A novel 3-dimensional graphene-based membrane with superior water flux and electrocatalytic properties for organic pollutant degradation
Wenyi
Wang
 *,
Chengcheng
Xie
,
Liuyong
Zhu
,
Bojin
Shan
,
Caini
Liu
and
Fangyan
Cui
*,
Chengcheng
Xie
,
Liuyong
Zhu
,
Bojin
Shan
,
Caini
Liu
and
Fangyan
Cui
State Key Laboratory of Separation Membranes and Membrane Processes, School of Material Science and Engineering, Tianjin Polytechnic University, Tianjin 300387, China. E-mail: wenyi-wang@hotmail.com; Fax: +86-22-83955055; Tel: +86-22-8395-5813
First published on 22nd November 2018
Abstract
Graphene-based separation membranes exhibit great potential for the application of wastewater purification, but it is difficult to achieve a balance between water flux and rejection. To overcome the “trade-off” effect between permeability and selectivity, a novel 3-dimensional graphene-based membrane with superior water flux was fabricated using a simple, viable and green method. In addition, electrocatalytic technology is coupled to the membrane separation process. Comprehensive characterization indicates that the TiO2 nanoparticles were successfully mounted on the moderately reduced graphene oxide (rGO) surface. By introducing oxygen-functionalized carbon nanotubes (oCNTs), the rGO-TiO2/oCNT membrane has more pronounced 3-dimensional water channels. More importantly, the rGO-TiO2/oCNT membrane acts as both a separation membrane and an anode to couple the membrane process to the electrocatalytic technique. The small-molecule contaminants that are difficult to remove by physical exclusion would be degraded when the matter passes through the channels with the electrocatalyst. The TiO2 nanoparticles act as both a nano-wedge to expand the water channels and an electrocatalyst to promote the degradation of the contaminants. The rGO-TiO2/oCNT membranes showed a removal rate up to 95% at a high liquid hourly space velocity of 88 h−1 under the applied electrocatalysis conditions, which is two times more than that obtained without the electrocatalyst. The rGO-TiO2/oCNT membrane could not only achieve a superior water flux (the maximum was 44.14 L m−2 h−1 bar−1) but also maintain excellent dye removal performance (>90%) via membrane process coupling electrocatalysis. The optimum component ratio of the rGO-TiO2/oCNT membrane was investigated, and it was found that 30% oCNT content accounts for a stable catalytic performance. The graphene-based membrane coupled electrocatalysis in this study would provide a new approach to break the bottleneck of the trade-off effect and inspire the design of a functional membrane.
1 Introduction
Membrane separation technology1 is highly sought after due to the grand challenge of water depletion and environmental issues. Since the advent of graphene in 2004, it has attached worldwide attention.2 Numerous studies about the application of graphene in separation science have sprung up in the past few years,3,4 because of the outstanding barrier properties of its dense honeycomb-like crystal lattice structure.5–8 Unfortunately, the “trade-off” effect restrains the performance of membranes1 and flawless graphene faces great challenges in preparation and dispersion. To achieve high-performance separation, novel membrane structures are required. Graphene oxide (GO), a graphene derivative, has been applied in various fields due to its good dispersibility and easy modification of oxygen functional groups.9 GO has become a special candidate that can self-assemble into membranes with a lamination structure, which possess high flexibility and mechanical strength even for those with submicron thickness.10,11 The gaps between graphene layers or the defects on the GO surface can construct nano-scale channels to efficiently sieve salt ions and other contaminants.12 Although GO-based membranes have excellent size exclusion performance, both the narrow water transport channel and the hydrophilic oxygen functional groups on the surface have a considerable impediment to the transport of water molecules rather than providing ultra-fast molecular transport like carbon wall of graphene,10,13 and eventually lead to a fairly disappointing water flux.In order to improve the water flux of GO-based membranes, reducing graphene oxide (rGO) or introducing nanoparticles onto the GO sheets seems to be a good idea. rGO was usually obtained using a strong reductant such as hydrazine,14 pyrolysis,15 alkali metal or its derivatives,16 ascorbic acid or other acids,17 which makes the membrane preparation process difficult because of poor rGO dispersity in solution. Although the introduced nanoparticles can increase the interlayer space between graphene layers,18,19 the complex process is difficult to control by using in situ growth and the improvement of flux or selectivity is still unsatisfactory. There are many strategies to improve the water flux of graphene-based membranes; unfortunately it is hard to achieve both a high flux and excellent rejection performance at the same time, especially for removing contaminants with a small molecular radius from wastewater. How to make a membrane with both outstanding flux and ability to effectively remove contaminants remains a major challenge for the application of graphene-based materials in the field of membrane separation.
In our work, an appropriate interlayer space of GO was designed to achieve ultra-high water flux and maintain a high removal rate for small molecules by electrocatalysis instead of physical exclusion. For this purpose, TiO2 nanoparticles and oxygen-functionalized carbon nanotubes (oCNTs) which have different low dimensions were chosen to construct stable water transport channels with 2-dimensional graphene. TiO2 can be loaded onto a GO surface and the GO can be reduced moderately by a one-step hydrothermal method.20–22 In addition, oCNTs can provide wider water corridors between the graphene layers just like a stable water channel “spacer”.23 Following the design presented above, a rGO-TiO2/oCNT membrane was fabricated using two simple, viable and green steps. Both TiO2 and oCNTs can expand the interlayer space between graphene layers. Theoretically, the expanded interlayer space and the reduced hydrophilic groups would lead to the decreased transport resistance of water so that the water flux of the membrane can be improved.
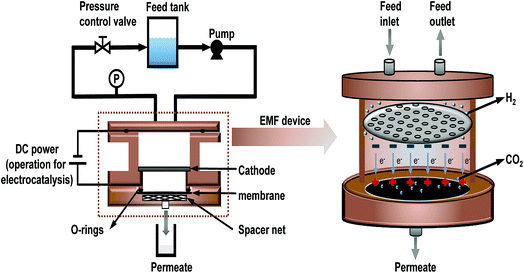
Importantly, relevant studies24–26 have indicated that TiO2 as a catalyst shows excellent photoelectrocatalytic performance due to its special band structure and the synergistic effect with graphene. Therefore, TiO2 was chosen to act as both an intercalation material and a catalyst. To investigate the electrocatalytic properties of rGO-TiO2/oCNT membranes, an electrocatalytic membrane filter (EMF) was designed, as shown in Fig. 1, in which the membrane acted as both a filter and an anode. Combined with electrocatalytic technology, the graphene-based membrane was expected to simultaneously achieve a superior water flux and removal of small-molecule contaminants (such as MO dyes). The method of membrane separation coupling electrocatalysis is promising to break the limit of the “trade-off” effect in the field of membranes. GO, oCNT, rGO-TiO2 and their derivative membranes were characterized by transmission electron microscopy (TEM), Fourier Transform Infrared spectroscopy (FT-IR), Raman spectroscopy, X-ray diffraction (XRD) and scanning electron microscopy (SEM). The rGO-TiO2/oCNT membranes were characterized by an additional electrochemical test. Additionally, the dye removal mechanism of 3-dimensional membranes and the synergistic electrocatalytic effect with DC voltage were discussed.
2 Materials and methods
2.1 Materials
Graphite powder, anhydrous ethanol and methyl orange (MO) were bought from Aladdin Reagent Company. Butyl titanate, H2SO4, HCl and acetic acid glacial were supplied by Tianjin Kermel Chemical Reagent Co., Ltd, China. KMnO4, NaNO3, HNO3 and H2O2 were obtained from Tianjin Fengchuan Chemical Reagents Technology Co., Ltd, China. Na2SO4 and NaCl were purchased from Tianjin Beilian Fine Chemicals Co., Ltd, China. The cellulose acetate microporous membrane (the nominal pore size: 0.22 μm) was provided by Tianjin Jinteng Experimental Equipment Co., Ltd, China. Multi-walled carbon nanotubes (MWCNTs, length 10–30 μm and outside diameter 10–20 nm) were purchased from Times Nano Ltd., Chengdu. The water used in the experiment was deionized (DI) water.2.2 Preparation of TiO2, GO and oCNTs
GO was produced via Hummers' method using graphite powder as the raw material.27 Briefly, 1 g of graphite powder and 0.8 g of NaNO3 were added to 100 ml of concentrated H2SO4 under strong stirring at below 15 °C, and then 4.5 g of KMnO4 was slowly added to the mixed solution within 1 h and further stirred for 0.5 h. Subsequently, the solution was placed in an oil bath and stirred at 60 °C for 10 h, after adding 100 ml of DI water to the solution, and then the temperature was immediately adjusted to 90 °C under stirring for 1 h to obtain a suspension. After the suspension cooled down to room temperature naturally, 5 ml of concentrated HCl and a large amount of DI water were added to the suspension to remove the residual salts and acids, and then the suspension was concentrated by centrifugation and the process was repeated three times. The suspension was diluted with DI water and centrifuged until the pH of the solution was closed to neutral. The neutral suspension was sonicated for more than 5 hours to obtain a GO dispersion and stored with a density of 2.58 g L−1 for use.The sol–gel method was utilized to prepare titanium dioxide (TiO2). In brief, 0.1 mol of butyl titanate was slowly added to a solution that contains 100 ml of anhydrous ethanol and 1 ml of acetic acid glacial under vigorous stirring at room temperature for 1 h to get a homogenous solution. Then another mixed solution containing 20 ml of anhydrous ethanol, 6 ml of DI water and 1 ml of HNO3 (mole ratio, 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added, and stirred for 2 h to form a sol. The sol solution was allowed to rest in air under ambient conditions for 24 h to obtain a gel, before which it was dried in an oven at 60 °C and ground to powder. Finally, the powder was calcined at 450 °C for 2 h to achieve TiO2.28
1) was added, and stirred for 2 h to form a sol. The sol solution was allowed to rest in air under ambient conditions for 24 h to obtain a gel, before which it was dried in an oven at 60 °C and ground to powder. Finally, the powder was calcined at 450 °C for 2 h to achieve TiO2.28
Pristine MWCNTs were usually grafted with hydrophilic functional groups by chemical modification to improve their dispersibility.29 In this process, 1 g of MWCNTs was added to 200 ml of the mixed solution of HNO3/H2SO4 in a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (mole ratio, 2
3 (mole ratio, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69). The solution mixture was sonicated at room temperature for 0.5 h and subsequently heated to reflux under magnetic stirring at 75 °C for 5 h. After being cooled down, oCNTs were filtered and rinsed several times using DI water until neutral. The obtained oCNTs were then dried at 45 °C under vacuum for use.
69). The solution mixture was sonicated at room temperature for 0.5 h and subsequently heated to reflux under magnetic stirring at 75 °C for 5 h. After being cooled down, oCNTs were filtered and rinsed several times using DI water until neutral. The obtained oCNTs were then dried at 45 °C under vacuum for use.
2.3 Preparation of rGO-TiO2 composites and rGO-TiO2/oCNT membranes
The rGO-TiO2 composites were fabricated via a one-step hydrothermal method under high temperature and high pressure.21 In brief, different masses of TiO2 nanoparticles (Table 1) and 20 mg of GO were added to 60 ml of a mixed solution of water and anhydrous ethanol in a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After an ultrasonic treatment at 25 °C for 2 h and stirring for 2 h, the solution was immediately transferred into a 100 ml Teflon-sealed autoclave and heated at 120 °C for 24 h to prepare the rGO-TiO2 materials.
1. After an ultrasonic treatment at 25 °C for 2 h and stirring for 2 h, the solution was immediately transferred into a 100 ml Teflon-sealed autoclave and heated at 120 °C for 24 h to prepare the rGO-TiO2 materials.
| Samples | Components | W TiO2/WGO | W oCNT/WGO | Loading mass (g m−2) |
|---|---|---|---|---|
| a rGO represents moderately reduced GO; oCNTs represent oxygen-functionalized carbon nanotubes. WTiO2/WGO and WoCNT/WGO mean the mass ratio of TiO2 and oCNTs to GO, respectively. | ||||
| GO | GO | 0 | 0 | 4.13 |
| rGO | rGO | 0 | 0 | 4.13 |
| rGT-2 | rGO-TiO2 | 25% | 0 | 4.13 |
| rGT-5 | rGO-TiO2 | 50% | 0 | 4.13 |
| rGT-10 | rGO-TiO2 | 100% | 0 | 4.13 |
| rGTC-1 | rGO-TiO2/oCNT | 100% | 10% | 4.13 |
| rGTC-3 | rGO-TiO2/oCNT | 100% | 30% | 4.13 |
| rGTC-5 | rGO-TiO2/oCNT | 100% | 50% | 4.13 |
| rGTC-7 | rGO-TiO2/oCNT | 100% | 70% | 4.13 |
| rGTC-10 | rGO-TiO2/oCNT | 100% | 100% | 4.13 |
The rGO-TiO2/oCNT membranes were fabricated from rGO-TiO2 and oCNTs via a vacuum-assisted method. A rGO-TiO2 solution and oCNT dispersion were mixed and diluted with deionized water until the concentration of rGO was 0.04 mg ml−1. The mixed solution was sonicated for 2 h and stirred for 0.5 h, and then placed in a vacuum-assisted filtration device to prepare rGO-TiO2/oCNT membranes, as shown in Scheme 1 (the cellulose acetate membrane was used as the support membrane). The prepared membranes were kept overnight at room temperature. To compare the characteristics of the rGO-TiO2/oCNT membranes, a rGO-TiO2 membrane and pure rGO membrane were fabricated by the same process. The rGO-TiO2 and rGO-TiO2/oCNT membranes were prepared respectively, as shown in Table 1. The corresponding samples were denoted as rGT and rGTC.
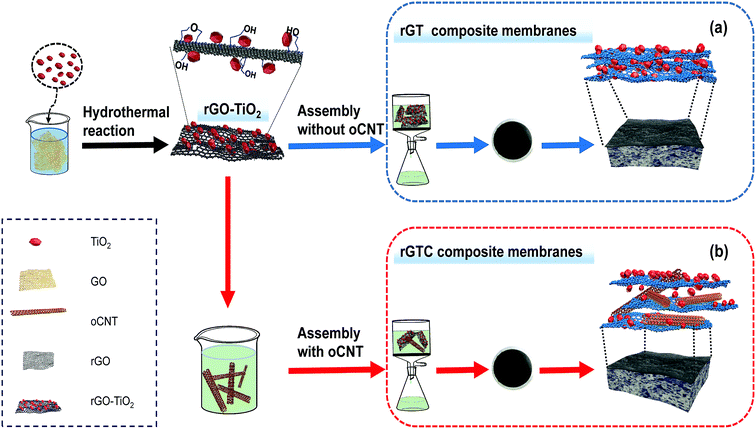 | ||
| Scheme 1 Illustration of the preparation of rGT and rGTC membranes via vacuum-assisted filtration and their structural diagram: (a) rGT and (b) rGTC. | ||
2.4 Characterization
The atomic force microscope (AFM) images of GO were obtained by using an Agilent-S5500 scanning probe optical microscope (AGILENT, USA) with the tapping mode. The morphology of each component was obtained by using a Transmission Electron Microscope (TEM) (Hitachi H7650, Japan). The Raman spectrum was obtained at a laser wavelength of 532 nm with an XploRA PLUS (Horiba, Japan). Fourier Transform Infrared spectroscopy (FTIR) was performed by using a Thermo Nicolet iS50 spectroscope (Thermo Nicolet Corporation, USA). The surface and cross-sectional topographies of the membranes were observed with a Scanning Electron Microscope (SEM) (Hitachi S4800, Japan). Ultraviolet-visible spectroscopy (UV-vis) (Shimadzu UV-2401PC, Japan) was performed to detect the contaminant concentration.In order to confirm whether the TiO2 nanoparticles were still carried on the surface of graphene after the sheets were fabricated into membrane, the elements of the membranes were tested by energy dispersive X-ray spectroscopy (EDX). The X-ray diffraction (XRD) (Bruker D8 DISCOVER, Germany) patterns were obtained with Cu Kα radiation (λ = 0.15406 nm). Electrochemical impedance spectroscopy (EIS) was performed with an electrochemical workstation (CS350H, China). EIS measurements were conducted in a three-electrode system, consisting of saturated Ag/AgCl as the reference electrode, graphite as the counter electrode, the membrane as the working electrode (2 cm−2) and 10 g L−1 NaCl as the electrolyte. And it was performed at open-circuit potential by applying an AC voltage of 10 mV within the frequency range from 106 to 100 Hz. The graphene-based membrane was infiltrated using DI water before EIS testing.
2.5 Permeation and selectivity tests of the membranes
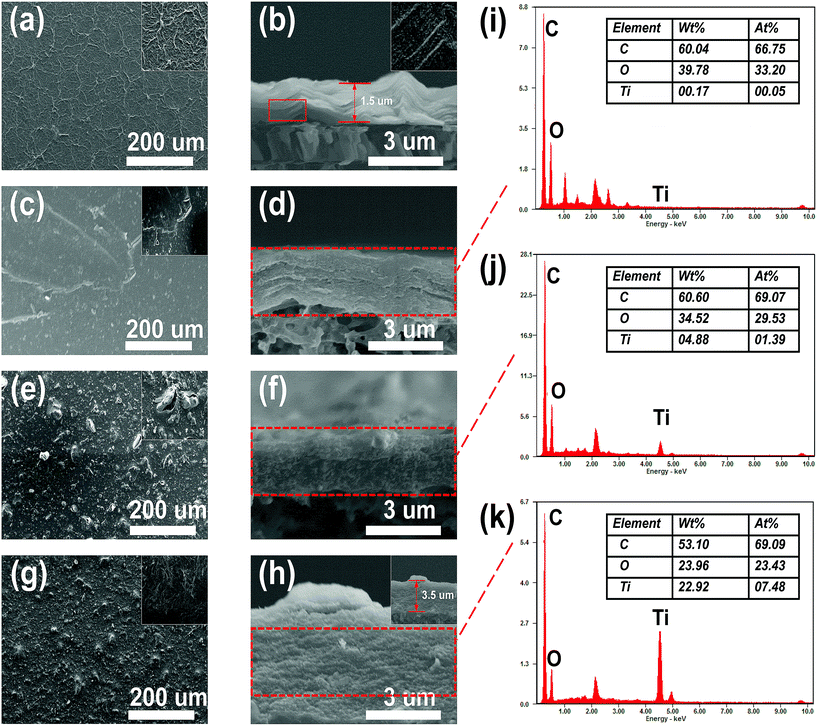
A modified dead-end filtration system with an effective test area of 9.6 cm2 was designed to investigate the filtration performance of the composite membranes, as depicted in Fig. 1. The permeation experiment was performed using pure water to evaluate the flux. The membrane was pre-pressed at 0.1 MPa for 1 h to obtain a stable water flux. Then the volume of permeation water was recorded at intervals of 10 min under 0.2 MPa, and the average was calculated.MO dyes as small-molecule organic contaminants (molecular weight: 327.33) face the challenge of being removed by the exclusion mechanism. Thus, the selectivity of the membrane was investigated using 20 ppm MO solution as the feed wastewater. After the permeation experiment, the feed solution was switched to MO solution and the test was carried at a pressure of 0.1 MPa to collect permeate solution. The concentration of MO in the feed solution and permeate solution was detected by using a UV-2401PC (ShimadzuUV-2450, Japan) so as to calculate the rejection rate for MO. The pure water flux and rejection rate could be calculated by using eqn (1) and (2) respectively,30 as follows:
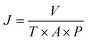 | (1) |
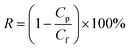 | (2) |
2.6 The electrocatalytic and separation coupling test
The experiment was performed in an EMF device based on the dead-end filtration system (as shown in Fig. 1) which coupled the technology of filtration and electrocatalysis to improve the removal rate of dye. The EMF was designed with two wire interfaces fixed with a rubber ring capable of connecting to the positive and negative poles of the power supply, respectively. The graphene-based membrane and a stainless steel plate with regular pores (with 2 mm diameter) were used as the anode and cathode, respectively. The distance between the anode and cathode is 20 mm. Similarly, a 20 ppm MO solution was used as the feed solution and 0.05 wt% sodium sulfate as the electrolyte. In this study, the graphene-based membranes with high water flux performance confirmed by the permeation test would be placed in an EMF cell, and treated with a constant voltage (electric-field intensity of 1.5 V cm−1) for 3 h. Before the coupling test, the water flux of the membrane was tested to make sure that the membrane was of good quality. The parameter termed liquid hourly space velocity (LHSV)31 was used in the process to standardize electrocatalytic residence time in the membranes. LHSV (h−1) was defined as the ratio of the hourly volume of the permeate (ml h−1) to the catalyst volume. Herein, an effective LHSV of 88 h−1 was applied in the coupling test of the graphene-based membrane (volume of roughly 9.6 × 0.0035 cm3). The dye removal rate was also calculated by using eqn (2).3 Results and discussion
3.1 Characterization of GO, TiO2, and oCNTs
The typical TEM image of GO and TiO2 nanoparticles and AFM images of GO are shown in Fig. 2. As shown in Fig. 2a and b, the oxygen-containing functional groups result in typical wrinkles on the GO surface, which are necessary for gaining thermodynamic stability of the graphene structure.32,33 The microsized diameter of the prepared GO is favorable for loading TiO2 particles. The prepared TiO2 has a size of roughly 20 nm (Fig. 2c). The vertical height of the GO plane was about 0.82 nm measured from the AFM image in Fig. 2d and the interlayer distance measured by AFM is consistent with the result of XRD (Fig. 5). It is recognized that the thickness of monolayer GO is larger than that of graphene (∼0.34 nm), because the oxygen-containing groups (e.g., epoxy, hydroxyl, carboxyl, phenol etc.) on the surface of GO has increased the interlayer spacing.34 The typical thickness of the single-layer GO is about 0.8 nm which is consistent with the interlayer spacing shown in Fig. 2d.35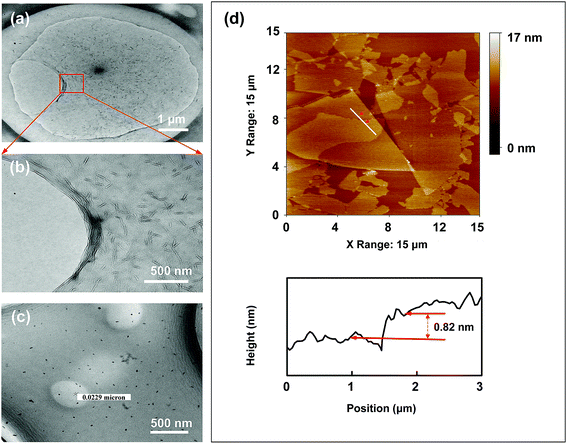 | ||
| Fig. 2 Typical TEM image of GO (a and b) and TiO2 nanoparticles (c); and AFM images of the prepared GO (d). | ||
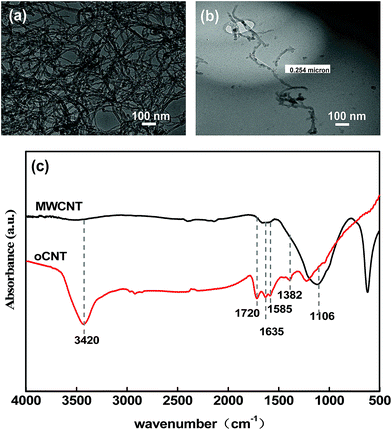
The TEM images of pristine MWCNTs and oCNTs are shown in Fig. 3 respectively. The pristine MWCNTs with a larger aspect ratio were seriously entangled. The oCNTs treated with an acid have better dispersity than MWCNTs because of a smaller diameter and length. The infrared spectrum of oCNTs was also inspected and is displayed in Fig. 3c. The adsorption peak at 1106 cm−1 in the MWCNT curve is assigned to C–C bonds.36 Compared with the original MWCNTs, the strong absorption peaks of the hydroxyl group (–OH) and carboxylic group (HO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) are found at 3400 cm−1 and 1720 cm−1, respectively, and those of the carbonyl group (C
O) are found at 3400 cm−1 and 1720 cm−1, respectively, and those of the carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) from the quinine or ring structure are also observed at 1635 cm−1.8 The peaks at 1585 cm−1 manifested in both MWCNT and oCNT curves are assigned to C
O) from the quinine or ring structure are also observed at 1635 cm−1.8 The peaks at 1585 cm−1 manifested in both MWCNT and oCNT curves are assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching.29,37 The peaks at 1382 cm−1 and 1226 cm−1 in the oCNT curve are attributed to C–O and C–O–H bonds, respectively. These absorption peaks correspond to various hydrophilic oxygen functional groups, indicating that the pristine MWCNTs have been modified to oCNTs by acid treatment, and the strong electrostatic repulsion between the oxygen-containing functional groups results in excellent dispersion stability of the oCNTs, providing important co-assembly conditions for membrane formation.
C stretching.29,37 The peaks at 1382 cm−1 and 1226 cm−1 in the oCNT curve are attributed to C–O and C–O–H bonds, respectively. These absorption peaks correspond to various hydrophilic oxygen functional groups, indicating that the pristine MWCNTs have been modified to oCNTs by acid treatment, and the strong electrostatic repulsion between the oxygen-containing functional groups results in excellent dispersion stability of the oCNTs, providing important co-assembly conditions for membrane formation.
3.2 Characterization of the prepared membranes
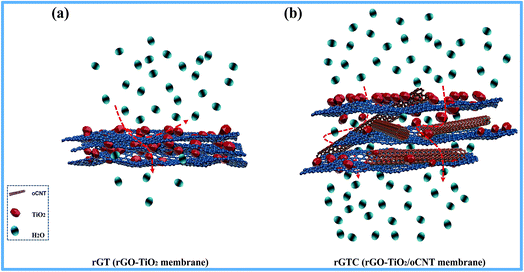
The structural and spectroscopic information of the rGO-TiO2 composite is displayed in Fig. 4. In Fig. 4a and b, the typical morphology suggests that TiO2 nanoparticles are successfully loaded on the surface of graphene sheets in line with the speculated structure of rGT in Scheme 1. It was noticed that the rGO retains the 2-dimensional plane structure instead of a curl-like structure, which retains some longer wrinkles and nanoporous defects after the hydrothermal reaction. The TiO2 nanoparticles are more likely to be adsorbed or bonded (Ti–O–C) in crinkled or marginal regions of rGO because of more oxygen-containing functional groups in there.20,21
 | ||
| Fig. 4 TEM image of rGT-10 (a and b). Raman spectra of GO, rGO and rGT-10 (c). FTIR spectra of GO, rGO, rGT-10, rGTC-3 and TiO2 (d). | ||
Raman spectroscopy provides additional insight into the structure of rGO-TiO2. As shown in Fig. 4c, the Raman spectra of GO, pure rGO and rGT-10 composites all manifest the characteristic peaks of D and G bands, corresponding to the degree of disorder/defects and order structure of graphene, respectively. The change of graphene loaded TiO2 was indirectly deduced by comparing the intensity ratios of ID/IG, which was related to the average size of graphene sp2 domains and reflected the extent of graphene defects.40 The order from large to small ID/IG ratios is rGO (1.21) > rGT-10 (1.10) > GO (1.04). The rGO had a higher ID/IG ratio than GO indicating that graphene sheets had more exposed defects after hydrothermal treatment, which accorded with the TEM results and other reports.16,41 Combined with infrared analysis (Fig. 4d) as discussed later, it is inferred that the defects of the GO surface cannot be repaired and even more defects are exposed through deoxygenation by hydrothermal treatment. Meanwhile, it can be inferred that the smaller and more sp2 domains on rGO nanosheets relatively reduce the intensity of the G-band corresponding to the first-order scattering of the E2g mode observed in the G-band, resulting in an increase in the ratio of ID/IG.42 Nevertheless, the ID/IG value of rGT-10 was higher than that of GO but less than rGO, inferring that rGT-10 was moderately reduced and exposed more structural disorders than GO after anchoring TiO2. This can be attributed to the interaction of rGO nanosheets with TiO2, such as the bond of Ti–O–C or the adsorption of TiO2, thereby masking the defects of graphene.22 Furthermore, the D and G bands of rGT-10, at 1350 cm−1 and 1591 cm−1 respectively, have an obvious Raman shift compared with that of GO (1359 cm−1 and 1588 cm−1). A similar Raman shift has been reported for the rGO-TiO2 composite prepared by in situ grafting,43–45 indicating the interactions between TiO2 and rGO.
Fig. 4d shows the FTIR spectra of GO, rGO, rGT-10, and rGTC membranes and TiO2 nanoparticles. In the spectra of the GO membrane, the characteristic peaks of oxygen-containing groups were found at 3400 cm−1, 1734 cm−1, 1370 cm−1, 1269 cm−1 and 1050 cm−1, respectively. Two peaks at 2920 cm−1 and 2850 cm−1 are ascribed to the –CH2– stretching vibration of the graphene skeleton, and the peak at 1629 cm−1 is caused by C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching and –OH bending.46,47 The peak of pure TiO2 at roughly 3440 cm−1 and 1629 cm−1 can be attributed to the vibration of Ti–OH caused by the absorption of moisture in the air. As for the infrared spectra of the rGT-10 membranes, the intensity of all absorption peaks corresponding to oxygen-containing groups became blunt and significantly decreased, and even the peak at 3400 cm−1 (–OH) has nearly disappeared. It is suggested that GO can be appropriately reduced via a hydrothermal method. Besides, the rGT-10 curve exhibits a broad peak at 400–700 cm−1, which has the same trend as that of pure TiO2 nanoparticles, corresponding to the stretching vibration of Ti–O–Ti bonds.48,49 It is noteworthy that this broad peak of rGT-10 shifts toward a low wavenumber and is sharper than that of pure TiO2 nanoparticles, for which a weak peak at 804 cm−1 can be attributed to Ti–O–C.21,22,50,51 In addition, FTIR spectroscopy reveals that the intensity of characteristic peaks of rGO slightly decreases compared with that of GO. However, the peak intensities at 3400 cm−1 (–OH) of rGT and rGTC-3 are much smaller than those of GO and rGO, and at the same time, the peaks at 1629 cm−1 shift to a longer wavelength position, indicating the interaction between TiO2 and the graphene layers. According to the Raman analysis, the reduction degree of rGO was decreased after loading TiO2. It can be further speculated that part of the –OH bonds would provide anchoring sites to TiO2 when GO was reduced to rGO, so that the adsorption peaks at 3400 cm−1 have drastically declined. Apart from the formation of Ti–O–C as mentioned before, there may be hydrogen bonding force caused by –OH between TiO2 and GO.20
C stretching and –OH bending.46,47 The peak of pure TiO2 at roughly 3440 cm−1 and 1629 cm−1 can be attributed to the vibration of Ti–OH caused by the absorption of moisture in the air. As for the infrared spectra of the rGT-10 membranes, the intensity of all absorption peaks corresponding to oxygen-containing groups became blunt and significantly decreased, and even the peak at 3400 cm−1 (–OH) has nearly disappeared. It is suggested that GO can be appropriately reduced via a hydrothermal method. Besides, the rGT-10 curve exhibits a broad peak at 400–700 cm−1, which has the same trend as that of pure TiO2 nanoparticles, corresponding to the stretching vibration of Ti–O–Ti bonds.48,49 It is noteworthy that this broad peak of rGT-10 shifts toward a low wavenumber and is sharper than that of pure TiO2 nanoparticles, for which a weak peak at 804 cm−1 can be attributed to Ti–O–C.21,22,50,51 In addition, FTIR spectroscopy reveals that the intensity of characteristic peaks of rGO slightly decreases compared with that of GO. However, the peak intensities at 3400 cm−1 (–OH) of rGT and rGTC-3 are much smaller than those of GO and rGO, and at the same time, the peaks at 1629 cm−1 shift to a longer wavelength position, indicating the interaction between TiO2 and the graphene layers. According to the Raman analysis, the reduction degree of rGO was decreased after loading TiO2. It can be further speculated that part of the –OH bonds would provide anchoring sites to TiO2 when GO was reduced to rGO, so that the adsorption peaks at 3400 cm−1 have drastically declined. Apart from the formation of Ti–O–C as mentioned before, there may be hydrogen bonding force caused by –OH between TiO2 and GO.20
As mentioned above, GO could be moderately reduced, and TiO2 nanoparticles can be mounted on the surface of rGO due to the formation of Ti–O–C bonds and the adsorption of hydrogen bonds. The peaks of oxygen-containing groups in the rGO and rGT-10 spectra indicate that a certain number of hydrophilic groups were still left on the graphene sheets to maintain the good dispersion, which was crucial to achieving a laminated layer structure.52 Additionally, the infrared spectrum of the rGTC-3 membrane shows a similar trend to that of rGT-10, but the characteristic peak of TiO2 at 400–700 cm−1 is smaller than that of rGT-10, for which the intensity is reduced and width is increased. Theoretically the oxygen-containing functional groups on the surface of the oCNTs can form a stable bond with the residual functional groups on the surface of rGO.37
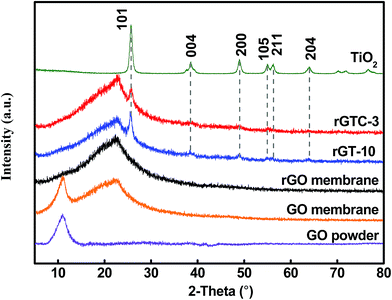
Simultaneously, the XRD of TiO2, pure GO, rGO, rGT-10 and rGTC-3 membranes is displayed in Fig. 5. The peaks of pure TiO2 correspond to different crystallographic planes of the anatase phase structure (JCPDS no. 21-1272: space group), suggesting that the TiO2 calcined at 450 °C formed the anatase single phase structure. The pure GO powder XRD pattern is also collected and only displays a typical well-defined (001) peak (2θ = 11°) that derives from the well-ordered layered structure.45 By using the Bragg equation,53 it can be deduced that the layer spacing of GO (0.82 nm) is consistent with the result of AFM (Fig. 2c). However, the derivative membranes of GO manifest two characteristic peaks, a sharp peak at 10.99° and a broad peak at 22.6°, respectively. The characteristic diffraction peaks at 2θ = 10.99° are attributed to the layer spacing of GO membranes with the stacking structure, while the broad diffraction peaks at 2θ = 22.6° may be due to the formation of short-range graphene structures with excessive GO stacking.54,55 After the hydrothermal treatment, it was found that a wide diffraction peak at 2θ = 22.6° that belongs to the graphitic structure of rGO and the characteristic peak of the GO (001) membrane disappear,45,56 indicating that GO is effectively reduced to rGO and the spacing of rGO layers is smaller than that of GO. From the XRD of rGT-10 and rGTC-3 membranes, we not only observed the symbolic peaks of rGO but also ensured the characteristic peaks of the TiO2 anatase phase, which indicates that TiO2 nanoparticles are loaded on graphene sheets and do not destroy the rGO stacking structure.43 Compared with pure TiO2, the diffraction peaks of TiO2 on rGTC and rGT membranes are still observable. However, the diffraction intensity of TiO2 of rGTC-3 membranes at 25.6° is lower than that of rGT-10 membranes, which is caused by the oCNTs covering the surface of the membranes that diminish the diffraction intensity of TiO2. Thus, it could be concluded that the materials of different spatial dimensions (GO, oCNTs, and TiO2) were successfully assembled into the composite membranes.
 | ||
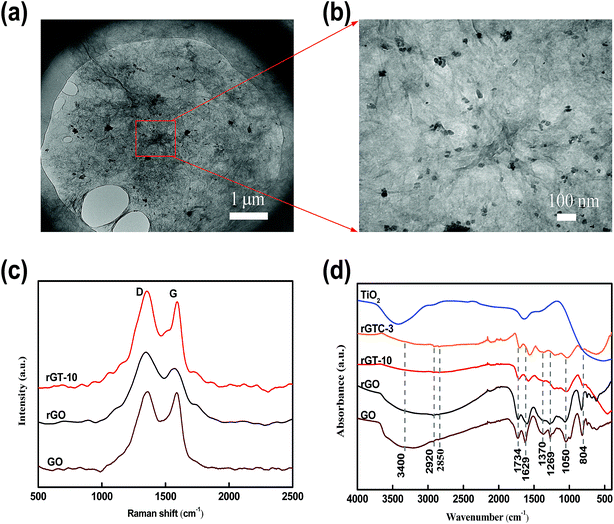
| Fig. 6 Surface and cross-sectional SEM images of GO (a and b), rGO (c and d), rGT-10 (e and f), and rGTC-3 (g and h); and the EDX spectra of rGO (i), rGT-10 (j) and rGTC-3 (k). | ||
As is known, the collapse of the cross section will generate surface stress on the surface of the membrane. And then, when the GO is reduced to rGO, a portion of the oxygen-containing functional groups are decomposed, resulting in rGO with a weak interlayer bonding force that is more easily torn by the surface stress. As a result, the membrane of nascent rGO shows micro-cracks, as shown in the inset in Fig. 6c. Moreover, compounded with TiO2, the surface of the rGT-10 membrane exhibits TiO2 nanoparticles with concomitant pore defects (Fig. 6e), and its cross-sectional morphology still shows the compacted board-like structure (Fig. 6f), which is consistent with other similar studies.57 It could be deduced that the TiO2 nanoparticles, as a 0-dimensional heterogeneous material having a larger size than the space of the rGO lamella, are embedded into the rGT membranes, resulting in local bulges or even defects in the TiO2 aggregation regions. Therefore, in order to confirm whether TiO2 nanoparticles were introduced into the graphene layers, the cross section was tested by EDX, as shown in Fig. 6i–k. By comparing the EDX results of rGO(Fig. 6i) and rGT-10 (Fig. 6j), it can be confirmed that TiO2 nanoparticles are intercalated into the layers of rGO. However, as for the rGTC-3 membranes with the 1-dimensional CNT dopant, the morphologies of the surface and cross section are completely different for rGT and rGO membranes. Obviously, there are no pore defects on the surface of the rGTC-3 (Fig. 6g) membrane compared with rGT-10 (Fig. 6e). The oCNT covering on the surface of the rGTC-3 membrane can be found in the inset in Fig. 6g. In particular, the cross section of rGTC-3 membranes shows a distinct layered fluffy structure rather than the board-like structure like rGT-10 (Fig. 6f) and rGO (Fig. 6d). The thickness of the rGTC-3 membrane (roughly 3.5 μm) is significantly increased compared to the GO membrane (shown in Fig. 6h), indicating a larger interlayer spacing in the rGTC membrane compared with rGT, rGO and GO membranes. Furthermore, TiO2 nanoparticles are uniformly distributed in the cross section of the rGTC membrane (Fig. 6h). And according to the cross-sectional EDX results of rGTC-3 membranes (Fig. 6k), there is plenty of Ti element besides the two major elements of C and O. Although the same mass of TiO2 was added in the preparation of rGTC and rGT membranes (Table 1), the EDX results show different Ti amounts in the cross section.
The morphology comparison of the surface and cross section of rGT and rGTC membranes indicates that the dopant of oCNTs would play a 1-dimensional supporting role in the 3-dimensional stable channel structure, so that the nanochannels of rGTC could not collapse to form a board-like structure, as depicted in Scheme 1(b). As a result, the cross section of rGTC-3 membranes with a stable 3-dimensional structure expose more TiO2 nanoparticles than the collapsed rGT membranes (Fig. 6j and k), and the surface pore defects of the rGT-10 membrane are suppressed (Fig. 6g).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
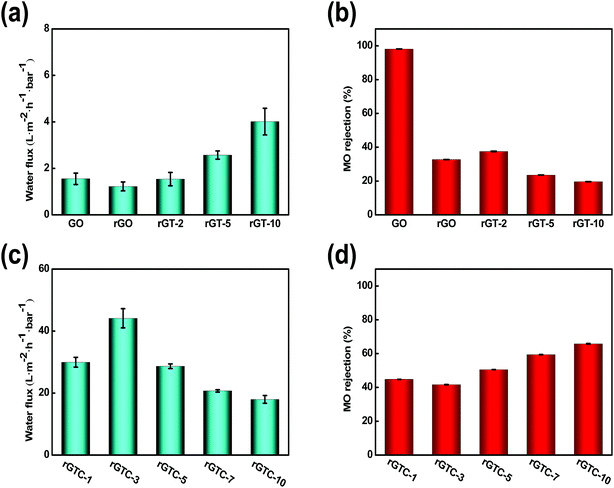
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), it tended to be broken to form a membrane. However, the performance of rGTC membranes was greatly enhanced by the intercalated oCNTs, as depicted in Fig. 7c and d. Compared with the water flux of the rGT-10 membrane, the flux of the rGTC membrane has been increased by 11 times, and that of the rGTC-3 membrane reaches 44.14 L m−2 h−1 bar−1.
2), it tended to be broken to form a membrane. However, the performance of rGTC membranes was greatly enhanced by the intercalated oCNTs, as depicted in Fig. 7c and d. Compared with the water flux of the rGT-10 membrane, the flux of the rGTC membrane has been increased by 11 times, and that of the rGTC-3 membrane reaches 44.14 L m−2 h−1 bar−1.
Combined with SEM characterization results, the reason for such ultra-high flux is that the nanochannels of rGTC would not collapse like that in rGT membranes (Fig. 6f), which could maintain the edges and pores of rGO providing more and wider channels for water transport. What's more, compared with rGT-10 (Fig. 7b), the rGTC membranes can achieve ultra-high water flux and the rejection rate has been increased by 2–3 times (Fig. 7c). As illustrated before, the oCNTs within rGTC can inhibit the generation of surface defects, which leads to a significantly increase in the rejection rate. Therefore, based on SEM (Fig. 6) and the results of permeation experiments (Fig. 7c), it can be concluded that the rGTC membrane is co-assembled with 2-dimensional graphene, 1-dimensional carbon nanotubes, and 0-dimensional TiO2, so 3-dimensional water channels are successfully constructed, as shown in Scheme 1(b). The addition of oCNTs can stabilize and support the water channel to avoid the collapse, while effectively suppressing the surface defects caused by uncoordinated spatial dimensions between TiO2 and the spacing of graphene layers, as mentioned before. Thus, the synergistic assembly of the three low dimensional materials creates such an extraordinary flux performance. However, the excess doping of oCNTs would decrease the water flux of the rGTC membrane, which can be observed from rGTC-5, rGTC-7 and rGTC-10 in Fig. 7c. The water flux of rGTC-10 membranes has been decreased by 59.2% compared to that of rGTC-3. One reason of this result is caused by excessive oCNTs in the graphene water channel and surface, which hinders the transport of water. The other one is that the hydrophilic groups on the surface of oCNTs have greater resistance for water far more than the role of expanding water channels.37 Correspondingly, the rejection rate of the rGTC membranes is gradually improved with the increase of the oCNTs, as shown in Fig. 7d.
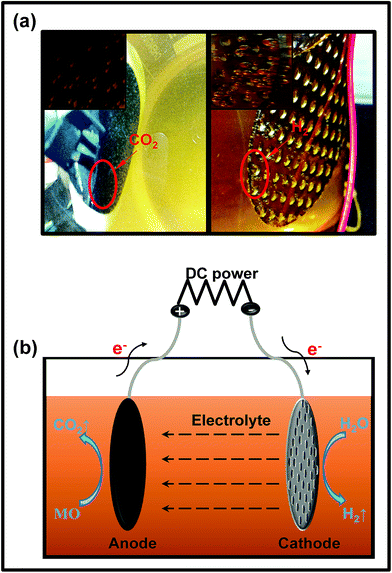 | ||
| Fig. 8 (a) The digital photographs of the simulated electrocatalytic experiment; (b) the mechanism of the electrocatalytic system. | ||
The rGTC-3 membrane is considered to be an inert electrode with high oxygen evolution potential. When it is used as the anode, anodization may occur, so that the mineral can be directly mineralized into CO2,59,60 as depicted in Fig. 8b. This experiment indicates that the rGTC membranes have electrocatalytic properties and can be matched with the principle of EMFs, as shown in Fig. 1. In accordance with the experimental phenomena of electrocatalysis (Fig. 8a), the electrode reaction under neutral conditions can be derived as follows:
Cathode:
| 4H2O + 4e− = 2H2 (g) + 4OH− (aq) | (3) |
Anode:
| MO (C14H14N3SO3Na) + H2O → CO2 (g) + 4H+ (aq) + 4e− | (4) |
| 2H2O − 4e− = O2 (g) + 4H+ (aq) |
Furthermore, the electrochemical properties of rGT and rGTC membranes can be specifically illustrated by electrochemical impedance spectroscopy (EIS). It is well known that the semicircular diameter in the EIS Nyquist diagram represents the charge transfer resistance (RCT).58 The diameter of the semicircle is inversely related to the charge transfer efficiency at the electrode/electrolyte interface,61 which reflects the electrochemical performance of the electrode to some extent.
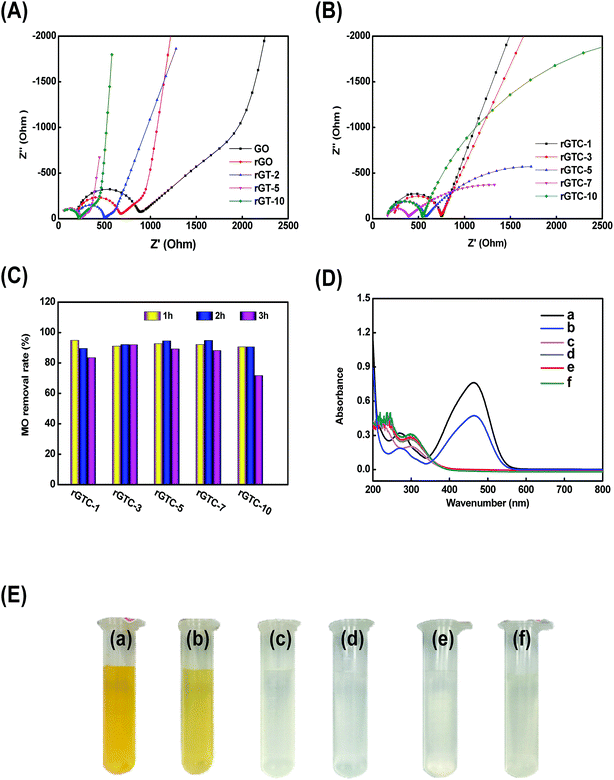
The EIS Nyquist plots of rGT and rGTC membranes are shown in Fig. 9A and B, respectively. With increasing the amount of TiO2 from 0% (rGO) to 100% (rGT-10), the radius of RCT is gradually decreased and changed from 653.2 Ω cm−2 to 201.6 Ω cm−2, which is much smaller than those of pure GO and rGO membranes (Fig. 9A). It is suggested that the special energy band structure of TiO2 crystals would be beneficial to improve the electrocatalytic performance of rGO-TiO2. Because the holes and electrons are easily excited to separate58 and the graphene has a high electron mobility, the electrons can easily transferred to the surface of graphene.45,62 The increased TiO2 content will result in a smaller RCT, indicating that more TiO2 may achieve a higher catalytic efficiency. If the amount of TiO2 is further increased (WTiO2/WGO, 200%), the membrane structure cannot be formed after being dried, and excess TiO2 may destroy the ordered stacking structure of graphene. The RCT of rGTC membranes is larger than that of rGT-10, as shown in Fig. 9B. One reason is probably that the carbon nanotubes make the spacing of graphene layers larger which indirectly increases the RCT of rGTC membranes, and the other possible reason is that the oxygen-containing functional groups could damage the oCNT surface.63 As the content of oCNTs increases from 10% to 100%, the RCT of rGTC decreases first and then increases (Fig. 9B), while the lowest RCT of the rGTC-7 membrane is 212.7 Ω cm−2. It is worth noting that as the oCNT content increases, the straight line portion of the Nyquist curve in the low frequency range gradually becomes irregular. As shown in Fig. 9B, when the amount of oCNTs exceeds 30% (rGTC-3), a second arc appears in the EIS curves of rGTC-5, rGTC-7 and rGTC-10, respectively, and the diameter gradually becomes larger. The results show that the graphene membrane containing excess oCNTs is very likely to generate a considerable capacitance which eventually evolves into a capacitive reactance,64 resulting in lower electron transport efficiency which may affect the electrocatalytic performance of the rGTC membrane.
The rGTC membrane was applied in an EMF device (Fig. 1) to ascertain the feasibility to further improve the MO removal rate by coupling with electrocatalysis technology. The permeate solution was collected under the conditions of 3.0 V constant voltages in the EMF device. As shown in Fig. 9C, the MO removal rates of the rGTC membranes were all abruptly increased to 90%, and the maximum value was 95%. Compared to the non-electrocatalytic rGTC membrane (Fig. 7d), the graphene-based membrane exhibits an extraordinary MO removal rate with electrocatalytic coupling, indicating that the technique of rGTC membrane coupling electrocatalysis can be realized and practically effective. The rGTC membrane could act as both an anode and a filter in the course of electrocatalysis. Combined with the electrocatalytic experimental analysis (Fig. 8), it can be concluded that the electrocatalytic degradation of MO occurred after electrocatalytic coupling. Thus, it can be deduced that the small-molecule organic contaminants could be rapidly degraded due to the electrocatalysis when they passed through the three-dimensional channels of the rGTC membrane. Overall, it has been proved that the superior removal effect of the fabricated graphene-based membranes for small-molecule organic contaminants such as MO dyes can be achieved while maintaining the ultra-high water flux, exceeding those of existing state-of-the-art graphene-based membranes and other work in the field of water treatment, as shown in Table 2. Although the water flux reported in recent work has improved under the promise of a high rejection rate, it is still unsatisfactory because it may be useful for large-molecular weight contaminants, such as reactive black 5 (Mw: 991.8), methyl blue (Mw: 799.8) and so on, but still challenging for small-molecular weight dyes. In contrast, the novel graphene-based membrane with three-dimensional channels in this study presented an excellent performance, which showed a superior water flux (maximum up to 44.14 L m−2 h−1 bar−1, as shown in Fig. 7c) and an outstanding dye removal rate (>90%, as shown in Fig. 9C) with electrocatalytic coupling. From the comparison of the performance of the rGTC membranes via an EMF experiment (Fig. 9C), it is clear that different oCNT contents have different electrocatalytic stability. rGTC-3 is the optimum material and the MO removal is maintained above 90%, implying that there is a suitable oCNT adulterating range for a stable catalytic performance and which is consistent with the EIS Nyquist plots in Fig. 9B. It has been reported that as the content of carbon nanotubes increased, the capacitance of the membrane would also increase.64 With the passage of time, excessive capacitance will decrease the electron transfer rate, thereby reducing the electrocatalytic efficiency. In the experimental process of coupling with electrocatalysis, the MO removal of rGTC-3 membranes could be maintained at more than 90% even after 4 h, as shown in Fig. 9D. After EMF treatment, the peaks of MO at 464 nm have disappeared, which is the maximum absorption wavelength of MO solution. Besides, the noticeable red shift of the secondary peak at 272 nm indicates that the chemical structure of MO was destroyed, when the molecules were transporting through the water channel. The corresponding photograph, including the feed solution, the permeation with electrocatalysis or not, is shown in Fig. 9E. Clearly, the decolorization of MO wastewater was almost up to 100% after EMF treatment (Fig. 9E(c–f)).
| Membrane samples | Feed solution (molecular weight) | Water flux (L m−2 h−1 bar−1) | Dye-removal rate | Pressure (bar) | References |
|---|---|---|---|---|---|
| GO@PAN | Congo red (696) | ∼2 | ∼100% | 1–3 | 33 |
| GO/APT | Rhodamine B (479) | 3.1 | 99.9% | 0.9 | 65 |
| PRGO/HNTs | Reactive black 5 (991.8) | 8.8 | 97.9% | 6 | 66 |
| CFGO/PA | New coccine (604) | 11.2 | 94.3% | 10 | 67 |
| TA/GOQD TFN | Methyl blue (799.8) | 11.7 | 97.6% | 2 | 68 |
| rGO/TiO2/PES | Reactive blue (377) | ∼8 | 81.4% | 5 | 69 |
| PA6@GO/TiO2@PA6 | Methyl blue and MO | 13.77 | 92% and 99% | 1 | 70 |
| PDDA/GO | Methyl blue | 6.42 | 99.2% | 5 | 71 |
| rGO-CNTs | Direct yellow (957) | 11.3 | 99% | 5 | 72 |
| MgSi@RGO/PAN | MO | 4.2 | 73.4% | 5 | 73 |
| rGO-TiO2 | MO | 4.01 | 19.65% | 2 | This study |
| rGO-TiO2/oCNTs | MO | 44.14 | 41.64% | 2 | This study |
| rGO-TiO2/oCNTs | MO | 44.14 | 90–95% (coupling with electrocatalysis) | <1 | This study |
3.3 The mechanism for high water flux and coupling with electrocatalysis
Generally, the abundant oxygen-containing groups and defects in the basal plane or edges of GO layers could be used for grafting modification or loading nanoparticles.22 In particular, graphene-based composites prepared via a hydrothermal method using nanoparticles as the additives are very attractive due to the simple operation and easily quantitative control. Therefore, the rGO-TiO2 composites were prepared and manufactured into novel membranes. The TiO2 nanoparticles were inserted as nano-wedges into the graphene sheets, and more importantly, as an electrocatalyst for the separation of electrons and holes to significantly improve the electrocatalytic efficiency. It was found from the rGT membranes that TiO2 nanoparticles and rGO cannot be synergistically constructed into non-defective membranes because the large particle size of TiO2 could cause the destruction of the nano-transport channels and the formation of obvious defects, as shown in Fig. 6. However, the prepared rGTC membranes can not only obtain ten times higher water flux but also improve the rejection rate for MO by two times compared with the rGT membrane (as shown in Fig. 7 and Table 2). There are two main reasons why rGTC membranes could achieve such ultra-high flux. Firstly, the intercalation of oCNTs into the graphene layers can alleviate the defects and thereby increase the flux. Since the particle size of TiO2 is larger than the spacing of graphene layers, the introduction of the one-dimensional oCNTs can expand the pore channels and form a buffer between the zero-dimensional nanoparticles and the 2-dimensional graphene, which thereby maintain a stable three-dimensional channel structure and successfully expand the interlayer space between graphene layers, as shown in Fig. 10. Secondly, most hydrophilic groups on the basal plane of GO were removed by hydrothermal treatment, which greatly reduces the water resistance during the transfer. In summary, the nanochannels with a three-dimensional structure, which are constructed from different dimensional materials including 0-dimensional TiO2, 1-dimensional oCNT, and 2-dimensional rGO, have good stability and superior water transport performance.As is known, the special band structure of TiO2 is widely used in the field of photoelectrocatalysis.20,62,74 Although the rGTC membrane with a 3-dimensional water channel structure achieves a lower rejection rate for MO, a higher MO removal rate will be obtained (higher than 90%) when coupled with electrocatalysis. As displayed in Scheme 2, when the rGTC membrane as an anode is electrified, the TiO2 loaded on the surface of graphene will be excited to promote electron–hole pair separation (eqn (5)).58 It is noteworthy that the electrocatalytic degradation is a continuous process rather than an event, when the MO molecules are transported in the channels. TiO2 can expand the interlayer space between adjacent graphene sheets like oCNTs, and also act as an electrocatalyst to promote the formation of hydroxyl radicals. Graphene not only acts as a barrier for molecular sieving, but also functions as an electron transporter to excite electron–hole pairs of TiO2 for separation. Similar to the photocatalytic mechanism, the separated electrons (e−) in the conduction band could be rapidly transferred to the graphene conduction band and transmitted on the surface of graphene, which may react with H2O generated by electrocatalysis to further generate O2− with reducing properties (eqn (6), (7), (9), (10) and (11)). As for the separated holes (h+) in the valence band, they can react with the adsorbed H2O to generate reactive intermediates ·OH with strong oxidizing properties (eqn (8)), which rapidly degrade MO in nanochannels into H2O, CO2 and organic residues, as shown in Scheme 2. Furthermore, the carbon nanotubes as an electron transporter have a certain absorption effect on the electrons on the surface of graphene45 and can effectively inhibit the recombination of electrons and holes, thereby improving the electrocatalytic efficiency.
| TiO2 → TiO2 (h+ + e−) | (5) |
| H2O + 2h+ = 1/2O2 (g) + 2H+ | (6) |
| O2 + e− → O2− | (7) |
| H2O + h+ → ·OH + H+ | (8) |
| O2− + H+ → HO2˙ | (9) |
| 2HO2˙ → H2O2 + O2 | (10) |
| O2− + H2O2 → ˙OH + OH− + O2 | (11) |
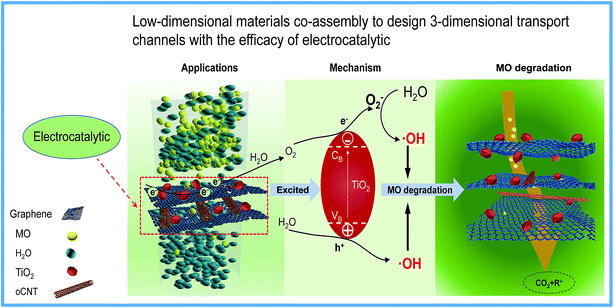 | ||
| Scheme 2 Atomic view of water penetration through rGTC composite membranes and the dye degradation mechanism of rGTC composite membranes by coupling with electrocatalysis. | ||
4 Conclusion
In our work, rGO-TiO2 composites were successfully prepared by a hydrothermal method without any surfactants, and a rGO-TiO2/oCNT membrane was prepared by a simple, low-cost and green method. It is the first time to co-assemble one-dimensional oCNTs with rGO-TiO2 nanocomposites to construct three-dimensional channels for water transportation. Furthermore, the three-dimensional channel structure is not limited to the use of oCNTs or TiO2 because this configuration takes advantage of the physical dimensional properties of the material. This approach would be expected to solve the bottleneck of imbalance between the water flux and rejection rate of the membranes. By adjusting the size of nanoparticles, a more stable 3-dimensional water channel structure has been formed with oCNTs and graphene, while achieving high selectivity of graphene-based membranes. On the other hand, utilizing the electrocatalytic properties of TiO2 and the excellent electronic conductivity of graphene and oCNTs, MO was degraded by using an EMF during the permeation of feed solution, thereby greatly improving the removal rate of dyes (more than 90%). It is the first time to realize the coupling technology of graphene-based membranes and electrocatalysis.In summary, a new idea is provided for the application of graphene in the fields of separation membranes and electrocatalysis. In the future, further work on the theory and properties of these membranes will be taken seriously because of their regulation properties and stability.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was supported by the Science & Technology Development Fund of Tianjin Education Commission for Higher Education (2018ZD14). Thanks to Prof. H. Shi and Prof. Q. Xie for their help on the data analysis and language improvement.References
- H. B. Park, J. Kamcev, L. M. Robeson, M. Elimelech and B. D. Freeman, Science, 2017, 356 Search PubMed.
- X. Du, I. Skachko, A. Barker and E. Y. Andrei, Nat. Nanotechnol., 2008, 3, 491–495 CrossRef CAS PubMed.
- J. Zhu, J. Hou, A. Uliana, Y. Zhang, M. Tian and B. V. D. Bruggen, J. Mater. Chem. A, 2018, 6, 3773–3792 RSC.
- T. Yang, H. Lin, X. Zheng, K. P. Loh and B. Jia, J. Mater. Chem. A, 2017, 5, 16537–16558 RSC.
- T. Saleh and V. Gupta, Nanomaterial and Polymer Membranes, Elsevier, 2016 Search PubMed.
- T. A. Saleh, A. Sarı and M. Tuzen, Chem. Eng. J., 2017, 307, 230–238 CrossRef CAS.
- T. A. Saleh and V. K. Gupta, Sep. Purif. Technol., 2012, 89, 245–251 CrossRef CAS.
- T. A. Saleh, M. M. Al-Shalalfeh and A. A. Al-Saadi, Sci. Rep., 2016, 6, 32185 CrossRef CAS.
- K. S. Novoselov, V. I. Fal′Ko, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, Nature, 2012, 490, 192–200 CrossRef CAS.
- R. R. Nair, H. A. Wu, P. N. Jayaram, I. V. Grigorieva and A. K. Geim, Science, 2012, 335, 442–444 CrossRef CAS.
- D. A. Dikin, S. Stankovich, E. J. Zimney, R. D. Piner, G. H. B. Dommett, G. Evmenenko, S. B. T. Nguyen and R. S. Ruoff, Carbon, 2007, 448, 457 CAS.
- J. R. Werber, C. O. Osuji and M. Elimelech, Nat. Rev. Mater., 2016, 1, 16018 CrossRef CAS.
- K. G. Zhou, K. S. Vasu, C. T. Cherian, M. Neekamal, J. C. Zhang, H. Ghorbanfekrkalashami, K. Huang, O. P. Marshall, V. G. Kravets and J. Abraham, Nature, 2018, 559, 236–240 CrossRef CAS PubMed.
- S. Park, J. An, J. R. Potts, A. Velamakanni, S. Murali and R. S. Ruoff, Carbon, 2011, 49, 3019–3023 CrossRef CAS.
- S. Mao, G. Lu, K. Yu, Z. Bo and J. Chen, Adv. Mater., 2010, 22, 3521–3526 CrossRef CAS.
- D. Luo, G. Zhang, J. Liu and X. Sun, J. Phys. Chem. C, 2011, 115, 11327–11335 CrossRef CAS.
- M. J. Fernándezmerino, L. Guardia, J. I. Paredes, S. Villarrodil, P. Solísfernández, A. Martínezalonso and J. M. D. Tascón, J. Phys. Chem. C, 2010, 114, 6426–6432 CrossRef.
- C. Xu, A. Cui, Y. Xu and X. Fu, Carbon, 2013, 62, 465–471 CrossRef CAS.
- C. Xu, Z. Chen and X. Fu, J. Mater. Chem., 2011, 21, 12889–12893 RSC.
- Y. Zhang, Z. R. Tang, X. Fu and Yj. Xu, ACS Nano, 2010, 4, 7303–7314 CrossRef CAS.
- H. Zhang, X. Lv, Y. Li, Y. Wang and J. Li, ACS Nano, 2010, 4, 380–386 CrossRef CAS.
- P. Wang, J. Wang, X. Wang, H. Yu, J. Yu, M. Lei and Y. Wang, Appl. Catal., B, 2013, 132–133, 452–459 CrossRef CAS.
- B. Lin, T. Feng, F. Chu, S. Zhang, N. Yuan, G. Qiao and J. Ding, RSC Adv., 2015, 5, 57216–57222 RSC.
- Y. Tang, Y. Li, W. Guo, J. Wang, X. Li, S. Chen, S. Mu, Y. Zhao and F. Gao, J. Mater. Chem. A, 2017, 6, 623–632 RSC.
- H. Sun, S. Liu, S. Liu and S. Wang, Appl. Catal., B, 2014, 146, 162–168 CrossRef CAS.
- J. Zhu, J. Wang, J. Hou, Y. Zhang, J. Liu and B. V. d. Bruggen, J. Mater. Chem. A, 2017, 5, 6776–6793 RSC.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- O. Akhavan and E. Ghaderi, J. Phys. Chem. C, 2016, 113, 20214–20220 CrossRef.
- C. Zeng, N. Hossieny, C. Zhang and B. Wang, Polymer, 2010, 51, 655–664 CrossRef CAS.
- W. Wang, L. Zhu, B. Shan, C. Xie, C. Liu, F. Cui and G. Li, J. Membr. Sci., 2017, 548, 459–469 CrossRef.
- S. A. Treese, P. R. Pujadó and D. S. J. Jones, Handbook of Petroleum Processing, Springer Netherlands, 2006 Search PubMed.
- Z. Zhao, Y. Dai, G. Ge, Q. Mao, Z. Rong and G. Wang, Chemcatchem, 2015, 7, 1070–1077 CrossRef CAS.
- J. Wang, P. Zhang, B. Liang, Y. Liu, T. Xu, L. Wang, B. Cao and K. Pan, ACS Appl. Mater. Interfaces, 2016, 8, 6211–6218 CrossRef CAS.
- M. J. McAllister, J.-L. Li, D. H. Adamson, H. C. Schniepp, A. A. Abdala, J. Liu, D. L. Milius, R. Car and A. R. K. Prud'homme, Chem. Mater., 2007, 19, 4396–4404 CrossRef CAS.
- H. C. Schniepp, J. L. Li, M. J. Mcallister, H. Sai, M. Herrera-Alonso, D. H. Adamson, R. K. Prud'Homme, R. Car, D. A. Saville and I. A. Aksay, J. Phys. Chem. B, 2006, 110, 8535–8539 CrossRef CAS.
- T. A. Saleh, Appl. Surf. Sci., 2011, 257, 7746–7751 CrossRef CAS.
- H. Kang, J. Shi, L. Liu, M. Shan, Z. Xu, N. Li, J. Li, H. Lv, X. Qian and L. Zhao, Appl. Surf. Sci., 2017, 428, 990–999 CrossRef.
- Y. Zhou, Q. Bao, L. A. L. Tang, Y. Zhong and K. P. Loh, Chem. Mater., 2009, 21, 2950–2956 CrossRef CAS.
- C. Yao, Y. Shin, L. Q. Wang, C. F. Windisch, W. D. Samuels, B. W. Arey, C. Wang, W. M. Risen and G. J. Exarhos, J. Phys. Chem. C, 2007, 111, 15141–15145 CrossRef CAS.
- J. Shen, T. Li, Y. Long, M. Shi, N. Li and M. Ye, Carbon, 2012, 50, 2134–2140 CrossRef CAS.
- H. Wang, J. T. Robinson, X. Li and H. Dai, J. Am. Chem. Soc., 2009, 131, 9910 CrossRef CAS.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. B. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS.
- T. N. Lambert, C. A. Chavez, B. Hernandez-Sanchez, P. Lu, N. S. Bell, A. Ambrosini, T. Friedman, T. J. Boyle, D. R. Wheeler and D. L. Huber, J. Phys. Chem. C, 2009, 113, 19812–19823 CrossRef CAS.
- B. J. Moon, Y. Oh, H. S. Dong, J. K. Sang, H. L. Sang, T. W. Kim, P. Min and S. Bae, Chem. Mater., 2016, 28, 1481–1488 CrossRef CAS.
- X. Zeng, Z. Wang, N. Meng, D. T. Mccarthy, A. Deletic, J. H. Pan and X. Zhang, Appl. Catal., B, 2017, 202, 33–41 CrossRef CAS.
- K. Cao, Y. Tian, Y. Zhang, X. Yang, C. Bai, Y. Luo, X. Zhao, L. Ma and S. Li, Nanoscale, 2014, 6, 13518–13526 RSC.
- Q. Li, B. Guo, J. Yu, J. Ran, B. Zhang, H. Yan and J. R. Gong, J. Am. Chem. Soc., 2011, 133, 10878–10884 CrossRef CAS.
- J. Yu, H. Yu, B. Cheng, X. Zhao, J. C. Yu and W. K. Ho, J. Phys. Chem. B, 2003, 107, 13871–13879 CrossRef CAS.
- E. Pajootan, M. Rahimdokht and M. Arami, J. Ind. Eng. Chem., 2017, 55, 149–163 CrossRef CAS.
- S. Sakthivel and H. Kisch, Angew. Chem., Int. Ed., 2010, 42, 4908–4911 CrossRef.
- W. Liu, J. Cai, Z. Ding and Z. Li, Appl. Catal., B, 2015, 174–175, 421–426 CrossRef CAS.
- Y. Han, Z. Xu and C. Gao, Adv. Funct. Mater., 2013, 23, 3693–3700 CrossRef CAS.
- L. Chen, G. Shi, J. Shen, B. Peng, B. Zhang, Y. Wang, F. Bian, J. Wang, D. Li, Z. Qian, G. Xu, G. Liu, J. Zeng, L. Zhang, Y. Yang, G. Zhou, M. Wu, W. Jin, J. Li and H. Fang, Nature, 2017, 50, 380–383 CrossRef.
- H. K. Jeong, Y. P. Lee, R. J. Lahaye, M. H. Park, K. H. An, I. J. Kim, C. W. Yang, C. Y. Park, R. S. Ruoff and Y. H. Lee, J. Am. Chem. Soc., 2008, 130, 1362–1366 CrossRef CAS.
- H. K. Jeong, P. L. Yun, H. J. Mei, E. S. Kim, J. J. Bae and Y. H. Lee, Chem. Phys. Lett., 2009, 470, 255–258 CrossRef CAS.
- C. Nethravathi and M. Rajamathi, Carbon, 2008, 46, 1994–1998 CrossRef CAS.
- L. Wang, X. Guo, K. Cao, B. Li, Y. Li, M. Zhang, R. Wen, X. Li, S. Li and L. Ma, J. Mater. Chem. A, 2017, 5, 8051–8061 RSC.
- Y. Yang, J. Li, H. Wang, X. Song, T. Wang, B. He, X. Liang and H. H. Ngo, Angew. Chem., 2011, 50, 2148–2150 CrossRef CAS PubMed.
- E. Brillas, I. Sirés and M. A. Oturan, Chem. Rev., 2009, 109, 6570 CrossRef CAS.
- C. Comninellis, Electrochim. Acta, 1994, 39, 1857–1862 CrossRef CAS.
- M. Adachi, M. Sakamoto, J. Jiu, Y. Ogata and S. Isoda, J. Phys. Chem. B, 2006, 110, 13872–13880 CrossRef CAS.
- W. S. Wang, D. H. Wang, W. G. Qu, L. Q. Lu and A. W. Xu, J. Phys. Chem. C, 2016, 116, 19893–19901 CrossRef.
- S. Mallakpour and S. Soltanian, RSC Adv., 2016, 6, 109916–109935 RSC.
- C. Z. Hu, Z. T. Liu, X. L. Lu, J. Q. Sun, H. J. Liu and J. H. Qu, J. Mater. Chem. A, 2018, 6, 4737–4745 RSC.
- C. Y. Wang, W. J. Zeng, T. T. Jiang, X. Chen and X. L. Zhang, Sep. Purif. Technol., 2018 DOI:10.1016/j.seppur.2018.04.079.
- L. Zhu, H. Wang, J. Bai, J. Liu and Y. Zhang, Desalination, 2017, 420, 145–157 CrossRef CAS.
- H. Zhang, B. Li, J. Pan, Y. Qi, J. Shen, C. Gao and B. V. D. Bruggen, J. Membr. Sci., 2017, 539, 128–137 CrossRef CAS.
- C. Zhang, K. Wei, W. Zhang, Y. Bai, Y. Sun and J. Gu, ACS Appl. Mater. Interfaces, 2017, 9, 11082–11094 CrossRef CAS.
- M. Safarpour, V. Vatanpour and A. Khataee, Desalination, 2016, 393, 65–78 CrossRef CAS.
- L. Chen, N. Li, Z. Wen, L. Zhang, Q. Chen, L. Chen, P. Si, J. Feng, Y. Li and J. Lou, Chem. Eng. J., 2018, 347, 12–18 CrossRef CAS.
- L. Wang, N. Wang, J. Li, J. Li, W. Bian and S. Ji, Sep. Purif. Technol., 2016, 160, 123–131 CrossRef CAS.
- Y. Han, Y. Jiang and C. Gao, ACS Appl. Mater. Interfaces, 2015, 7, 8147 CrossRef CAS.
- B. Liang, C. Guan, J. Wang, J. Qu, K. Pan, L. Wang, P. Zhang and X. Wang, Carbon, 2016, 103, 94–100 CrossRef CAS.
- D. Zhao, G. Sheng, C. Chen and X. Wang, Appl. Catal., B, 2012, 111–112, 303–308 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |