Open Access Article
Open Access ArticleNear-infrared light-responsive hydrogels via peroxide-decorated MXene-initiated polymerization†
Na
Tao‡
a,
Depan
Zhang‡
a,
Xilong
Li
a,
Dongyang
Lou
a,
Xiaoyi
Sun
 a,
Chuanwan
Wei
b,
Juan
Li
a,
Chuanwan
Wei
b,
Juan
Li
 *a,
Junliang
Yang
*a,
Junliang
Yang
 c and
You-Nian
Liu
c and
You-Nian
Liu
 a
a
aCollege of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China. E-mail: juanli@csu.edu.cn
bSchool of Chemistry and Chemical Engineering, University of South China, Hengyang 421001, China
cHunan Key Laboratory for Super-Microstructure and Ultrafast Process, School of Physics and Electronics, Central South University, Changsha 410083, China
First published on 2nd October 2019
Abstract
Two-dimensional MXene Ti3C2Tx nanosheets with peroxide decoration (p-Ti3C2Tx) are synthesized by a sonication-assisted MILD etching method. The obtained MXenes can generate hydroxyl radical species and act as an initiator for free-radical polymerization of a series of acrylic monomers without the use of light illumination or co-initiators. The monomers analyzed include acrylamide, N-isopropylacrylamide (NIPAM), N,N-dimethylacrylamide, methyl methacrylate, and hydroxyethyl methacrylate. By simply mixing N-isopropylacrylamide monomers and p-Ti3C2Tx nanosheets under deoxygenated conditions, PNIPAM-based nanocomposite hydrogels are synthesized using a high concentration of the monomer. The nanocomposite hydrogels have a photothermal conversion efficiency of 34.7% and photothermal stability superior to that of pristine Ti3C2Tx. Taking advantage of the thermal responsive behavior of PNIPAM, the nanocomposite hydrogels are successfully exploited as remotely near-infrared light controlled “smart” windows, fluidic valves and photodetectors.
Introduction
“Smart” responsive hydrogels are now being widely studied in various fields such as soft robotics,1,2 sensors,3,4 microdevices,5 drug delivery,6,7 cell/tissue engineering scaffolds,8,9 and reaction vessels.10,11 Exposure to different stimuli (for example light, temperature, pH, and electric field) may cause these hydrogels to swell/shrink, bend/unbend, or swim/crawl, mimicking natural creatures with responsive behavior.12–15 In particular, light is a distinct stimulus to tune the properties of a hydrogel spatiotemporally in a remote controlled manner with great ease and convenience.6,16–19 During the past decade, increasing attention has been paid to near-infrared (NIR) light-absorbing materials. As opposed to UV-Vis light, NIR light has advantages of deep penetration, low energy, and little damage to structures. Additionally, some NIR-absorbing materials have strong photothermal conversion ability resulting in a rapid temperature increase at the irradiation site.20–22 Moreover, NIR can trigger phase separation or phase transition of thermo-responsive polymer networks, causing various physical and morphological changes. Hence, NIR-responsive hydrogels are being exploited for different applications, including smart windows, microfluidic valves, 3D microfabrication of complex objects, and drug delivery.18,20,23–26MXenes have been an emerging class of graphene-like two-dimensional nanomaterials since their first discovery in 2011.27 Owing to their properties of a functionalized surface, small band gaps, and excellent photothermal properties and conductivity, they have diverse applications in batteries, supercapacitors, photocatalysis, electrocatalysis, and biomedicine.28–33 Ti3C2Tx, a representative MXenes, has abundant functional groups (i.e. –OH, –O or –F) and is well dispersible in aqueous solution. Ti3C2Tx has outstanding photothermal properties, and Ti3C2Tx-based hydrogels have been applied in the remote light-controlled microfluidic pipeline.34,35 Ti3C2Tx-incorporated hydrogels were also recently applied as strain sensors and exhibited outstanding tensile strain sensitivity, with a gauge factor one order of magnitude higher than that of pristine hydrogels.36 Notably, Ti3C2Tx participates in many chemical processes (such as CO oxidation, oxygen evolution, and oxygen reduction), by acting as catalysts, cocatalysts or supporting substrates. Ti3C2Tx itself can catalyze in situ polymerization of aromatic EDOT or pyrrole using the mechanism of charge-transfer-induced polymerization for the polymerization process.37 To the best of our knowledge, the catalytic role of MXenes in radical polymerization has not been reported yet.37–39
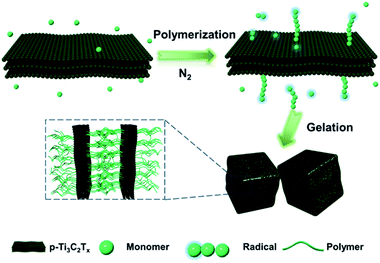
Herein, we aim to design MXene-based NIR-responsive nanocomposite (NC) hydrogels towards remote-controlled devices. To be specific, we synthesized a novel kind of Ti3C2Tx nanosheet with peroxide decoration (p-Ti3C2Tx), which itself acts as an initiator in the polymerization of acrylic monomers without any light irradiation or co-initiators. The preparation procedure for the MXene-based hydrogels is quite simple and efficient (Scheme 1). Briefly, after adding monomers into a freshly prepared p-Ti3C2Tx nanosheet suspension in an ice bath, polymerization occurs within several minutes after the N2 purging process. Gelation is observed at a high concentration of the monomer. In view of the widespread use of inorganic semiconductor nanoparticles (such as V2C, perovskite nanocrystals, graphitic carbon nitride, TiO2 nanoparticles, and titania nanosheets) as photo-catalysts in polymerization,40–45 it is surprising to observe this rapid p-Ti3C2Tx-initiated polymerization in the absence of light illumination.
Results and discussion
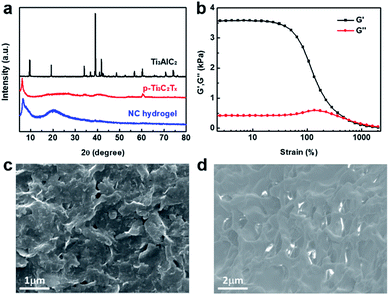
In our work, p-Ti3C2Tx nanosheets are synthesized by a modified MILD etching method (LiF/HCl) at room temperature.46 The modified etching process is performed under bath sonication, defined as a sonication-assisted MILD etching method. As a result, delamination is simultaneously completed along with the etching process. The p-Ti3C2Tx nanosheets exhibit a new peak at 2θ ≈ 6° in the XRD pattern (Fig. 1a), while the 2θ peak at 39° (characteristic of the precursor Ti3AlC2) almost disappears in the p-Ti3C2Tx sample. The appearance of a low angle peak is attributed to a typical (002) plane for most reported MXenes, in agreement with previous studies.46 This result indicates the high etching efficiency in the sonication-assisted MILD etching process. These obtained nanosheets are highly water dispersible. The polymerization is first examined with N-isopropylacrylamide (NIPAM). By adding NIPAM monomers into the nanosheet suspension, the homogeneous mixture is further purged with N2 for 5 minutes. Polymerization is easily detected from the increase of viscosity within minutes. To be specific, the reaction vessel is well protected against light during the whole N2 purging and polymerization process. Using a high concentration of the monomer, a hydrogel is formed. Typically, in the presence of p-Ti3C2Tx, gelation of NIPAM takes place at a concentration above 8 wt%.The gelation of NC hydrogels was established by rheology tests. The storage modulus (G′) is dominant over the loss modulus (G′′) over a broad strain range, demonstrating that NC hydrogels have a good linear viscoelastic region (Fig. 1b). Nevertheless, G′ of hydrogels decreases dramatically when the strain is more than 40%. The G′′ surpasses G′ when the strain is over 600%, indicating the destruction of hydrogels. Nevertheless, alternate-step strain sweeps (30 s at a strain of 800% for destruction, 3 min at a strain of 0.1% for recovery) reveal the good recovery properties of NC hydrogels for 6 cycles (Fig. S1†). This self-recovery is a characteristic of physical crosslinking. For poly(N-isopropylacrylamide) (PNIPAM) hydrogels, hydrogen bonds, hydrophobic interaction and chain entanglement cooperatively induce gelation.47
Before polymerization, pristine p-Ti3C2Tx has a layered sheet structure (Fig. 1c). In contrast, the NC hydrogel sample obtained after polymerization displays a distinct interconnected network structure (Fig. 1d). This indicates that the layered p-Ti3C2Tx nanosheets are wrapped by polymer chains. Although the nanosheets are embedded in the polymer matrix, the NC hydrogel still exhibits periodic XRD peaks of p-Ti3C2Tx (Fig. 1a).
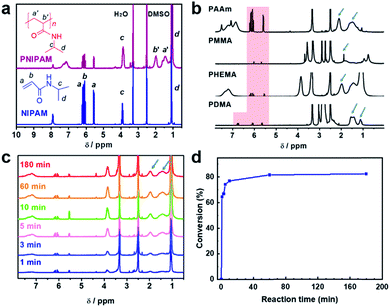
The polymerization is confirmed by 1H-nuclear magnetic resonance (1H-NMR) measurement. The 1H-NMR spectrum of the NC hydrogel has new broad signals around 1.5 ppm and 2.0 ppm, attributed to the protons of polymer backbone chains. On the other hand, peaks around 5.5–6.3 ppm, attributed to the protons of the monomeric double bond, are greatly decreased after polymerization (Fig. 2a).
Besides NIPAM described above, other monomers including acrylamide (AAm), N,N-dimethylacrylamide (DMA), methyl methacrylate (MMA) and hydroxyethyl methacrylate (HEMA) are also successfully polymerized in the presence of Ti3C2Tx (Fig. 2b). Like NIPAM, AAm and DMA are able to form hydrogels in the presence of p-Ti3C2Tx (Fig. S2†). 1H-NMR measurement also confirmed the polymerization of different monomers (Fig. 2b and Table S1†). In this work, we selected NIPAM for the preparation of the NC polymer or hydrogel unless specified.
The kinetics of the reaction was explored by monitoring the proton signals as a function of reaction time using NMR measurement. As shown in Fig. 2c, the chemical shift signals from the backbone protons increase with polymerization time. Meanwhile, the signals from protons of the monomeric double bond decrease with the polymerization time. The monomer conversion of NIPAM increases with time gradually (Fig. 2d). These results are similar to the features of classical free-radical polymerization. However, the conversion of NIPAM to PNIPAM was decreased to 73.5% in the NC hydrogel prepared using a monomer concentration of 8 wt% (Fig. S3†). This decrease is caused by the slow diffusion of monomers in the hydrogels.
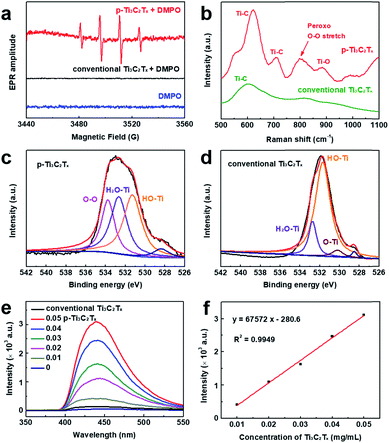
To verify the mechanism of initiation, electron paramagnetic resonance (EPR) was used to analyze the radical species. In addition, another kind of Ti3C2Tx nanosheet was prepared by the conventional MILD etching method as a control.46 Using DMPO as the spin trapping agent, p-Ti3C2Tx from the sonication-assisted process displays an obvious ˙OH signal (Fig. 3a), while conventional Ti3C2Tx shows no EPR signals. Although several studies reported highly reactive hydroxyl radicals could be generated by photocatalysis as Ti3C2Tx is partially oxidized to TiO2,48–50 the experiments for the MXene-initiated radical polymerization in this work are performed in the darkness and the absence of light illumination. Interestingly, the catalytic activity of p-Ti3C2Tx in the polymerization disappeared after being stored over one week at room temperature. Conventional Ti3C2Tx is unable to catalyze the polymerization, either for the as-prepared samples or for stored samples. This phenomenon demonstrates that p-Ti3C2Tx nanosheets themselves generate the reactive species for initiation.
To gain insight into the structure of p-Ti3C2Tx nanosheets, Raman and X-ray photoelectron spectroscopy (XPS) were employed. The peroxo O–O stretching vibration in Raman spectroscopy is found at 796 cm−1 for p-Ti3C2Tx instead of conventional Ti3C2Tx (Fig. 3b),51 which strongly supports the fact that peroxide is decorated on the surface of Ti3C2Tx. The XPS survey spectrum demonstrated the presence of Ti, C, F and O in both kinds of Ti3C2Tx (Fig. S4†). The high-resolution XPS O 1s spectrum of p-Ti3C2Tx can be split into 4 peaks including absorbed oxygen, HO–Ti, H2O–Ti (absorbed H2O) and O–O at 528.2, 531.4, 532.8, and 533.8 eV, respectively (Fig. 3c).52,53 The p-Ti3C2Tx nanosheets exhibit an obvious O–O peak (Fig. 3c), differing from conventional Ti3C2Tx (Fig. 3d). In addition, this peak disappears after storage for over one week at room temperature. In Fig. S5,† an O–Ti/TiO2 peak becomes dominant in inactive p-Ti3C2Tx, suggesting a conversion to TiO2.54 Thus, we deduce that the surface decoration of peroxide O–O in the p-Ti3C2Tx nanosheets is ascribed to the generation of reactive radical species.
A further understanding of the occurrence of peroxo species in Ti3C2Tx nanosheets was gained when we found that the sonication process in the etching method has a profound effect on the formation of peroxide-decorated Ti3C2Tx. It was reported that titanium peroxide can be formed if TiO2 reacts with H2O2.55–57 Taking into account the fact that H2O2 is produced from cavitation bubbles during sonication55,58 and –OH groups are anchored onto the surface of Ti3C2Tx during the etching process, it is well-reasoned that p-Ti3C2Tx is formed in the sonication-assisted MILD etching process. Conventional Ti3C2Tx is inactive to initiate polymerization because no sonication treatment is involved in the preparation process.
In order to quantify p-Ti3C2Tx nanosheets, the luminol assay was used. Luminol is an extensively used chemiluminescence reagent which generates a blue glow in the presence of an appropriate oxidizing agent. Peroxide, Fe2+/Fe3+ and various biochemical substrates like ALP and glucose were detected successfully by using the luminol system. In this work, different concentrations of p-Ti3C2Tx nanosheets are incubated with a luminol solution. In the case of luminol mixed with p-Ti3C2Tx, the fluorescence intensity increases with the increase of the concentration of p-Ti3C2Tx (Fig. 3e). A good linear relationship between the fluorescence intensity and the concentration of p-Ti3C2Tx ranging from 0.01 to 0.05 mg mL−1 was obtained (Fig. 3f). The optimal concentration of Ti3C2Tx to initiate polymerization is above 0.05 mg mL−1.
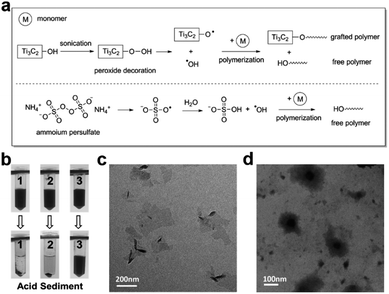
The proposed mechanism of polymerization is shown in Fig. 4a. After the initiation by p-Ti3C2Tx, two kinds of radicals (hydroxyl and peroxyl radicals) may be generated. After chain propagation, two kinds of polymers (grafted polymer and free polymer) are therefore created. Although some peroxide salts (such as ammonium persulfate) can initiate the polymerization of acrylic monomers with a similar mechanism (EPR spectrum in Fig. S6†), the p-Ti3C2Tx nanosheets themselves are functional materials and have the ability to trigger a surface-initiated “grafting-from” polymerization. The resulting graft polymers were examined using acidic sediment. Pristine p-Ti3C2Tx is not stable to be dispersed in an acidic solution due to the decreased negative charges at lower pH (Fig. 4b). In addition, p-Ti3C2Tx is also not stable at acidic pH when physically mixed with free PNIPAM. In contrast, the NC polymer dispersion remains well dispersed at acidic pH. This result demonstrates that the Ti3C2Tx nanosheets of the NC polymer dispersion are covalently grafted with PNIPAM. The steric stability of grafted PNIPAM ensures the high stability of the NC polymer dispersion.
Furthermore, the grafted polymer can be observed by TEM and AFM. The size of p-Ti3C2Tx nanosheets is about 200 nm with a single or multilayered structure (Fig. 4c). The morphology of the NC polymer dispersion is different from that of pristine p-Ti3C2Tx nanosheets. Instead, clusters of thin nanosheets of p-Ti3C2Tx are found in the polymer dispersion (Fig. S7†). After being stained with phosphotungstic acid, NC polymer shows a dark core with a grey shell coating in the TEM image (Fig. 4d). Meanwhile, the height of p-Ti3C2Tx nanosheets in the NC polymer is larger than that of pristine p-Ti3C2Tx as shown in the AFM image, suggesting the occurrence of polymer grafting on the surface of nanosheets (Fig. S8†). Unfortunately, the molecular weight of the polymers in the NC polymer dispersion is not determined in this work and remains a challenge due to the difficulty of the separation and/or detachment of the grafted polymer from the MXenes in the NC polymer dispersion.
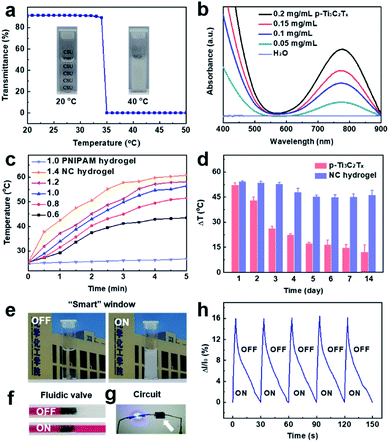
As is well-known, PNIPAM is a typical thermosensitive polymer and shows temperature-dependent optical transmittance. In this work, the PNIPAM-based NC hydrogels are synthesized using p-Ti3C2Tx as the initiator. By monitoring the transmittance as a function of time, the NC hydrogel has a LCST of 34 °C, close to the transition temperature of the pure PNIPAM hydrogel.59 The little influence of p-Ti3C2Tx nanosheets on the LCST is probably because of the low content of p-Ti3C2Tx (0.1 mg mL−1) in the hydrogel matrix. Below the LCST, the NC hydrogel is transparent, whereas above the LCST, the hydrogels become turbid indicating the dehydration of the PNIPAM networks (Fig. 5a).
To verify the photothermal performance of PNIPAM-based NC hydrogels, the absorbance spectra were measured. The NC hydrogels with different concentrations of p-Ti3C2Tx nanosheets show strong NIR absorption (650–900 nm, Fig. 5b). The photothermal conversion of NC hydrogels is proportional to the power density of irradiation (Fig. 5c). Under exposure to 808 nm laser irradiation, the temperature of NC hydrogels gradually increase as a function of irradiation time and power. The photothermal-conversion efficiency (η) of NC hydrogels was calculated to be 34.7% at 808 nm irradiation (Fig. S9†). In addition, the NC hydrogels retain similar photothermal effects after 5 cycles (Fig. S10†). The photothermal properties of the NC hydrogel under light beyond the infrared region were also studied. Compared to other light wavelengths (405, 532 and 648 nm), NIR (808 nm) triggers a pronounced photothermal effect in the NC hydrogel (Fig. S11†).
We evaluated the photothermal stability of Ti3C2Tx nanosheets and PNIPAM-based NC hydrogels under 808 nm irradiation (1.0 W cm−2) for 5 min. The results indicated that the pristine p-Ti3C2Tx nanosheets were almost oxidized and degraded gradually at low temperature (4 °C) in one week (Fig. 5d). In contrast, p-Ti3C2Tx embedded in the hydrogel matrix shows remarkable photothermal stability. The oxidation kinetics of p-Ti3C2Tx is quite slow in the polymer hydrogel matrix, which allows the MXenes to have a prolonged shelf life and reproducible functional properties in its applications.54 The long-term stability of the NC hydrogel depends on the inherent oxidation potential and environmental factors. The strategy of slowing down the kinetics in the semi-solid hydrogel media could greatly improve the stability of MXenes against oxidation.
In this work, the excellent photothermal performance and stability endow the PNIPAM-based NC hydrogel with potential applications such as NIR-responsive “smart” windows and fluidic valves based on its opaque character and volume shrinkage. As a proof of concept, simple models of a “smart” window and fluidic valve are rationally designed (Fig. 5e and f). The NC hydrogels also exhibit higher electrical conductivity than the pristine PNIPAM hydrogel. For example, when the p-Ti3C2Tx concentration is about 1.0 mg mL−1, the conductivity of the NC hydrogel increases from 0.0098 S m−1 to 0.019 S m−1. The enhancement in conductivity of the hydrogels depends on the content of p-Ti3C2Tx.60 The LED of a demo circuit glows throughout the NC hydrogels (Fig. 5g). Therefore, the NC hydrogel is designed to be a NIR detector. Upon irradiation of NIR for 3 s, the current increases abruptly (Fig. 5h). The reason for the increase of current may be ascribed to photoconductive effects and the accelerated electron mobility with a temperature rise.61–63 A decay of current to the original value is observed after turning off the laser. Repeatable signal patterns are observed in response to NIR irradiation, suggesting the potential application of NC hydrogels as infrared photodetectors and photovoltaics. Additionally, since both MXenes and PNIPAM are biocompatible,32,33,64 the NC hydrogel may be further designed as a photothermal agent for localized anticancer treatment or enzyme bioreactors.
Conclusions
In summary, peroxide-decorated Ti3C2Tx nanosheets are synthesized via the sonication-assisted MILD etching method. The nanosheets are able to generate reactive radical species, which initiate polymerization of acrylic monomers in a simple N2 purging procedure. Our approach to initiate the acrylic monomers has good universality, as confirmed by over 5 monomers. Physically crosslinked PNIPAM-based NC hydrogels are facilely formed using a high monomer concentration. The NC hydrogels are sensitive to temperature and NIR, which can serve as a means to adjust transmittance, volume, and conductivity. Their excellent photothermal performance and electrical conductivity guarantee their future application as remotely light controlled “smart” windows, fluidic valves, and photodetectors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21574147 and 21374133), Hunan Provincial Natural Science Foundation of China (No. 2018JJ2483), and Innovation-Driven Project of Central South University (No. 2017CX020).Notes and references
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Mueller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed.
- F. Ilievski, A. D. Mazzeo, R. E. Shepherd, X. Chen and G. M. Whitesides, Angew. Chem., Int. Ed., 2011, 50, 1890–1895 CrossRef CAS PubMed.
- W. Bai and D. A. Spivak, Angew. Chem., Int. Ed., 2014, 53, 2095–2098 CrossRef CAS PubMed.
- J. P. Yuan, D. Wen, N. Gaponik and A. Eychmuller, Angew. Chem., Int. Ed., 2013, 52, 976–979 CrossRef CAS PubMed.
- M. Hippler, E. Blasco, J. Y. Qu, M. Tanaka, C. Barner-Kowollik, M. Wegener and M. Bastmeyer, Nat. Commun., 2019, 10, 232 CrossRef PubMed.
- I. Tomatsu, K. Peng and A. Kros, Adv. Drug Delivery Rev., 2011, 63, 1257–1266 CrossRef CAS PubMed.
- X. Liu, J. Zhang, M. Fadeev, Z. Li, V. Wulf, H. Tian and I. Willner, Chem. Sci., 2019, 10, 1008–1016 RSC.
- J. D. Kim, J. S. Heo, T. Park, C. Park, H. O. Kim and E. Kim, Angew. Chem., Int. Ed., 2015, 54, 5869–5873 CrossRef CAS PubMed.
- G. Kocer, J. ter Schiphorst, M. Hendrikx, H. G. Kassa, P. Leclere, A. Schenning and P. Jonkheijm, Adv. Mater., 2017, 29, 1606407 CrossRef PubMed.
- D. D. Diaz, D. Kuhbeck and R. J. Koopmans, Chem. Soc. Rev., 2011, 40, 427–448 RSC.
- H. Wu, J. Zheng, A.-L. Kjøniksen, W. Wang, Y. Zhang and J. Ma, Adv. Mater., 2019, 31, 1806204 CrossRef PubMed.
- L.-W. Xia, R. Xie, X.-J. Ju, W. Wang, Q. Chen and L.-Y. Chu, Nat. Commun., 2013, 4, 2226 CrossRef PubMed.
- A. Sidorenko, T. Krupenkin, A. Taylor, P. Fratzl and J. Aizenberg, Science, 2007, 315, 487–490 CrossRef CAS PubMed.
- Y. S. Zhang and A. Khademhosseini, Science, 2017, 356, eaaf3627 CrossRef PubMed.
- X. J. Wang, C. W. Wei, J. H. Su, B. He, G. B. Wen, Y. W. Lin and Y. Zhang, Angew. Chem., Int. Ed., 2018, 57, 3504–3508 CrossRef CAS PubMed.
- Y. Zheng, Y. Liang, D. Zhang, Z. Zhou, J. Li, X. Sun and Y.-N. Liu, Chem. Commun., 2018, 54, 13805–13808 RSC.
- P. P. Zhang, F. Liu, Q. H. Liao, H. Z. Yao, H. Y. Geng, H. H. Cheng, C. Li and L. T. Qu, Angew. Chem., Int. Ed., 2018, 57, 16343–16347 CrossRef CAS PubMed.
- Z. F. Sun, Y. Yamauchi, F. Araoka, Y. S. Kim, J. Bergueiro, Y. Ishida, Y. Ebina, T. Sasaki, T. Hikima and T. Aida, Angew. Chem., Int. Ed., 2018, 57, 15772–15776 CrossRef CAS PubMed.
- L. Chen, X. Yao, Z. Gu, K. Zheng, C. Zhao, W. Lei, Q. Rong, L. Lin, J. Wang, L. Jiang and M. Liu, Chem. Sci., 2017, 8, 2010–2016 RSC.
- H. K. Bisoyi, A. M. Urbas and Q. Li, Adv. Opt. Mater., 2018, 6, 1800458 CrossRef.
- Y. Liang, Y. Hao, Y. Wu, Z. Zhou, J. Li, X. Sun and Y.-N. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 21381–21390 CrossRef CAS PubMed.
- M. Qiu, D. Wang, W. Liang, L. Liu, Y. Zhang, X. Chen, D. K. Sang, C. Xing, Z. Li, B. Dong, F. Xing, D. Fan, S. Bao, H. Zhang and Y. Cao, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 501–506 CrossRef CAS PubMed.
- X. H. Qin, X. P. Wang, M. Rottmar, B. J. Nelson and K. Maniura-Weber, Adv. Mater., 2018, 30, 1705564 CrossRef PubMed.
- Y. J. Ke, C. Z. Zhou, Y. Zhou, S. C. Wang, S. H. Chan and Y. Long, Adv. Funct. Mater., 2018, 28, 1800113 CrossRef.
- H. Yang, W. R. Leow, T. Wang, J. Wang, J. C. Yu, K. He, D. P. Qi, C. J. Wan and X. D. Chen, Adv. Mater., 2017, 29, 1701627 CrossRef PubMed.
- L. Wang, K. Liang, X. Jiang, M. Yang and Y. N. Liu, Chem.–Eur. J., 2018, 24, 6557–6563 CrossRef CAS PubMed.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- H. Wang, Y. Wu, X. Yuan, G. Zeng, J. Zhou, X. Wang and J. W. Chew, Adv. Mater., 2018, 30, 1704561 CrossRef PubMed.
- H. Lin, Y. Chen and J. L. Shi, Adv. Sci., 2018, 5, 1800518 CrossRef PubMed.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS.
- H. Luo, W. Feng, C. Liao, L. Deng, S. Liu, H. Zhang and P. Xiao, J. Appl. Phys., 2018, 123, 104103 CrossRef.
- J. Liu, X. Jiang, R. Zhang, Y. Zhang, L. Wu, W. Lu, J. Li, Y. Li and H. Zhang, Adv. Funct. Mater., 2019, 29, 1807326 CrossRef.
- C. Xing, S. Chen, X. Liang, Q. Liu, M. Qu, Q. Zou, J. Li, H. Tan, L. Liu, D. Fan and H. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 27631–27643 CrossRef CAS PubMed.
- J. Xuan, Z. Wang, Y. Chen, D. Liang, L. Cheng, X. Yang, Z. Liu, R. Ma, T. Sasaki and F. Geng, Angew. Chem., Int. Ed., 2016, 55, 14569–14574 CrossRef CAS PubMed.
- C. Yang, D. Xu, W. Peng, Y. Li, G. Zhang, F. Zhang and X. Fan, Nanoscale, 2018, 10, 15387–15392 RSC.
- Y.-Z. Zhang, K. H. Lee, D. H. Anjum, R. Sougrat, Q. Jiang, H. Kim and H. N. Alshareef, Sci. Adv., 2018, 4, eaat0098 CrossRef PubMed.
- C. Chen, M. Boota, X. Xie, M. Zhao, B. Anasori, C. E. Ren, L. Miao, J. Jiang and Y. Gogotsi, J. Mater. Chem. A, 2017, 5, 5260–5265 RSC.
- O. Mashtalir, K. M. Cook, V. N. Mochalin, M. Crowe, M. W. Barsoum and Y. Gogotsi, J. Mater. Chem. A, 2014, 2, 14334–14338 RSC.
- M. Boota, B. Anasori, C. Voigt, M.-Q. Zhao, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2016, 28, 1517–1522 CrossRef CAS PubMed.
- C. Liao, Q. Wu, T. Su, D. Zhang, Q. Wu and Q. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 1356–1360 CrossRef CAS PubMed.
- M. Liu, Y. Ishida, Y. Ebina, T. Sasaki and T. Aida, Nat. Commun., 2013, 4, 2029 CrossRef PubMed.
- J. Liu, T. An, Z. Chen, Z. Wang, H. Zhou, T. Fan, D. Zhang and M. Antonietti, J. Mater. Chem. A, 2017, 5, 8933–8938 RSC.
- Y.-C. Wong, J. D. A. Ng and Z.-K. Tan, Adv. Mater., 2018, 30, 1800774 CrossRef PubMed.
- J. Chen, K. Chen, D. Tong, Y. Huang, J. Zhang, J. Xue, Q. Huang and T. Chen, Chem. Commun., 2015, 51, 314–317 RSC.
- B. Kumru, M. Shalom, M. Antonietti and B. V. K. J. Schmidt, Macromolecules, 2017, 50, 1862–1869 CrossRef CAS.
- M. Alhabeb, K. Maleski, B. Anasori, P. Lelyukh, L. Clark, S. Sin and Y. Gogotsi, Chem. Mater., 2017, 29, 7633–7644 CrossRef CAS.
- Y. Tamai, H. Tanaka and K. Nakanishi, Macromolecules, 1996, 29, 6750–6760 CrossRef CAS.
- C. Peng, H. Wang, H. Yu and F. Peng, Mater. Res. Bull., 2017, 89, 16–25 CrossRef CAS.
- A. Shahzad, K. Rasool, M. Nawaz, W. Miran, J. Jang, M. Moztahida, K. A. Mahmoud and D. S. Lee, Chem. Eng. J., 2018, 349, 748–755 CrossRef CAS.
- C. Peng, X. Yang, Y. Li, H. Yu, H. Wang and F. Peng, ACS Appl. Mater. Interfaces, 2016, 8, 6051–6060 CrossRef CAS PubMed.
- X. Li, Y. Qiao, S. Guo, Z. Xu, H. Zhu, X. Zhang, Y. Yuan, P. He, M. Ishida and H. Zhou, Adv. Mater., 2018, 30, 1705197 CrossRef PubMed.
- Z. Wang, J. Xuan, Z. Zhao, Q. Li and F. Geng, ACS Nano, 2017, 11, 11559–11565 CrossRef CAS PubMed.
- B. P. Swain and R. O. Dusane, Mater. Chem. Phys., 2006, 99, 240–246 CrossRef CAS.
- T. Habib, X. Zhao, S. A. Shah, Y. Chen, W. Sun, H. An, J. L. Lutkenhaus, M. Radovic and M. J. Green, npj 2D Mater. Appl., 2019, 3, 8 CrossRef.
- T. Tuziuti, K. Yasui, Y. Iida, H. Taoda and S. Koda, Ultrasonics, 2004, 42, 597–601 CrossRef CAS PubMed.
- Y. Wang, X. Meng, X. Yu, M. Zhang and J. Yang, Appl. Catal., B, 2013, 138–139, 326–332 CrossRef CAS.
- C. Ogino, M. F. Dadjour, Y. Iida and N. Shimizu, J. Hazard. Mater., 2008, 153, 551–556 CrossRef CAS PubMed.
- P. Kanthale, M. Ashokkumar and F. Grieser, Ultrason. Sonochem., 2008, 15, 143–150 CrossRef CAS PubMed.
- L. Hou and P. Wu, Soft Matter, 2014, 10, 3578–3586 RSC.
- H. Liao, X. Guo, P. Wan and G. Yu, Adv. Funct. Mater., 2019, 29, 1904507 CrossRef.
- X. L. Zhao, X. B. Ding, Z. H. Deng, Z. H. Zheng, Y. X. Peng and X. P. Long, Macromol. Rapid Commun., 2005, 26, 1784–1787 CrossRef CAS.
- S. C. Tong, J. Sun, C. H. Wang, Y. L. Huang, C. J. Zhang, J. Q. Shen, H. P. Xie, D. M. Niu, S. Xiao, Y. B. Yuan, J. He, J. L. Yang and Y. L. Gao, Adv. Electron. Mater., 2017, 3, 1700058 CrossRef.
- H. Xia, S. Tong, C. Zhang, C. Wang, J. Sun, J. He, J. Zhang, Y. Gao and J. Yang, Appl. Phys. Lett., 2018, 112, 233301 CrossRef.
- M. Zhou, S. Liu, Y. Jiang, H. Ma, M. Shi, Q. Wang, W. Zhong, W. Liao and M. M. Q. Xing, Adv. Funct. Mater., 2015, 25, 4730–4739 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc03917a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2019 |