °C hexanes again (30 mL). The solid was isolated and dried in vacuo to yield green polymeric material, 18. In the case of 18a (1.31 M), polymeric material was also isolated from precipitation into 20 °C hexanes. 1H NMR (500 MHz, C6D6): δ [peak width at half height] (ppm) 185.4 [661 Hz] (br s, C5H4–CH(CH3)–CH2), 179.6 [1087 Hz] (br s, C5H4–CH2–CH2), 146.7 [985 Hz] (br s, C5H4–CH2–CH2), 22.1 (m, CH(CH3)), −246.3 [1790 Hz] (br s, C5H4). MALDI-TOF MS (linear, +mode): 2128 (n = 8), 2371 (n = 9), 2614 (n = 10), 2857 (n = 11), 3100 (n = 12), 3343 (n = 13), 3586 (n = 14), 3829 (n = 15), 4072 (n = 16), 4315 (n = 17), 4558 (n = 18), 4801 (n = 19), 5044 (n = 20), 5287 (n = 21), 5530 (n = 22), 5773 (n = 23), 6016 (n = 24), 6259 (n = 25), 6502 (n = 26), 6744 (n = 27), 6987 (n = 28), 7230 (n = 29), 7473 (n = 30), 7716 (n = 31), 7959 (n = 32) (for assignment of end groups see Fig. S7†). Further characterisation can be found for specific concentrations in Tables 2 and 3.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Engineering and Physical Sciences Research Council (EPSRC, EP/N030702/1) for funding. V. T. A. is grateful to the European Union for a Marie Curie Fellowship (H2020-MSCA-IF-2016_748371), and the Government of Canada for an NSERC postdoctoral fellowship. I. M. thanks the Government of Canada for a C150 Research Chair and the University of Bristol for support. We also thank Natalie Fey and Jeremy N. Harvey for computational contributions and useful discussion.Notes and references
- G. R. Whittell, M. D. Hager, U. S. Schubert and I. Manners, Nat. Mater., 2011, 10, 176–188 CrossRef CAS PubMed.
- J. Xiang, C.-L. Ho and W.-Y. Wong, Polym. Chem., 2015, 6, 6905–6930 RSC.
- Y. Yan, J. Zhang, L. Ren and C. Tang, Chem. Soc. Rev., 2016, 45, 5232–5263 RSC.
- H. Gu, S. Mu, G. Qiu, X. Liu, L. Zhang, Y. Yuan and D. Astruc, Coord. Chem. Rev., 2018, 364, 51–85 CrossRef CAS.
- M. Gallei and C. Rüttiger, Chem.–Eur. J., 2018, 24, 10006–10021 CrossRef CAS PubMed.
- T.-L. Choi, K.-H. Lee, W.-J. Joo, S. Lee, T.-W. Lee and M. Y. Chae, J. Am. Chem. Soc., 2007, 129, 9842–9843 CrossRef CAS PubMed.
- J. Zhang, Y. P. Chen, K. P. Miller, M. S. Ganewatta, M. Bam, Y. Yan, M. Nagarkatti, A. W. Decho and C. Tang, J. Am. Chem. Soc., 2014, 136, 4873–4876 CrossRef CAS PubMed.
- J. Collot, J. Gradinaru, N. Humbert, M. Skander, A. Zocchi and T. R. Ward, J. Am. Chem. Soc., 2003, 125, 9030–9031 CrossRef CAS PubMed.
- K. Y. Zhang, S. Liu, Q. Zhao and W. Huang, Coord. Chem. Rev., 2016, 319, 180–195 CrossRef CAS.
- F.-I. Wu, X.-H. Yang, D. Neher, R. Dodda, Y.-H. Tseng and C.-F. Shu, Adv. Funct. Mater., 2007, 17, 1085–1092 CrossRef CAS.
- J. M. Stanley and B. J. Holliday, Coord. Chem. Rev., 2012, 256, 1520–1530 CrossRef CAS.
- D. A. Rider, K. Liu, J. C. Eloi, L. Vanderark, L. Yang, J.-Y. Wang, D. Grozea, Z.-H. Lu, T. P. Russell and I. Manners, ACS Nano, 2008, 2, 263–270 CrossRef CAS PubMed.
- I. Korczagin, R. G. H. Lammertink, M. A. Hempenius, S. Golze and G. J. Vancso, Adv. Polym. Sci., 2006, 200, 91–117 CrossRef CAS.
- Y. Wang, L. Salmon, J. Ruiz and D. Astruc, Nat. Commun., 2014, 5, 1–12 CAS.
- H.-J. Kim, J.-H. Lee and M. Lee, Angew. Chem., Int. Ed., 2005, 44, 5810–5814 CrossRef CAS PubMed.
- K. L. Robinson and N. S. Lawrence, Anal. Chem., 2006, 78, 2450–2455 CrossRef CAS PubMed.
- B. J. Holliday, T. B. Stanford and T. M. Swager, Chem. Mater., 2006, 18, 5649–5651 CrossRef CAS.
- T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813–817 CrossRef CAS PubMed.
- D. A. Foucher, B. Z. Tang and I. Manners, J. Am. Chem. Soc., 1992, 114, 6246–6248 CrossRef CAS.
- L. Espada, K. H. Pannell, V. Papkov, L. Leites, S. Bukalov, I. Suzdalev, M. Tanaka and T. Hayashi, Organometallics, 2002, 21, 3758–3761 CrossRef CAS.
- Y. Ma, W.-F. Dong, M. A. Hempenius, H. Möhwald and G. J. Vancso, Nat. Mater., 2006, 5, 724–729 CrossRef CAS PubMed.
- H. Wang, X. Wang, M. A. Winnik and I. Manners, J. Am. Chem. Soc., 2008, 130, 12921–12930 CrossRef CAS PubMed.
- J. Massey, K. N. Power, I. Manners and M. A. Winnik, J. Am. Chem. Soc., 1998, 120, 9533–9540 CrossRef CAS.
- J. A. Massey, K. N. Power, M. A. Winnik and I. Manners, Adv. Mater., 1998, 10, 1559–1562 CrossRef CAS.
- T. Gädt, N. S. Ieong, G. Cambridge, M. A. Winnik and I. Manners, Nat. Mater., 2009, 8, 144–150 CrossRef PubMed.
- X. Wang, G. Guerin, H. Wang, Y. Wang, I. Manners and M. A. Winnik, Science, 2007, 317, 644–647 CrossRef CAS PubMed.
- H. Qiu, Y. Gao, C. E. Boott, O. E. C. Gould, R. L. Harniman, M. J. Miles, S. E. D. Webb, M. A. Winnik and I. Manners, Science, 2016, 352, 697–701 CrossRef CAS PubMed.
- M. Ginzburg, M. J. MacLachlan, S. M. Yang, N. Coombs, T. W. Coyle, N. P. Raju, J. E. Greedan, R. H. Herber, G. A. Ozin and I. Manners, J. Am. Chem. Soc., 2002, 124, 2625–2639 CrossRef CAS PubMed.
- K. Kulbaba, A. Cheng, A. Bartole, S. Greenberg, R. Resendes, N. Coombs, A. Safa-Sefat, J. E. Greedan, H. D. H. Stöver, G. A. Ozin and I. Manners, J. Am. Chem. Soc., 2002, 124, 12522–12534 CrossRef CAS PubMed.
- R. L. N. Hailes, A. M. Oliver, J. Gwyther, G. R. Whittell and I. Manners, Chem. Soc. Rev., 2016, 45, 5358–5407 RSC.
- N. J. Long, in Metallocenes: An introduction to sandwich complexes, Wiley-Blackwell, 1st edn, 1998 Search PubMed.
- W. Finckh, B. Z. Tang, D. A. Foucher, D. B. Zamble, R. Ziembinski, A. Lough and I. Manners, Organometallics, 1993, 12, 823–829 CrossRef CAS.
- H. Braunschweig, F. Breher, M. Kaupp, M. Gross, T. Kupfer, D. Nied, K. Radacki and S. Schinzel, Organometallics, 2008, 27, 6427–6433 CrossRef CAS.
- H. Braunschweig, M. Gross and K. Radacki, Organometallics, 2007, 26, 6688–6690 CrossRef CAS.
- S. Baljak, A. D. Russell, S. C. Binding, M. F. Haddow, D. O'Hare and I. Manners, J. Am. Chem. Soc., 2014, 136, 5864–5867 CrossRef CAS PubMed.
- W. Buchowicz, L. B. Jerzykiewicz, A. Krasińska, S. Losi, A. Pietrzykowski and P. Zanello, Organometallics, 2006, 25, 5076–5082 CrossRef CAS.
- S. Trtica, M. H. Prosenc, M. Schmidt, J. Heck, O. Albrecht, D. Görlitz, F. Reuter and E. Rentschler, Inorg. Chem., 2010, 49, 1667–1673 CrossRef CAS PubMed.
- R. A. Musgrave, A. D. Russell, D. W. Hayward, G. R. Whittell, P. G. Lawrence, P. J. Gates, J. C. Green and I. Manners, Nat. Chem., 2017, 9, 743–750 CrossRef CAS.
- V. I. Tel'noi and I. B. Rabinovich, Usp. Khim., 1977, 46, 1337–1367 Search PubMed.
- D. E. Herbert, U. F. J. Mayer and I. Manners, Angew. Chem., Int. Ed., 2007, 46, 5060–5081 CrossRef CAS PubMed.
- R. A. Musgrave, A. D. Russell and I. Manners, Organometallics, 2013, 32, 5654–5667 CrossRef CAS.
- S. Barlow, M. J. Drewitt, T. Dijkstra, J. C. Green, D. O'Hare, C. Whittingham, H. H. Wynn, D. P. Gates, I. Manners, J. M. Nelson and J. K. Pudelski, Organometallics, 1998, 17, 2113–2120 CrossRef CAS.
- E. Khozeimeh Sarbisheh, H. Bhattacharjee, M. P. T. Cao, J. Zhu and J. Müller, Organometallics, 2017, 36, 614–621 CrossRef CAS.
- H. Bhattacharjee and J. Müller, Coord. Chem. Rev., 2016, 314, 114–133 CrossRef CAS.
- R. A. Musgrave, A. D. Russell, G. R. Whittell, M. F. Haddow and I. Manners, Organometallics, 2015, 34, 897–907 CrossRef CAS.
- B. Bagh, G. Schatte, J. C. Green and J. Müller, J. Am. Chem. Soc., 2012, 134, 7924–7936 CrossRef CAS PubMed.
- R. Resendes, J. M. Nelson, A. Fischer, F. Jäkle, A. Bartole, A. J. Lough and I. Manners, J. Am. Chem. Soc., 2001, 123, 2116–2126 CrossRef CAS PubMed.
- R. Rulkens, D. P. Gates, D. Balaishis, J. K. Pudelski, D. F. McIntosh, A. J. Lough and I. Manners, J. Am. Chem. Soc., 1997, 119, 10976–10986 CrossRef CAS.
- A. Duda and A. Kowalski, in Handbook of Ring-opening Polymerization, ed. P. Dubois, O. Coulembier and J.-M. Raquez, Wiley-VCH Verlag GmbH & Co., Weinheim, 2009 Search PubMed.
- P. Kubisa and S. Penczek, Prog. Polym. Sci., 1999, 24, 1409–1437 CrossRef CAS.
- R. Tuba and R. H. Grubbs, Polym. Chem., 2013, 4, 3959–3962 RSC.
- G. Odian, in Principles of Polymerization, John Wiley & Sons, New Jersey, 4th edn, 2004 Search PubMed.
- P. Olsén, J. Undin, K. Odelius, H. Keul and A.-C. Albertsson, Biomacromolecules, 2016, 17, 3995–4002 CrossRef PubMed.
- R. L. N. Hailes, R. A. Musgrave, A. F. R. Kilpatrick, A. D. Russell, G. R. Whittell, D. O'Hare and I. Manners, Chem.–Eur. J., 2019, 25, 1044–1054 CrossRef CAS PubMed.
- C. Angelakos, D. B. Zamble, D. A. Foucher, A. J. Lough and I. Manners, Inorg. Chem., 1994, 33, 1709–1718 CrossRef CAS.
- A. C. T. Kuate, M. Alexandru, M. Freytag, C. Racles, M. Cazacu, P. G. Jones and M. Tamm, J. Organomet. Chem., 2014, 751, 628–637 CrossRef.
- J. A. Massey, K. Kulbaba, M. A. Winnik and I. Manners, J. Polym. Sci., Part B: Polym. Phys., 2000, 38, 3032–3041 CrossRef CAS.
- M. Cypryk, Y. Gupta and K. Matyjaszewski, J. Am. Chem. Soc., 1991, 113, 1046–1047 CrossRef CAS.
- D. Foucher, R. Ziembinski, R. Petersen, J. Pudelski, M. Edwards, Y. Ni, J. Massey, C. R. Jaeger, G. J. Vancso and I. Manners, Macromolecules, 1994, 27, 3992–3999 CrossRef CAS.
- A. B. Pangborn, M. A. Giardello, R. H. Grubbs, R. K. Rosen and F. J. Timmers, Organometallics, 1996, 15, 1518–1520 CrossRef CAS.
- D. B. G. Williams and M. Lawton, J. Org. Chem., 2010, 75, 8351–8354 CrossRef CAS PubMed.
- T. K. Panda, M. T. Gamer and P. W. Roesky, Organometallics, 2003, 22, 877–878 CrossRef CAS.
- S. C. You, M. Gubler and M. Neuenschwander, Helv. Chim. Acta, 1994, 77, 1346–1362 CrossRef CAS.
- S. Collins, Y. Hong and N. J. Taylor, Organometallics, 1990, 9, 2695–2703 CrossRef CAS.
- Bruker-AXS, SAINT V7.68A, Madison, Wisconsin Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Computational methods, additional schemes, NMR and MALDI-TOF spectra, DLS and DSC data, crystallographic data. CCDC 1919308–1919311. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc02624j |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2019 |