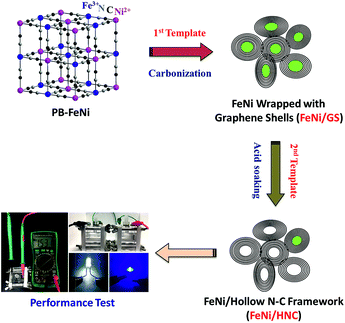
Coupling FeNi alloys and hollow nitrogen-enriched carbon frameworks leads to high-performance oxygen electrocatalysts for rechargeable zinc–air batteries†
Haihong
Wu‡
ab,
Min
Zeng‡
a,
Zhiyun
Li‡
a,
Xiang
Zhu
 *c,
Chengcheng
Tian
d,
Chungu
Xia
*a,
Lin
He
*c,
Chengcheng
Tian
d,
Chungu
Xia
*a,
Lin
He
 *a and
Sheng
Dai
*a and
Sheng
Dai
 d
d
aState Key Laboratory for Oxo Synthesis and Selective Oxidation, Suzhou Research Institute of Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou, 730000, China. E-mail: cgxia@licp.cas.cn; helin@licp.cas.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cDepartment of Chemistry, Texas A&M University, College Station, Texas 77843, USA. E-mail: xiang@tamu.edu/zhuxiang.ecust@gmail.com
dChemical Sciences Division, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA
First published on 6th September 2018
Abstract
A dual-template strategy for facile preparation of a bifunctional oxygen electrocatalyst for high-performance rechargeable zinc–air batteries has been reported. Coupling FeNi alloys with hollow nitrogen-doped carbon frameworks results in exceptionally high electrocatalytic oxygen reduction and evolution activities. In 1 M KOH, the resulting new material exhibits a superior oxygen evolution activity with a low overpotential of 250 mV to deliver 10 mA cm−2 current density, at which the obtained oxygen reduction performance is also comparable to that of commercial Pt/C and the half-wave potential reaches as high as 0.87 V. As a result, the bifunctional oxygen electrocatalysis performance thus obtained (0.61 V, 1 M KOH) ranks among the best of non-precious oxygen electrocatalysts. Using this new catalyst as an air electrode, the as-prepared rechargeable Zn–air battery shows a high current density of 215 mA cm−2 at a voltage of 1.0 V, large peak power density (310 mW cm−2), high potential efficiency (64.7% at 10 mA cm−2) and prolonged operation durability. This approach provides a means to control the surface features, thereby tuning the catalytic properties of the material, and may open up new possibilities for the rational design and synthesis of new materials for electrochemical applications.
Introduction
The development of renewable energy generation and storage systems, such as rechargeable metal–air batteries, is vital for mitigating the adverse effects of the increasing global energy crisis and environmental issues.1–3 Zn–air batteries have attracted tremendous attention due to their high theoretical energy density of 1086 W h kg−1, low cost and environmentally friendly components, suggesting great promise for application in portable electronic devices and electric vehicles.4–10 However, their energy efficiencies are still far from satisfactory and have been significantly limited by large overpotentials derived from the sluggish oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) kinetics at the air cathode.11,12 Thus far, precious Pt-based materials and Ru/Ir-based compounds have been applied as state-of-the-art electrocatalysts for the ORR and OER, respectively; however, their intrinsic drawbacks, such as high cost and poor durability, have restricted their potential for scale-up preparation.1–3 In this regard, great research efforts have been devoted to the rational design and synthesis of highly efficient and cost-effective bifunctional oxygen electrocatalysts for Zn–air batteries.Among various types of catalysts, transition-metals (e.g. Fe, Co, Ni) and their derivatives (such as FeCo and FeNi alloys) have been studied as promising alternatives because of their theoretically high catalytic activities and rich reserves.13,14 However, solely using these materials usually results in poor bifunctional oxygen catalytic performance. For example, FeNi and FeCo alloys have been extensively studied for the OER, whereas their ORR activities are shown to be very low owing to the lack of ORR-active sites within the architectures.15–20 In this context, coupling these nanoparticles with ORR-active nitrogen-doped carbon (N–C) materials, to work in concert, could be a promising methodology to improve their bifunctional electrocatalytic performance.21–24 Unfortunately, merging these two different kinds of attractive sites in one material remains a challenge, mainly because it is difficult to bridge their huge synthesis differences.
Herein, we report a dual-template strategy to create FeNi alloys and hollow N–C framework hybrids as an efficient bifunctional electrocatalyst for the OER and ORR in alkaline media. The key to our success lies in the formation of attractive FeNi-based core–shell nanostructures by the thermal treatment of a FeNi-based Prussian blue analogue. These core–shell structures further give rise to the formation of hollow N–C frameworks by simple acid etching of FeNi nanoparticles. As a result, the obtained new catalyst exhibits an excellent OER activity in 1 M KOH with a low overpotential (η10) of 250 mV to deliver 10 mA cm−2 current density, at which its ORR activity is also comparable to that of commercial Pt/C and the half-wave potential (E1/2) reaches as high as 0.87 V. Additionally, using this catalyst as an air electrode, the obtained rechargeable Zn–air battery exhibits a high current density of 215 mA cm−2 at a voltage of 1.0 V, large peak power density (310 mW cm−2) and prolonged operation stability.
Results and discussion
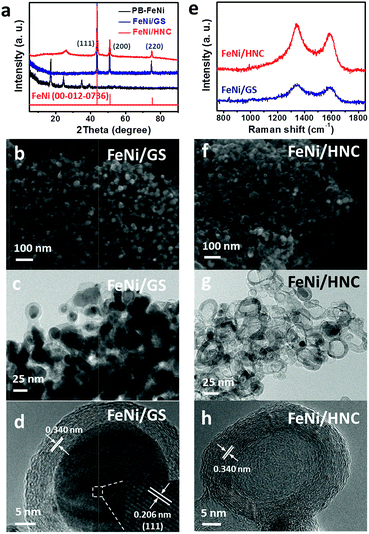
As shown in Scheme 1, a FeNi-based Prussian blue analogue (PB-FeNi) was prepared and employed as the sacrificial template. Simple coprecipitation of nickel acetate and K3Fe(CN)6 solutions affords PB-FeNi nanoparticles (synthesis details can be found in the ESI†).25 The structural features of PB-FeNi were first studied using the X-ray diffraction (XRD) technique. As shown in Fig. 1a, the pattern of PB-FeNi matches well with that of nitrosyl nickel iron cyanide hydrate Ni[Fe(CN)5NO]2·2H2O (JCPDS card no. 00-022-0463). A high carbon yield of ca. 40 wt% (over 600 °C) was indicated by the thermogravimetric analysis (TGA) (Fig. S1†). Pyrolysis at 600 °C under Ar was subsequently performed to convert PB-FeNi to the desired FeNi alloys wrapped with graphene-like shells (FeNi/GS).The formation of graphitic structures was supported by the observed D (ca. 1346 cm−1) and G bands (ca. 1580 cm−1) in the Raman spectrum of FeNi/GS (Fig. 1e).26 A graphene-like shell with an interlayer distance of 3.40 Å was clearly observed in the high-resolution transmission electron microscopy (HR-TEM) image (Fig. 1d). Most importantly, the existence of FeNi alloys was indicated by the XRD pattern (JCPDS card no. 00-012-0736) (Fig. 1a). This result was further confirmed by the HR-TEM measurements. As shown in Fig. 1d, an obvious lattice fringe with a d-spacing of 2.06 Å, consistent with the (111) plane of the FeNi alloy, was observed. Energy dispersive X-ray spectroscopy (EDS) confirms the presence of Fe, Ni, C and N within the material (FeNi/GS). Interestingly, mapping of their relative distributions indicates the presence of the N-doped graphene-like shells as well (Fig. S2†).
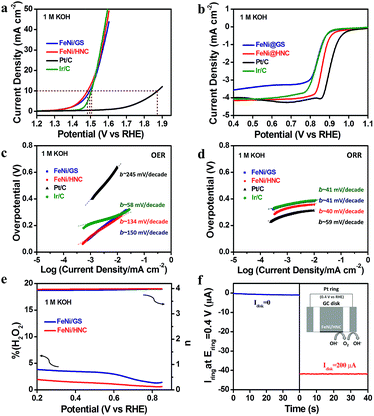
We then examined the OER activity of the FeNi/GS in 1 M KOH using a typical rotating disc electrode (RDE) technique. A glassy carbon electrode was employed as the working electrode (catalyst loading: ca. 0.3 mg cm−2). As expected, a high catalytic activity for the OER was achieved as a result of the existence of rich FeNi alloys, exceeding that of a commercial noble Ir/C catalyst. Fig. 2a displays linear sweep voltammetry (LSV) curves. The anodic current recorded on the FeNi/GS renders a sharp onset potential at 1.34 V (versus the reversible hydrogen electrode, RHE), which is better than that of the Ir/C (Eonset = 1.44 V). The overpotential (η10) of FeNi/GS to deliver 10 mA cm−2 current density, which is a metric related to solar water-splitting devices, reaches as low as 266 mV. This value is lower than that of Ir/C (η10 = 275 mV) and also comparable to those of best-performing non-precious OER catalysts, for example NiCo bimetal–organic framework nanosheets (η10 = 250 mV),27 Ni2P/NiOx core–shell structures (η10 = 280 mV)28 and Co4N porous nanowire arrays (η10 = 257 mV).29 A detailed comparison of various highly active OER catalysts is shown in Table S1.†
Motivated by the above promising OER performance, we further studied the ORR performance in 1.0 M KOH. The polarization curve of FeNi/GS on the rotating ring disk electrode (RRDE) exhibits a positive onset potential (Eonset) of 0.98 V and a high half-wave potential (E1/2) of 0.84 V (Fig. 2b), which is 70 mV smaller than that of 20 wt% commercial Pt/C (Eonset = 1.06 V and E1/2 = 0.91 V). We reasoned that the lack of active ORR sites within the matrix may account for this lower performance. As reported, N-doped graphene materials have been extensively studied as alternative efficient metal-free ORR catalysts.6,30 We thus hypothesized that selective etching of FeNi alloys inside FeNi/GS with acid solutions towards the formation of hollow N-doped carbon frameworks could help improve the overall ORR performance.
Toward this end, the as-obtained FeNi/GS was further used as the second template. After simply etching with 1 M HCl for 8 h, significant mass losses of both Fe (10.2 vs. 22.7 wt%) and Ni (15.1 vs. 34.5 wt%) were observed for the new material (FeNi/HNC) from the inductively coupled plasma (ICP) analysis. Acid etching for a longer time of 16 h affords the lowest content of Fe and Ni (Table S6†). As a result, the porosity of the resultant material was greatly enhanced (177 m2 g−1 for FeNi/HNC and 38 m2 g−1 for FeNi/GS), as confirmed by the N2 adsorption–desorption curves at 77 K (Fig. S3†). The obtained pore size distribution curves successfully support the existence of mesopores within the matrix of FeNi/HNC. Highly porous architectures could help facilitate the transport of gas and the electrolyte to the active sites, thereby boosting the ORR performance. Furthermore, HR-TEM images clearly indicate the generation of a hollow carbon framework (Fig. 1g and h), in which some OER-active FeNi alloys wrapped with graphene-like layers were also observed. The XRD results support the existence of FeNi alloys as well (Fig. 1a). X-ray photoelectron spectroscopy (XPS) was thus performed to study the surface chemical features. As depicted in Fig. S4a and S4b,† the lowest binding energy peaks in both Ni and Fe spectra correspond to metallic Ni (852.7 eV) and Fe (707.2 eV) from FeNi alloys. The peaks at 853.8 eV and 855.4 eV are ascribed to Ni (2P3/2) of Ni2+ and Ni3+, respectively. And the Fe 2P spectrum shows two peaks at 709.7 eV and 712.7 eV.31–33 In addition, a high N content of 4.1 at% was obtained for the FeNi/HNC. And two major N species, including pyridinic and pyrrolic N, existed. Accordingly, high ORR activity for this new mesoporous FeNi/HNC with hollow N-enriched carbon frameworks could be expected.34–36
The RRDE test was performed in 1.0 M KOH by using the FeNi/HNC as a new electrode material so as to evaluate its ORR activity. Interestingly, the FeNi/HNC exhibits an enhanced ORR activity with a E1/2 of 0.87 V, which is only 40 mV lower than that of the Pt/C. Moreover, the Tafel slope of FeNi/HNC (40 mV dec−1) is lower than that of Pt/C (59 mV dec−1) (Fig. 2d), suggesting a faster kinetic process. The excellent ORR activity of FeNi/HNC is comparable to those of previously reported high-performance non-precious catalysts (Table S2†). The CV curves under N2 and O2 atmospheres as well as RDE polarization curves in 1 M KOH at different electrode rotation speeds are recorded and shown in Fig. S6,† further confirming the obtained high activity. We reasoned that the existence of rich N-doped hollow spheres and intrinsic mesoporous nature of FeNi/HNC may account for this enhanced performance. The peroxide yield was measured to be lower than 5.0% at all potentials (Fig. 2e). As a result, a high electron transfer number (>3.9) is calculated, suggesting four-electron reduction of oxygen.
Although some FeNi alloys were removed by acid washing, the OER performance of the FeNi/HNC maintains very well. This new material affords a current density of 10 mA cm−2 at 1.48 V with an exceptional overpotential (η10) of 250 mV. We further examined the effect of etching time on the OER activity and found that no significant progress was achieved when the etching time was extended to 16 h (Fig. S7†). Nevertheless, the fact that the existence of FeNi alloys plays a crucial role in achieving such a high OER activity can be confirmed by poison testing (Fig. S8†). In the presence of KSCN, significant loss of OER activity at a much higher overpotential (340 mV) was observed for the FeNi/HNC due to the coordination between FeNi sites and SCN− anions. Additionally, the use of 1 M KOH/5 mM KSCN as the electrolyte affords a decreased ORR activity as well.
Tafel plots were then examined to study the intrinsic catalytic kinetics of the OER on the FeNi/HNC. The Tafel slope value of FeNi/HNC (134 mV dec−1) is lower than that of FeNi/GS (150 mV dec−1), but still higher than that of Ir/C (58 mV dec−1) (Fig. 2c). A more detailed investigation of the reaction mechanism, using the rotating ring-disk electrode (RRDE) technique with a Pt ring electrode potential of 1.50 V (RHE), was carefully performed.37 Under this condition, the peroxide intermediates generated at the FeNi/HNC surface during the OER can be oxidized. Negligible hydrogen peroxide formation was indicated by a very low ring current (<6 μA) (Fig. S9†). Thus, a four electron pathway for water oxidation i.e., 4OH− → O2 + 2H2O + 4e− can be suggested.37 Additionally, we further employed a RRDE with a ring potential of 0.40 V (RHE) to reduce the generated O2 molecules, rendering a continuous OER (disk electrode) → ORR (ring electrode) process, and to further verify that the resulting current originates from the OER rather than from other side reactions.37–39 As shown in Fig. 2f, when a disk current of 0 (Idisk = 0) was applied, a negligible ring current was obtained. However, the constant use of a disk current of 200 μA (Idisk = 200 μA) successfully afforded a ring current of ∼41.6 μA, indicating that the generated O2 molecules were quickly reduced.37 Therefore, the oxidation current originates from the OER with a high faradaic efficiency of 98%. The obtained promising OER performance may be further supported by the electrochemically active surface area (ECSA) of FeNi/HNC, which was evaluated using the electrochemical double-layer capacitance (Cdl) and surface roughness factor (Rf).40–43 As shown in Fig. S10,† the Cdl and Rf of FeNi/HNC were determined to be 12.8 mF cm−2 and 213.3, which are three times higher than those of FeNi/GS (Cdl = 3.9 mF cm−2 and Rf = 65.0). The ECSA is therefore calculated to be 71.1 m2 g−1, when a specific capacitance value Cs = 0.060 mF cm−2 is adopted.44 The high ECSA value of FeNi/HNC further confirms its favorable reaction kinetics since previous studies have demonstrated that large ECSAs could facilitate the transport of the electrolyte into catalytically active surfaces.45
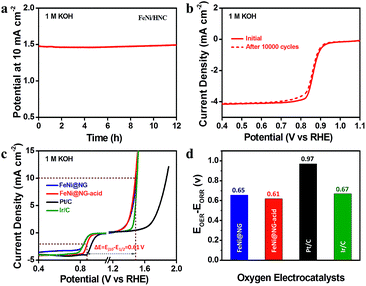
Durability has been considered as another key parameter governing the long-term practical application of electrocatalysts in energy conversion and storage systems. The potential of FeNi/HNC shifted positively by less than 20 mV to maintain 10 mA cm−2 for 12 hours, and negligible ORR performance loss was observed after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, suggesting prolonged operation stabilities (Fig. 3a and b). The overall oxygen electrocatalytic activity on FeNi/HNC was evaluated using the difference between OER and ORR metrics (ΔE = Ej=10 − E1/2). The smaller the ΔE value is, the closer the catalyst is to being an ideal reversible oxygen electrode.5 As shown in Fig. 3c–d and Table S3,† the ΔE obtained for the FeNi/HNC (0.61 V) is among the best, suggesting its high potential for application as an electrode material in rechargeable Zn–air batteries.
000 cycles, suggesting prolonged operation stabilities (Fig. 3a and b). The overall oxygen electrocatalytic activity on FeNi/HNC was evaluated using the difference between OER and ORR metrics (ΔE = Ej=10 − E1/2). The smaller the ΔE value is, the closer the catalyst is to being an ideal reversible oxygen electrode.5 As shown in Fig. 3c–d and Table S3,† the ΔE obtained for the FeNi/HNC (0.61 V) is among the best, suggesting its high potential for application as an electrode material in rechargeable Zn–air batteries.
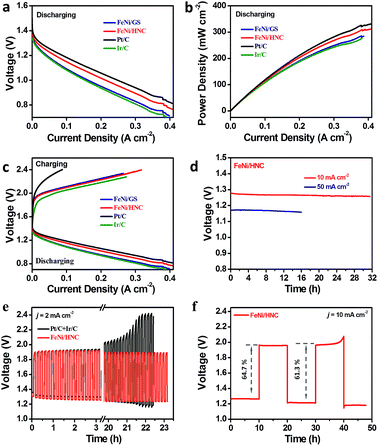
FeNi/HNC was then studied as the air cathode material to evaluate the battery performance (Fig. S12,† detailed information can be found in the ESI†). 6 M KOH solution was employed as the electrolyte, and an open circuit voltage of ca. 1.43 V (Fig. 4a) was obtained for the home-made zinc–air battery. The polarization curve and the corresponding power density plot are shown in Fig. 4a and b. Remarkably, a large current density of 215 mA cm−2 is obtained at a voltage of 1.0 V. The maximum power density reaches as high as 310 mW cm−2, ranking among the best performances (Table S4†). Furthermore, an exceptionally high stability was also observed for our zinc–air battery. When galvanostatically discharged at a current density of 10 mA cm−2 for 32 h, even 50 mA cm−2 for 16 h, no obvious voltage drop was observed (Fig. 4d). The slight voltage loss is associated with the degradation of the zinc anode. As shown in Fig. 4e, the FeNi/HNC shows a long cycle life of over 23 hours without any obvious voltage change. In contrast, the controlled Zn–air batteries prepared by using Pt/C and Ir/C as electrode materials exhibit very poor cycle lifes with a large overpotential increase. When galvanostatic charge and discharge curves were recorded at a higher current density of 10 mA cm−2 for a longer cycle period of 20 hours, a promising cycle performance with a small overpotential increase of 70 mV was obtained for the FeNi/HNC as well (Fig. 4f, Table S5†). High voltage efficiencies of 64.7% and 61.3% were achieved, respectively.
Conclusions
In summary, a dual-template strategy was developed to generate a high-performance bifunctional oxygen electrocatalyst for rechargeable zinc–air batteries. The key to our success lies in the simultaneous coupling of OER-active FeNi alloys and ORR-active N-doped hollow carbon within one material framework. The resulting new catalyst exhibits an excellent oxygen evolution activity with a low overpotential of 250 mV to deliver 10 mA cm−2 current density in 1 M KOH, at which the obtained ORR performance is comparable to that of commercial Pt/C and the half-wave potential reaches as high as 0.87 V. As a result, the bifunctional performance thus obtained for this new material (0.61 V, 1 M KOH) ranks among the best of non-precious oxygen electrocatalysts. Furthermore, using this catalyst as the desired air electrode material, the as-prepared rechargeable Zn–air battery exhibits a high current density of 215 mA cm−2 at a voltage of 1.0 V, large peak power density (310 mW cm−2), high potential efficiency (64.7% at 10 mA cm−2) and prolonged operation durability. This approach provides a means to control the surface features, thereby tuning the catalytic properties of the material, and may open up new possibilities for the rational design and synthesis of new materials for electrochemical applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported financially by the National Program for Thousand Young Talents of China, National Natural Science Foundation of China (21633013 and 21703267) and Foundation Research Project of Jiangsu Province (BK20171242 and BK20170423). X. Z. and S. D. were supported by the Materials Sciences and Engineering Division, Office of Basic Energy Sciences, U.S. Department of Energy under contract with UT-Battelle.Notes and references
- Z. L. Wang, D. Xu, J. J. Xu and X. B. Zhang, Chem. Soc. Rev., 2014, 43, 7746–7786 RSC.
- F. Cheng and J. Chen, Chem. Soc. Rev., 2012, 41, 2172–2192 RSC.
- Y. Li and H. Dai, Chem. Soc. Rev., 2014, 43, 5257–5275 RSC.
- Y. Li, M. Gong, Y. Liang, J. Feng, J. E. Kim, H. Wang, G. Hong, B. Zhang and H. Dai, Nat. Commun., 2013, 4, 1805 CrossRef PubMed.
- T. Y. Ma, J. Ran, S. Dai, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2015, 54, 4646–4650 CrossRef CAS PubMed.
- J. Zhang, Z. Zhao, Z. Xia and L. Dai, Nat. Nanotechnol., 2015, 10, 444–452 CrossRef CAS PubMed.
- Q. Liu, Y. Wang, L. Dai and J. Yao, Adv. Mater., 2016, 28, 3000–3006 CrossRef CAS PubMed.
- X. Liu, M. Park, M. G. Kim, S. Gupta, G. Wu and J. Cho, Angew. Chem., Int. Ed., 2015, 54, 9654–9658 CrossRef CAS PubMed.
- L. Li and A. Manthiram, Adv. Energy Mater., 2016, 6, 1502054 CrossRef.
- J. Fu, D. U. Lee, F. M. Hassan, L. Yang, Z. Bai, M. G. Park and Z. Chen, Adv. Mater., 2015, 27, 5617–5622 CrossRef CAS PubMed.
- Y. Zheng, Y. Jiao, Y. Zhu, Q. Cai, A. Vasileff, L. H. Li, Y. Han, Y. Chen and S.-Z. Qiao, J. Am. Chem. Soc., 2017, 139, 3336–3339 CrossRef CAS PubMed.
- T. Cheng, W. Bin, W. Hao-Fan and Z. Qiang, Adv. Mater., 2017, 29, 1703185 CrossRef PubMed.
- W. T. Hong, M. Risch, K. A. Stoerzinger, A. Grimaud, J. Suntivich and Y. Shao-Horn, Energy Environ. Sci., 2015, 8, 1404–1427 RSC.
- F. Song, L. Bai, A. Moysiadou, S. Lee, C. Hu, L. Liardet and X. Hu, J. Am. Chem. Soc., 2018, 140, 7748–7759 CrossRef CAS PubMed.
- U. Y. Qazi, C. Z. Yuan, N. Ullah, Y. F. Jiang, M. Imran, A. Zeb, S. J. Zhao, R. Javaid and A. W. Xu, ACS Appl. Mater. Interfaces, 2017, 9, 28627–28634 CrossRef CAS PubMed.
- G. Zhang, G. Wang, H. Liu, J. Qu and J. Li, Nano Energy, 2018, 43, 359–367 CrossRef CAS.
- Y. Yang, Z. Lin, S. Gao, J. Su, Z. Lun, G. Xia, J. Chen, R. Zhang and Q. Chen, ACS Catal., 2017, 7, 469–479 CrossRef CAS.
- X. Zhu, T. Jin, C. Tian, C. Lu, X. Liu, M. Zeng, X. Zhuang, S. Yang, L. He, H. Liu and S. Dai, Adv. Mater., 2017, 29, 1704091 CrossRef PubMed.
- P. Cai, Y. Hong, S. Ci and Z. Wen, Nanoscale, 2016, 8, 20048–20055 RSC.
- F. Gengtao, C. Zhiming, C. Yifan, L. Yutao, T. Yawen and J. B. Goodenough, Adv. Energy Mater., 2017, 7, 1601172 CrossRef.
- G. L. Tian, M. Q. Zhao, D. Yu, X. Y. Kong, J. Q. Huang, Q. Zhang and F. Wei, Small, 2014, 10, 2251–2259 CrossRef CAS PubMed.
- J. Wang, H. Wu, D. Gao, S. Miao, G. Wang and X. Bao, Nano Energy, 2015, 13, 387–396 CrossRef CAS.
- C. Hu and L. Dai, Angew. Chem., Int. Ed., 2016, 55, 11736–11758 CrossRef CAS PubMed.
- G. Wu, A. Santandreu, W. Kellogg, S. Gupta, O. Ogoke, H. Zhang, H. L. Wang and L. Dai, Nano Energy, 2016, 29, 83–110 CrossRef CAS.
- M. Zeng, Y. Liu, F. Zhao, K. Nie, N. Han, X. Wang, W. Huang, X. Song, J. Zhong and Y. Li, Adv. Funct. Mater., 2016, 26, 4397–4404 CrossRef CAS.
- Z. Zhang, M. Dou, H. Liu, L. Dai and F. Wang, Small, 2016, 12, 4193–4199 CrossRef CAS PubMed.
- S. Zhao, Y. Wang, J. Dong, C. T. He, H. Yin, P. An, K. Zhao, X. Zhang, C. Gao, L. Zhang, J. Lv, J. Wang, J. Zhang, A. M. Khattak, N. A. Khan, Z. Wei, J. Zhang, S. Liu, H. Zhao and Z. Tang, Nat. Energy, 2016, 1, 16184 CrossRef CAS.
- L. A. Stern, L. Feng, F. Song and X. Hu, Energy Environ. Sci., 2015, 8, 2347–2351 RSC.
- P. Chen, K. Xu, Z. Fang, Y. Tong, J. Wu, X. Lu, X. Peng, H. Ding, C. Wu and Y. Xie, Angew. Chem., Int. Ed., 2015, 54, 14710–14714 CrossRef CAS PubMed.
- D. Guo, R. Shibuya, C. Akiba, S. Saji, T. Kondo and J. Nakamura, Science, 2016, 351, 361–365 CrossRef CAS PubMed.
- G. Wu, W. Chen, X. Zheng, D. He, Y. Luo, X. Wang, J. Yang, Y. Wu, W. Yan, Z. Zhuang, X. Hong and Y. Li, Nano Energy, 2017, 38, 167–174 CrossRef CAS.
- V. Gomez, S. Irusta, O. B. Lawal, W. Adams, R. H. Hauge, C. W. Dunnill and A. R. Barron, RSC Adv., 2016, 6, 11895–11902 RSC.
- Z. Zhao, H. Wu, H. He, X. Xu and Y. Jin, J. Mater. Chem. A, 2015, 3, 7179–7186 RSC.
- X. Zhu, Y. Zhu, C. Tian, T. Jin, X. Yang, X. Jin, C. Li, H. Wang, H. Liu and S. Dai, J. Mater. Chem. A, 2017, 5, 4507–4512 RSC.
- Y. Li, T. Li, M. Yao and S. Liu, J. Mater. Chem., 2012, 22, 10911–10917 RSC.
- J. C. Li, P. X. Hou, S. Y. Zhao, C. Liu, D. M. Tang, M. Cheng, F. Zhang and H. M. Cheng, Energy Environ. Sci., 2016, 9, 3079–3084 RSC.
- T. Y. Ma, S. Dai, M. Jaroniec and S. Z. Qiao, J. Am. Chem. Soc., 2014, 136, 13925–13931 CrossRef CAS PubMed.
- T. Y. Ma, J. L. Cao, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2016, 55, 1138–1142 CrossRef CAS PubMed.
- D. Chen, C. L. Dong, Y. Zou, D. Su, Y. C. Huang, L. Tao, S. Dou, S. Shen and S. Wang, Nanoscale, 2017, 9, 11969–11975 RSC.
- D. U. Lee, J. Y. Choi, K. Feng, H. W. Park and Z. Chen, Adv. Energy Mater., 2014, 4, 1301389 CrossRef.
- Y. Li, P. Hasin and Y. Wu, Adv. Mater., 2010, 22, 1926–1929 CrossRef CAS PubMed.
- K. Gong, F. Du, Z. Xia, M. Durstock and L. Dai, Science, 2009, 323, 760–764 CrossRef CAS PubMed.
- J. Tian, Q. Liu, A. M. Asiri and X. Sun, J. Am. Chem. Soc., 2014, 136, 7587–7590 CrossRef CAS PubMed.
- C. C. L. McCrory, S. Jung, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2013, 135, 16977–16987 CrossRef CAS PubMed.
- H. Kong, J. Song and J. Jang, Chem. Commun., 2010, 46, 6735–6737 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8se00362a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |