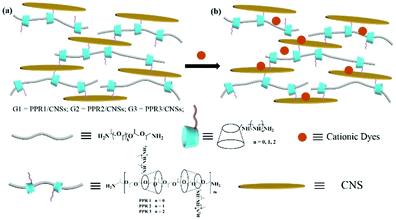
Construction and efficient dye adsorption of supramolecular hydrogels by cyclodextrin pseudorotaxane and clay†
Yi
Zhang
a,
Lu
Liang
a,
Yong
Chen
a,
Xu-Man
Chen
a and
Yu
Liu
 *ab
*ab
aCollege of Chemistry, State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin 300071, P. R. China. E-mail: yuliu@nankai.edu.cn
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, P. R. China
First published on 28th November 2018
Abstract
Supramolecular hydrogels, which are usually used to develop excellent smart soft materials, are widely applied in miscellaneous fields due to their inherent reversible properties, unique functions and mechanical properties. Compared with covalently linked hydrogels, supramolecular hydrogels have advantages of easy preparation, stimulus responsiveness and good biocompatibility. Herein, after threading amino-modified β-cyclodextrins onto poly(propyleneglycol)bis(2-amionopropylether) (PPG-NH2) chains, the resultant pseudorotaxanes non-covalently interacted with a clay nanosheet (CNS) matrix to construct supramolecular hydrogels bearing negative charges, and the mechanical properties of these hydrogels were positively correlated with the number of amino groups on the pseudorotaxane. Significantly, these hydrogels presented good adsorption properties for cationic dyes. The adsorption capacity (Qe) of the hydrogels towards rhodamine B (RhB), crystal violet (CV), and methylene blue (MB) could reach 181–228 mg g−1, and most of the dyes were adsorbed within 5 min. Thus, these hydrogels may have potential applications in the field of waste water treatment.
Introduction
Recently, supramolecular hydrogels constructed from ordered self-assemblies via supramolecular interactions including hydrogen bonds, π–π stacking, electrostatic interaction, metal–ligand interaction, van der Waals forces and hydrophobic interaction have attracted more attention.1 Unlike the classic chemical gels that lack stimulus responsiveness, supramolecular hydrogels not only have good mechanical properties (shear thinning, self-healing, etc.) but also can respond to several external stimuli such as heat, light, electricity and pressure.2 Among the various building blocks, pseudorotaxanes that are made from the threading of cyclodextrins (CDs) onto an axial polymer chain3 have been widely used in the construction of supramolecular hydrogels because of excellent biocompatibility, easy chemical modification and the mobility of the CD macrocycles on the axis.4 Harada et al. reported a series of CD-based polyrotaxanes.5 In a prior study, they synthesized and characterized several polymer gels based on polyrotaxane and poly(acrylamide) cross-linked with boronate linkages, and these gels were used for a scratch-healing top coating of hard materials due to their excellent self-healing properties.6 In addition, through the non-covalent cross-linking of tetraphenylethylene bridged CD oligomer with PEG, a strong blue fluorescence and injectable supramolecular hydrogel was successfully constructed, and the shear thinning properties and stimulus responsiveness of the gel enabled its potential application in many fields of soft materials.7 Kato and Ito reported a novel polyrotaxane formed by γ-CD threaded on a poly(dimethylsiloxane) chain, and γ-CDs on polyrotaxane were intermolecularly crosslinked in solution to get a new class of slip ring gels.8On the other hand, typically CNSs (LAPONITE® XLG) are a kind of sheet-like inorganic nanosheets with negative charges on their surface. Electrostatic forces among the nanosheets allow them to be dispersed in water to form clear and colorless aqueous dispersions. Because of the characteristic distribution of surface charges, the aqueous dispersion has different physical states such as viscoelastic sols and gels.9 Interestingly, the incorporation of water-soluble cationic polymers with CNSs can form a stable three-dimensional hydrogel network with good mechanical strength and other exciting properties. Aida et al. used a kind of dendrimer with multiple guanidino groups and CNSs to form a high-water content supramolecular hydrogel.10 We prepared supramolecular gels from CNSs and pseudorotaxanes that are composed of per(6-guanidino-6-deoxy)-β-CD and poly(propyleneglycol) chains with satisfactory mechanical properties and self-healing properties.2b Moreover, we also constructed a supramolecular hydrogel featuring reversible luminescence switching behavior by intercalating the dithienylethene-bridged bispyridinium dye into CNSs to activate the hydrogel fluorescence emission.11 More recently, we prepared supramolecular hydrogels composed of pseudorotaxane, clay and dyes, showing a multi-color luminescent emission including white light.12 Herein we employed a series of CD-based pseudorotaxanes (PPRs, Scheme 1), which were constructed by threading amino-modified β-CDs onto a poly(propyleneglycol)bis(2-amionopropylether) (PPG-NH2) chain, as cross-linkers in conjunction with a CNS (LAPONITE® XLG) matrix to non-covalently produce supramolecular hydrogels. The water content, stability, mechanical performance and thixotropy were investigated. Significantly, these supramolecular hydrogels were found to be able to quickly and efficiently adsorb cationic dyes from aqueous solution. This finding develops a series of convenient and stable gels as cheap and efficient adsorbents in the treatment of waste water containing organic dyes.
Results and discussion
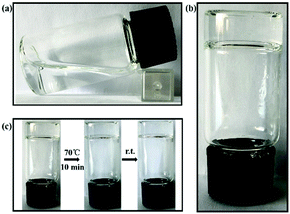
Pseudorotaxanes PPR 1–PPR 3 were prepared by threading ethylenediamine-modified β-CDs, diethylenetriamine-modified β-CDs or triethylenetetramine-modified β-CDs onto poly(propylene glycol)bis(2-aminopropylether) (PPG-NH2, Mw ≈ 2000) chains. The number of CD units on the PPG-NH2 chain could be calculated from the 1H NMR data (Fig. S1–S3, ESI†) by comparing the integral area of H1 protons of β-CD at 5.03 ppm (one β-CD unit has 7 H1 protons) with that of methyl protons of PPG-NH2 at 1.12 ppm (one PPG-NH2 chain has 103 methyl protons). The results showed that ca. 13.5, 11.3 and 9.2 β-CD units were threaded onto the main chain of PPG-NH2 in the cases of PPR 1, PPR 2 and PPR 3, respectively. Therefore, we can deduce that the average number of threaded β-CD cavities on the PPG-NH2 chain tended to decrease with the increase of the ethylenetriamine units linked to the β-CD rim. This concept will efficiently facilitate the design and synthesis of new polycationic supramolecular polypseudorotaxanes. A possible reason may be that the strengthened electrostatic repulsion among amino groups extended the distance between two β-CD cavities. Moreover, the 2D ROESY spectra (see Fig. S4–S6, ESI†) showed the clear NOE correlations between the interior protons of the β-CD cavity and methyl protons of PPG-NH2, indicating the threading of PPG-NH2 into the β-CD cavities.After mixing 4 mg of PPR 1–PPR 3 with 100 mg of CNSs in 4 mL of water, transparent hydrogels (G1–G3) could be easily obtained (Fig. 1b). Significantly, the water content of the hydrogel was calculated to be as high as 97.5%, and the content of organic compounds was as low as 0.097%. In addition, these hydrogels presented good thermal stabilities, and no phase transition could be observed even on heating the hydrogel up to 70 °C for 10 min (Fig. 1c). This property is better than those of most of the reported supramolecular hydrogels that usually underwent a gel-to-sol transition after heating.
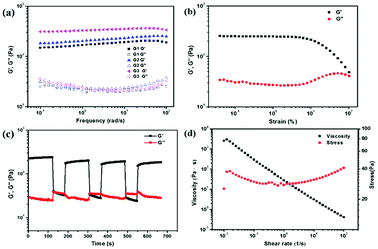
The mechanical properties of supramolecular hydrogels were investigated by the rheology measurement, where the amplitude sweep experiments were carried out to find a linear elastic-viscous area at strain (γ) = 0.01–100%. Taking G2 as an example (Fig. 2b), the supramolecular hydrogels underwent a gel-to-sol state transition at the critical strain region (γ > 100%), which showed the breakdown of the hydrogel network. The value of the storage modulus G′ and loss modulus G′′ remained essentially unchanged. G′ was higher than G′′ when strain γ varied from 0.01 to 8%, demonstrating that the supramolecular hydrogel was stable and not prone to be destroyed under this experimental condition. So the oscillating strain was fixed at 1% for the frequency tests.
The frequency sweep tests were also carried out at ω = 100–0.1 rad s−1, where the experiments were performed from high to low frequency to reduce both the test time and the impact of time on the gel properties in the testing process. As shown in Fig. 2a, the storage modulus G′ and loss modulus G′′ of G1–G3 were almost constant in the range of ω = 100–0.1 rad s−1, and the G′ values increased in the order of G1 < G2 < G3. A possible reason may be that the relatively high positive charge density of PPR 3 was more favorable to the cross-linking of neighboring nanosheets. In addition, the continuous step strain tests (Fig. 2c) showed that, when treated with a high strain (γ = 100%, ω = 6.28 rad s−1), the G′ value of the supramolecular hydrogels decreased, and the hydrogel converted to a sol state. However, when the strain was decreased (γ = 0.1%, ω = 6.28 rad s−1), both the value of G′ and G′′ recovered rapidly to the original values within 60 s, accompanied by the regeneration of the hydrogel. Significantly, this strain-responsive sol–gel transformation was reversible. This result demonstrated that the gel had the ability of self-healing. Moreover, the steady shear rheological experiments (Fig. 2d) showed that the viscosity of the supramolecular hydrogel decreased significantly with increasing shear rate when a shear force was applied, indicating that the hydrogels had shear-thinning properties. All these results demonstrated that the non-covalent interactions between the negatively charged CNSs and the positively charged PPR played a vital role in the formation of supramolecular hydrogels, and the mechanical properties of the hydrogels increased with the elongation of the amino arms modified on the CD rims.
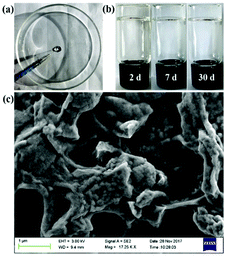
Owing to the shear-thinning property, the supramolecular hydrogels could be injected through a thin syringe, demonstrating their good injectability via the application of slight forces. This may enable their potential use as an injectable material (Fig. 3a). In addition, the supramolecular hydrogels also showed good stability (Fig. 3b), and no obvious change could be observed even after keeping the hydrogels for a month. The morphological information of the supramolecular hydrogels came from scanning electron microscopy (SEM), which showed a number of cross-linked porous networks (Fig. 3c). In the self-healing experiment, the damaged supramolecular hydrogels could gradually recover (Fig. S16, ESI†), indicating a satisfactory self-healing property of the supramolecular hydrogels.
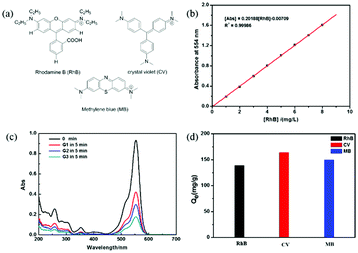
It is well documented that supramolecular hydrogels with a three-dimensional network structure have broad application prospects in tissue engineering,13 light-emitting materials14 and dye adsorption.15 Among them, the hydrogels used for dye adsorption from waste water have the advantages of low cost, simple design, easy operation and environment-friendliness. Herein, the dye adsorption abilities of G1–G3 were investigated. Zeta potential measurements gave an average zeta potential of G1–G3 of −33.71 mV (Fig. S7–S9, ESI†), indicating the possibility of G1–G3 adsorbing cationic dyes. Therefore, some cationic dyes including rhodamine B (RhB), crystal violet (CV), and methylene blue (MB) were selected as model substrates (Fig. 4a). In a typical experiment, 0.010 g xerogel was added to 5 mL of cationic dye solution (500 mg L−1). After the mixture was stirred at room temperature for 10 s, the UV-vis spectra of the dye solution at different time points were measured until the adsorption equilibrium was reached (Fig. 4). According to eqn (1) and the standard curve, the amount of adsorbed dyes at equilibrium could be calculated.
 | (1) |
Taking the case of RhB as an example, on the basis of the absorbance maximum of RhB at 554 nm, the standard curve could be plotted as Fig. 4b. Subsequently, the UV-vis absorption spectra of the RhB + G2 system at different time points were measured (Fig. 4c), demonstrating that G2 presented a fast and effective adsorption of RhB, and most of the dye was adsorbed by G2 within 5 min. Similar results were also obtained in other hydrogel/dye pairs. Towards RhB, the adsorption efficiency of G1, G2 and G3 could reach 55%, 68% and 81% within 5 min, and theadsorption capacity (Qe) was calculated as 139 mg g−1, 152 mg g−1, and 198 mg g−1 at 5 min, and 181 mg g−1, 197 mg g−1 and 211 mg g−1 at 180 min according to eqn (1), respectively (Fig. 4b). Significantly, the adsorption capacity (Qe) of G1 to RhB, CV and MB could reach 139 mg g−1, 164 mg g−1, and 150 mg g−1 at 5 min and 181 mg g−1, 199 mg g−1, and 201 mg g−1 at 180 min, respectively (Fig. 4d). The adsorption efficiency and the adsorption capacity (Qe) in 180 min for the three dyes are shown in Table S1 ESI.† Therefore, the adsorption trend of these hydrogels towards RhB was concluded as that the adsorption capacity and efficiency increased with an increased number of amino groups on the pseudorotaxanes. More interestingly, the solution of CV or MB almost became colorless after adsorption by the hydrogels (Fig. S10–S15, ESI†), and the adsorption efficiencies of the three hydrogels towards CV and MB were all >90% at adsorption equilibrium. We deduced that the three-dimensional cross-linked porous network of the hydrogels as well as the electrostatic interactions between the hydrogel and dye may contribute to the quick and efficient removal of cationic dyes in aqueous solution.
Experimental
All chemicals were commercially available unless noted otherwise. β-CD was recrystallized twice from water and dried in vacuo at 80 °C for 24 h prior to use. Ethylenediamine, diethylenetriamine, triethylenetetramine and poly(propylene glycol) bis(2-aminopropylether) (PPG-NH2, Mw ≈ 2000) were purchased from Aladdin. All reagents were commercially available and used without further purification.161H NMR spectra and ROESY spectra were recorded in D2O using a Bruker AV400 spectrometer at 25 °C. UV-vis spectra were measured in quartz cells (light path, 10 mm) using a Shimadzu UV-2401PC spectrophotometer equipped with a Thermo HAAKE-SC100 temperature controller. The rheological characterization of the hydrogels was carried out using an AR-G2 rheometer (TA instruments, Etten-Leur, The Netherlands) equipped with a 1° steel cone geometry of 20 mm diameter and a solvent trap. The gap was set at 1.0 mm. The zeta potential was measured using a ZetaPALS + BI-90 instrument (Brookhaven Co. USA).
Preparation of PPRs
PPR 1 was prepared in 23% yield according to a reported method.12 PPR 2 and PPR 3 were prepared as follows: PPG-NH2 was added to a saturated aqueous solution of diethylenetriamine-modified β-CD (or triethylenetetraamine-modified β-CD), and the mixture was stirred for 3 d (or 7 d) at room temperature to produce PPR 2 (or PPR 3). During this process, the solution became turbid from being clear. And PPR 2 (or PPR 3) was obtained as a white precipitate in 9% (or 3%) yield, which was collected by filtration, washed with water and dried in vacuo.Preparation of the hydrogels
100 mg of CNSs was suspended in 4 mL of water and stirred at 25 °C for 10 min. Then PPR (4 mg) was added to the stirred solution, and the mixture was kept at room temperature for 2 d to get a supramolecular hydrogel.Conclusions
In summary, a series of supramolecular hydrogels were successfully constructed through a simple but facile method from polyamine mono-substituted cyclodextrin-based polypseudorotaxane and CNSs. These hydrogels possessed high water content, high transparency, and good thermal stability and mechanical properties. More importantly, the mechanical strength of the supramolecular hydrogels could be modulated by adjusting the number of amino groups on the cyclodextrins. Owing to the cooperative contribution of the three-dimensional cross-linked network structure of the hydrogels and the electrostatic interaction between hydrogels and dyes, the hydrogels presented good adsorption properties for a wide range of dyes, which would enable the potential application of the hydrogels in the fields of organic dye removal and waste water treatment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank NSFC (21432004, 21672113, 21772099 and 21861132001) for financial support.Notes and references
-
(a) G. Yu, X. Yan, C. Han and F. Huang, Chem. Soc. Rev., 2013, 42, 6697–6722 RSC
; (b) Q. Zhao, Y. Chen, S.-H. Li and Y. Liu, Chem. Commun., 2018, 54, 200–203 RSC
; (c) L. Tang, W. Liu and G. Liu, Adv. Mater., 2010, 22, 2652–2656 CrossRef CAS PubMed
; (d) Z. Qi and C. A. Schalley, Acc. Chem. Res., 2014, 47, 2222–2233 CrossRef CAS PubMed
; (e) X. Dai, Y. Zhang, L. Gao, T. Bai, W. Wang, Y. Cui and W. Liu, Adv. Mater., 2015, 27, 3566–3571 CrossRef CAS PubMed
; (f) P. Cordier, F. Tournilhac, C. Soulié-Ziakovic and L. Leibler, Nature, 2008, 451, 977–980 CrossRef CAS PubMed
.
-
(a) E. A. Appel, J. del Barrio, X. J. Loh and O. A. Scherman, Chem. Soc. Rev., 2012, 41, 6195–6214 RSC
; (b) Z. Li, Y.-M. Zhang, H.-Y. Wang, H. Li and Y. Liu, Macromolecules, 2017, 50, 1141–1146 CrossRef CAS
; (c) Y. Takashima, Y. Sawa, K. Iwaso, M. Nakahata, H. Yamaguchi and A. Harada, Macromolecules, 2017, 50, 3254–3261 CrossRef CAS
; (d) F. van de Manakker, L. M. J. Kroon-Batenburg, T. Vermonden, C. F. van Nostrum and W. E. Hennink, Soft Matter, 2010, 6, 187–194 RSC
.
-
(a) A. Harada, J. Li and M. Kamachi, Nature, 1992, 356, 325–327 CrossRef CAS
; (b) J. Li, A. Harada and M. Kamachi, Polym. J., 1994, 26, 1019–1026 CrossRef CAS
; (c) A. Harada, Acc. Chem. Res., 2001, 34, 456–464 CrossRef CAS
; (d) Y. Chen and Y. Liu, Chem. Soc. Rev., 2010, 39, 495–505 RSC
; (e) Q. Zhao, S.-H. Li and Y. Liu, Prog. Chem., 2018, 30, 673–683 Search PubMed
.
- S. A. Nepogodiev and J. F. Stoddart, Chem. Rev., 1998, 98, 1959–1976 CrossRef CAS PubMed
.
-
(a) A. Harada, J. Li, M. Kamachi, Y. Kitagawa and Y. Katsube, Carbohydr. Res., 1997, 305, 127–129 CrossRef CAS
; (b) A. Harada and M. Kamachi, J. Chem. Soc., Chem. Commun., 1990, 1322–1323 RSC
; (c) A. Harada, J. Li and M. Kamachi, Chem. Lett., 1993, 237–240 CrossRef CAS
.
- M. Nakahata, S. Mori, Y. Takashima, H. Yamaguchi and A. Harada, Chem, 2016, 1, 766–775 CAS
.
- Q. Zhao, Y. Chen and Y. Liu, Chin. Chem. Lett., 2018, 29, 84–86 CrossRef CAS
.
- K. Kato, K. Inoue, M. Kidowaki and K. Ito, Macromolecules, 2009, 42, 7129–7136 CrossRef CAS
.
- J. Labanda, J. Sabaté and J. Llorens, Colloids Surf., A, 2007, 301, 8–15 CrossRef CAS
.
- Q. Wang, J. L. Mynar, M. Yoshida, E. Lee, M. Lee, K. Okuro, K. Kinbara and T. Aida, Nature, 2010, 463, 339–343 CrossRef CAS PubMed
.
- G. Liu, Y.-M. Zhang, X. Xu, L. Zhang and Y. Liu, Adv. Opt. Mater., 2017, 5, 1700149 CrossRef
.
- Y. Zhang, Y. Cheng, J.-J. Li, L. Liang and Y. Liu, Acta Chim. Sin., 2018, 76, 622–626 CrossRef
.
- H. Wang and S. C. Heilshorn, Adv. Mater., 2015, 27, 3717–3736 CrossRef CAS PubMed
.
- K. V. Rao, K. K. R. Datta, M. Eswaramoorthy and S. J. George, Adv. Mater., 2013, 25, 1713–1718 CrossRef CAS PubMed
.
-
(a) G. M. Peters, L. P. Skala and J. T. Davis, J. Am. Chem. Soc., 2016, 138, 134–139 CrossRef CAS PubMed
; (b) T. N. Plank, L. P. Skala and J. T. Davis, Chem. Commun., 2017, 53, 6235–6238 RSC
; (c) H. Zhang, J.-R. Wu, X. Wang, X.-S. Li, M.-X. Wu, F. Liang and Y.-W. Yang, Dyes Pigm., 2019, 162, 512–516 CrossRef CAS
; (d) T. Zhou, H. Yu, M. Liu and Y.-W. Yang, Chin. J. Chem., 2015, 33, 125–130 CrossRef CAS
.
- Y. Liu, C.-C. You and B. Li, Chem. – Eur. J., 2001, 7, 1281–1288 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8sm02203h |
| This journal is © The Royal Society of Chemistry 2019 |