MOFs-derived ZnCo–Fe core–shell nanocages with remarkable oxygen evolution reaction performance†
Yang
Fu
a,
Weijun
Wang
a,
Jingwei
Wang
a,
Xiangnan
Li
a,
Run
Shi
a,
Owen
Peng
a,
Bananakere Nanjegowda
Chandrashekar
a,
Kai
Liu
 b,
Abbas
Amini
b,
Abbas
Amini
 cd and
Chun
Cheng
cd and
Chun
Cheng
 *a
*a
aDepartment of Materials Science and Engineering, Southern University of Science and Technology, Shenzhen 518055, P. R. China. E-mail: chengc@sustc.edu.cn
bState Key Laboratory of Low-Dimensional Quantum Physics, Department of Physics, Tsinghua-Foxconn Nanotechnology Research Center, State Key Laboratory of New Ceramics and Fine Processing, School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China
cCentre for Infrastructure Engineering, Western Sydney University, Penrith, NSW 2751, Australia
dDepartment of Mechanical Engineering, Australian College of Kuwait, Mishref 13015, Kuwait
First published on 22nd April 2019
Abstract
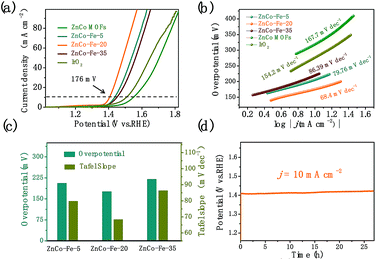
Various renewable energy systems, such as water splitting cells and metal–air batteries, are based on the oxygen evolution reaction (OER). The replacement of common noble-metal platinum catalysts with highly efficient, cheap, and operationally stable electrocatalysts opens huge potential applications for OER; thus, there have been increasing efforts to develop alternative efficient OER catalysts via low-cost fabrication processes. Herein, we report a facile self-templated method to synthesize ZnCo–Fe core–shell nanocages derived from metal–organic frameworks (MOFs) as an efficient catalyst for OER. The unique structure of ZnCo–Fe core–shell nanocages offers a large active surface area with abundant active sites and efficient charge transfer capability as the hollow structure facilitates the active direct contact of materials with electrolytes. We introduced these catalysts at different ratios of Fe, which had a significant effect on the electrocatalytic performance of OER. In other words, the introduction of Fe not only changed the composition but also changed the morphology to expose numerous active sites for OER. Owing to the synergistic effect between the element composition and abundant active sites, the optimized ZnCo–Fe-20 sample presented a superior electrocatalytic OER performance with a very low overpotential (η = 176 mV at 10 mA cm−2) and a small Tafel slope (69.3 mV dec−1). This developed strategy can be easily extended to synthesize other MOF-derived polymetallic oxide-based hybrid electrodes for OER as a practical route for the design of cheap and efficient electrocatalysts.
Introduction
The development of clean and renewable energy technologies is critically important for the replacement of fossil fuels. The oxygen evolution reaction (OER) plays an important role in renewable energy technologies, such as electrochemical water-splitting and rechargeable metal–air batteries, while the kinetically sluggish process hinders the conversion efficiency.1 The excellent OER electrocatalyst must possess properties including a low overpotential, small Tafel slope, and good stability as the reaction proceeds. At present, noble-metal-based catalysts, such as IrO2 and RuO2, are well-known active OER electrocatalysts. However, their high price and poor durability limit their use in large-scale applications.2,3 Many efforts have been made to explore new low cost and highly efficient electrocatalysts for OER. For this purpose, transition-metal oxides, hydroxides, and their derivatives have been intensively studied owing to their earth-abundant nature, low cost and tenability.4–8 An important proportion of the efficient transition metal-based catalysts require a relatively large overpotential (η > 250 mV) to deliver a catalytic current density (j) of 10 mA cm−2. For example, Rui and his co-workers synthesized hybrid Ni-based metal–organic framework (MOFs) nanosheets decorated with Fe-MOF nanoparticles. Owing to the 2D nanosheet and the synergistic effect between Ni active centers and Fe species, the catalyst exhibited a good OER performance (overpotential was about 265 mV at 10 mA cm−2).9 Mao and his colleagues reported a high-performance electrocatalyst composed of 3D crumpled graphene–cobalt oxide nanohybrids, which demonstrated a low overpotential of 340 mV for OER at a current density of 10 mA cm−2.10 Xue and co-workers also designed optimized bimetallic MOFs A2.7B-MOF-FeCo1.6 exhibiting excellent OER activity with a low overpotential of 288 mV at 10 mA cm−2.11In recent years, hollow materials with multiple shell layers or compositions have maximized structural advantages compared to single component hollow structures, thus exhibiting a remarkable performance in electrochemical applications.12 Specifically, MOFs with unique hybrid structures can produce a large specific surface area and expose plenty of active sites for OER.13,14 When used as catalysts, MOFs have many advantages such as well-defined morphology, uniform pore size, and excellent chemical stability.15 It has been reported that MOFs such as ZIF-67 and ZIF-8 were directly used as OER electrocatalysts, while pristine MOFs did not show a high catalytic efficiency for OER performance because of their poor electrical conductivity.16,17 After the development of two-dimensional (2D) layered materials, layered double hydroxide (LDHs)-based nanomaterials recently received much attention and have been regarded as promising OER catalyst candidates, owing to their ultrathin structures with a high specific surface and highly accessible active sites that enable short pathways for mass and carries transport.18,19 For instance, Joong Hee Lee's group designed sheet-like Zn1−xFex–oxyselenide and Zn1−xFex–LDH nanostructured materials with remarkable oxygen evolution reduction ability.20 It is therefore expected that the combination of MOF and 2D hybrids can consolidate their synergistic effects when applied together as OER electrocatalysts. Although many studies have investigated MOF and LDH-based nanomaterials as catalysts, MOF-derived hybrids have not received much attention.21–23
Herein, we report a facile self-templated method to synthesize MOF-derived ZnCo–Fe hybrid transition oxides, simplified as ZnCo–Fe, as efficient catalysts for OER. The synthesized ZnCo–Fe sample had a robust core–shell nanocage structure with a ZnCo MOF core and thin shell of CoFe2O4 and ZnFe2O4 mixtures, inheriting the pristine uniform and regular morphology of ZnCo MOF templates. The smart-designed structure of the core–shell nanocages offered a large surface area for abundant active sites and good stability in water for electrocatalysts. Attributed to this unique design, the ZnCo–Fe sample showed an excellent electrocatalytic performance for OER. In particular, the ZnCo–Fe-20 sample demonstrated a superior OER performance with an overpotential of 176 mV at a current density of 10 mA cm−2 and a small Tafel slope of 69.3 mV dec−1. The strategy developed here can be simply utilized for synthesizing other MOF-derived polymetallic oxide-based hybrid electrodes for OER and facilitating a practical platform for electrocatalysts with higher efficiencies.
Results and discussion
The synthetic procedure for the ZnCo–Fe nanocages is schematically depicted in Fig. 1a. First, ZnCo MOFs were fabricated on a large scale as templates at room temperature using a modified precipitation method.24 Then, the ZnCo MOFs were converted to nanocages through a cation exchange reaction. Briefly, protons were generated from the hydrolysis of ferrous sulfate in the mixture of ethanol and water. The protons consumed the MOF templates and gradually released Zn2+ and Co2+ into solution. The ZnCo MOF precursor was etched by the protons, and the released Zn2+ and Co2+ further reacted with Fe2+ to form a ternary metal oxide as the ultrathin shell. Moreover, the residual ZnCo MOF precursor was etched thoroughly to finally generate the ZnCo–Fe nanocages. Due to the proper control of the ratio of mixture solvents (ethanol and water) as well as the amount of Fe2+, well-defined nanocages (simplified as ZnCo–Fe) were derived from the ZnCo MOFs after annealing treatment. ZnCo–Fe samples with 5, 20, and 35 mg of iron(II) sulfate were prepared for comparison. The crystallographic structure and phase information of the as-prepared ZnCo–Fe-20 samples were investigated using X-ray diffraction (XRD) as presented in Fig. 1b. The XRD data revealed the typical diffraction peaks for the (002), (112), (222), and (044) planes of ZnCo MOFs, which confirmed the successful synthesis of the precursor templates.25 The ZnCo–Fe-20 product was determined to be a hybrid of zinc iron oxide (JCPDS card no. 22-1012) and cobalt iron oxide (JCPDS card no. 22-1086), which was consistent with the expectation of our catalyst design. The peaks in the ZnCo–Fe-20 XRD spectrum were weak and broad. This may suggest a small size feature and possibly a low crystallization property of the as-formed products. In fact, the peaks from the MOFs in the ZnCo–Fe samples with relatively weak intensity suggest that a small amount of MOFs remained in the ZnCo–Fe-20 samples.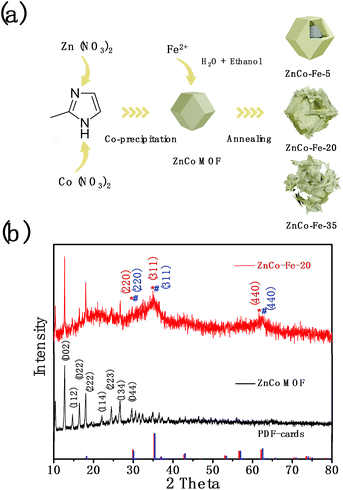 | ||
| Fig. 1 (a) Schematic of the synthesis process of ZnCo–Fe nanocages derived from ZnCo MOFs. (b) XRD patterns of the ZnCo MOF precursor and ZnCo–Fe nanocages. | ||
To clarify the underlying fabrication mechanism, the morphologies and components of the as-prepared intermediate and final products were characterized by electron microscopy. The as-prepared ZnCo MOFs showed a well-defined rhombic dodecahedron morphology with a smooth surface at a large scale and a uniform particle size of about 350 nm (Fig. 2a and e). The even contrast within the single particles (Fig. 2e) suggests a solid feature for these MOFs. Because Zn has a similar ionic radius and electronegativity to Co, the ZnCo MOFs had a similar shape to that of ZIF-67.26 It is noteworthy to mention that the amount of iron(II) sulfate plays a critical role in controlling the morphology and content of ZnCo–Fe. Fig. 2b–d and f–h show the MOF-derived ZnCo–Fe samples prepared using different amounts of iron(II) sulfate. Basically, they inherited the rhombic dodecahedron morphology of the ZnCo MOF precursor, while their smooth surfaces turned into ultrathin and rough nanosheets. This resulted in the formation of a core/shell (ZnCo–Fe-5, Fig. 2b; ZnCo–Fe-20, Fig. 2c) as well as hollow and even-collapsed (ZnCo–Fe-35, Fig. 2d) nanocage morphologies. For ZnCo–Fe-5, a relatively small amount of Fe salt (5 mg) could not produce enough protons to consume the ZnCo MOF templates and release Co2+ into the solution, thus resulting in gentle wrinkles on the surface of ZnCo–Fe-5 (Fig. 2b). Comparatively, broken nanosheets with a pristine metal organic framework were observed in ZnCo–Fe-35 under an excessive amount of Fe salt (35 mg). The ZnCo MOFs were wholly consumed (Fig. 2d). When the usage of iron(II) sulfate was limited to a moderate amount (ZnCo–Fe-20, 20 mg of iron(II) sulfate), the converted nanostructures exhibited a well-defined core–shell nanocage morphology wrapped with relatively thick shells (Fig. 2c).
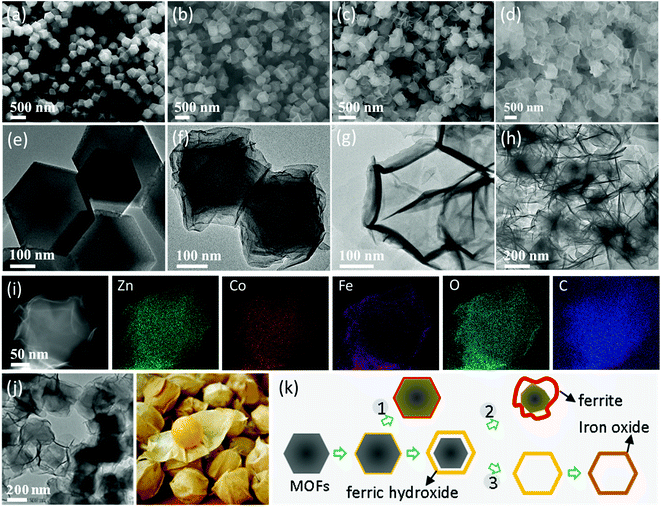 | ||
| Fig. 2 Morphology and elemental analysis as well as the growth process. (a–d) SEM images of ZnCo-MOFs and ZnCo–Fe samples. (e–h) TEM images of ZnCo MOFs and intermediate products of ZnCo–Fe-35 with aging time (5, 10, and 15 min). (i) HAADF-STEM image of one single core/shell nanocage taken in Fig. 2f and its element mapping of Zn, Co, Fe, O, and C. (j) TEM image of typical Zn/Co–Fe-20 and Physalis alkekengi L. fruit. (k) Three basic procedures for the products of ZnCo–Fe-5, 20, and 35, respectively. | ||
To deepen the understanding of the synthesis process, the intermediate samples of ZnCo–Fe-35 were aged for 5, 10, and 15 minutes. Their component distribution and detailed structures were checked by a TEM equipped with an EDS accessory. It was found that as the aging time increased, ultrathin shells formed on the surface of pristine ZnCo MOFs and the inner part gradually disappeared (Fig. 2f). Consequently, nanocages with a well-defined rhombic dodecahedron shape were constructed (Fig. 2g). The nanocages collapsed into nanosheets for aging times beyond 15 minutes (Fig. 2h). Fig. 2i shows the high-angle annular dark-field scanning TEM (HAADF-STEM) image of a typical ZnCo–Fe-20 along with its elemental mapping. It was observed that Zn, Co, O, and C were uniformly distributed except for Fe, which was distributed on the surface of MOFs, indicating the preferential deposition of ferric hydroxide shells. In the annealing process, the ferric hydroxide shells reacted with the left-over Co/Zn core to form the ultrathin nanocages. These results verify the proposed fabrication process (Fig. 1a) and confirm the fact that we have successfully synthesized the core–shell structures of ZnCo–Fe nanocages with CoFe2O4 and ZnFe2O4 mixtures around the surface as well as maintained MOFs as the core. These findings are consistent with the XRD results in Fig. 1b. Fig. S1† provides the HRTEM results of the shell outside of ZnCo–Fe-20, which shows the poor crystallinity of the nanosheets. This result coincides with the broad and weak peaks in the XRD data (Fig. 1b). In order to have a better understanding of the structure variation, the XRD patterns of the as-fabricated materials (ZnCo–Fe-5 and ZnCo–Fe-35) are presented in Fig. S2.† From the XRD results, we found that the ZnCo–Fe-5 samples possessed diffraction peaks for the ZnCo MOF precursor and also weak diffraction peaks for ZnFe2O4 and CoFe2O4. This suggests that a large amount of the ZnCo MOFs remained in the ZnCo–Fe-5 samples. In addition, the diffraction peaks of the ZnCo MOFs in the ZnCo–Fe-35 samples seemed invisible, while the diffraction peaks of ZnFe2O4 and CoFe2O4 were relatively strong. These results suggest that the ZnCo MOFs were consumed. Fig. 2j shows evidence for the typical core–shell morphology of ZnCo–Fe-20 being similar to that of the structure of the Physalis alkekengi L. plant whose fruit is wrapped in a layer of thin husk. Based on the above TEM observation and EDS results, it was concluded that ZnCo–Fe-5 as well as the 20 and 35 products followed 1–3 reaction processes (Fig. 2k). ZnCo–Fe-5 has a thin layer of CoFe2O4 and ZnFe2O4 mixtures and an annealed ZnCo MOF core 1. ZnCo–Fe-20 has a relatively thicker layer of CoFe2O4 and ZnFe2O4 mixtures and a size-reduced annealed ZnCo MOF core 2. ZnCo–Fe-35 only has a thin layer of iron oxides because the ZnCo MOF core is completly dissolved 3. X-ray photoelectron spectroscopy (XPS) analysis was performed to further investigate the elemental composition and electronic states of the elements in the as-synthesized samples. As depicted in Fig. 3a, the full XPS spectrum of ZnCo–Fe-20 indicated the presence of Zn, Co, Fe, O, and C elements. In the XPS spectrum of Zn 2p, the peaks at 1044.3 and 1022.8 eV were assigned to Zn 2p3/2 and Zn 2p1/2 (Fig. 3b).27 In the spectrum of Co 2p (Fig. 3c), the binding energy at 781.3 eV and 797.2 eV belonged to Co 2p2/3 and Co 2p1/2, respectively. In addition, the peaks at 786.8 and 802.7 eV were assigned to a satellite peak, which indicate the presence of Co2+. The binding energy peak at 782.7 eV was also attributed to Co2+.28 As for the Fe 2p spectrum shown in Fig. 3d, the binding energy at around 713 eV and 725 eV belonged to Fe 2p2/3 and Fe 2p1/2, respectively, which confirms the presence of Fe3+.29 The XPS data analysis indicates that in ZnCo–Fe-20, Zn, Co, and Fe were in the form of Zn2+, Co2+, and Fe3+ oxidation states, which is in agreement with the XRD results in Fig. 1b.
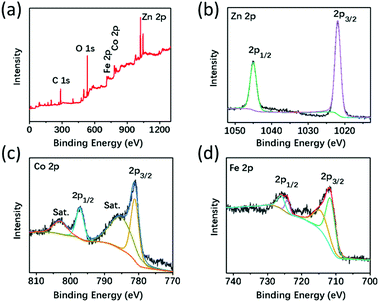 | ||
| Fig. 3 Compositional analysis. (a) Full XPS spectra of the ZnCo–Fe-20 sample, (b) Zn 2p, (c) Co 2p, and (d) Fe 2p. | ||
The core–shell nanocage architectures of ZnCo–Fe-20, as shown in Fig. 2c, were expected to have an enhanced electrochemical property because of their high surface area with plenty of active sites. Therefore, we investigated the OER activity of the ZnCo–Fe samples in a 1.0 M KOH aqueous electrolyte solution using a standard three-electrode system. In order to make a comparison, ZnCo–Fe-5 and ZnCo–Fe-35 were tested using the same mass dropping on glassy carbon. The electrocatalytic activities were evaluated by employing linear sweep voltammetry (LSV) between 1.0 and 1.8 V at a scan rate of 5 mV s−1 (Fig. 4a). The results show that the ZnCo–Fe-5 and ZnCo–Fe-35 samples generated a current density of 10 mA cm−2 at the overpotentials of 206 and 220 mV, respectively. The optimal catalytic performance of ZnCo–Fe-20 achieved the smallest overpotential of 176 mV at a current density of 10 mA cm−2. Therefore, the catalytic activity of the ZnCo–Fe products varied at different amounts of Fe. As presented in Fig. 4b, the corresponding Tafel slope of ZnCo–Fe-20 (68.4 mV dec−1) was lower than those of ZnCo–Fe-5 (79.76 mV dec−1) and ZnCo–Fe-35 (86.39 mV dec−1). For a better picture, the OER performance of the three samples is summarized in Fig. 4c. The enhanced OER performance of ZnCo–Fe-20 can be explained as follows: first, ZnCo–Fe-20 enabled the accessibility of OER active sites by providing a relatively large surface area (1809.85 m2 g−1) for the active materials (Fig. S3, see ESI†). The pore size distribution shown in Fig. S4† obviously reveals the average size distribution at around 350 nm. In addition, compared to ZnCo–Fe-5, ZnCo–Fe-20 possessed a thicker shell, resulting in more active materials for the CoFe2O4 and ZnFe2O4 mixtures (Fig. 2k). Compared to ZnCo–Fe-35, ZnCo–Fe-20 had a hybrid of CoFe2O4 and ZnFe2O4 with higher electrochemically activity than pure iron oxide (Fig. 2k).30,31 Therefore, ZnCo–Fe-20 demonstrated the best OER performance, as shown in Fig. 4c. Overall, the as-fabricated ZnCo–Fe-20 showed a significantly enhanced OER performance compared to CoFe based LDH,32,33 Co–Fe–O/hydroxyl oxide,34 Ni/Fe-based MOF,35 Co3O4/HNCP-40,36 FeCo–P/C,37 plasma-enhanced Fe1Co3/VO-800,38 and CoNi/CoFe2O4/NF39 (detailed comparison to previous reports is provided in Table S1, see ESI†). As presented in Fig. 4d, the voltage needed to reach a current density of 10 mA cm−2 was almost constant after 27 hours, proving that our sample showed an excellent stability toward the OER process. Moreover, in order to investigate the composition and structural stability of the ZnCo–Fe nanocages after the long-time stability test, we performed the characterization measurements including XRD, SEM, TEM, and EDS mapping. The SEM and TEM images (as shown in Fig. S5 and S6†) of the as-prepared ZnCo–Fe nanocages after the OER process indicated that the nanocages could maintain the initial morphology, except that the cores of the ZnCo MOF in some ZnCo–Fe nanocages were dissolved. This did not affect the performance of the catalysts as the outer shell is the active content. Meanwhile, we also observed ultrathin nanosheets around the nanocages, indicating the main structure was relatively stable after the OER process. The XRD data (Fig. S7†) showed that the corresponding diffraction peaks of the ZnCo MOFs and the hybrid of zinc iron oxide and cobalt iron oxide were mainly retained after the electrolytic processes of OER. Furthermore, we observed a uniform distribution of Zn, Co, Fe, and O elements from the EDS mappings. These results confirm that the composition, structure, as well as the morphology were retained during the OER process, thereby further validating the excellent stability of the ZnCo–Fe catalysts.
For OER kinetics, electrochemical impedance spectroscopy (EIS) (Fig. S8†) measurements were first performed at controlled potentials to study the electron-transfer kinetics. In addition, the inset of Fig. S8† shows the electrochemical impedance spectroscopy fitting results. The ZnCo–Fe-20 electrode possessed a smaller charge transfer resistance than ZnCo MOFs. Furthermore, the electrochemically active surface areas (ECSAs) of the ZnCo MOFs, ZnCo–Fe-5, ZnCo–Fe-20, and ZnCo–Fe-35 were herein evaluated and compared using their double-layer capacitances (Cdl; Fig. S9–12†). The calculated Cdl value of ZnCo–Fe-20 showed the highest Cdl (14.3 mF cm−2) compared to ZnCo MOFs (9.38 mF cm−2), ZnCo–Fe-5 (9.44 mF cm−2), and ZnCo–Fe-35 (11.29 mF cm−2), demonstrating the highest electrochemically active surface areas of the ZnCo–Fe-20 for the OER performance. Based on the above results, the following reasons explain why the optimized ZnCo–Fe-20 could afford a superior OER performance. First, the unique design of the core–shell ZnCo–Fe nanocages offered a large surface area for various exposed electrocatalytic sites. Moreover, similar to the Co3O4/CoFe oxide-based catalyst,40 the introduction of Fe was essential for the high catalytic activity, which was favorable for the OER performance. The existence of zinc in the traditional metal organic frameworks restrained Co aggregation more effectively and improved the stability of the structure.41,42 Meanwhile, Co and Fe elements, as the catalytic active metal for OER, played an important role in the enhanced OER performance.43 The addition of the Fe element also improved the electrical conductivity of the material. The synergistic effect among Zn, Co, and Fe elements led to a better catalytic performance for the OER. Also, the hollow structure increased the contact area to the electrolyte, thus improving the conductivity and mass transport. Overall, the present study highlights a new strategy for generating a cheap, highly active, and efficient OER catalyst.
Conclusions
We report a self-templated approach to synthesize MOF-derived ZnCo–Fe nanocages as efficient hybrid polymetallic oxide catalysts for OER. The TEM observation and EDS mapping verified that the deposition of iron hydroxide and the Zn/Co ion exchange to the solution, which resulted in core/shell ZnCo–Fe nanocages and even stacking nanosheets, respectively, at different amounts of Fe2+. The optimized ZnCo–Fe-20 sample not only inherited the advantage of a large surface area for the ZnCo MOF template, which enabled the accessibility of OER active sites and enhanced the mass and charge transfer, but also combined hybrid polymetallic oxides for the synergistic effect among Zn, Co, and Fe elements. These findings resulted in a highly promoted OER performance. Our study provided an alternative and facile way to develop cheap, high-efficiency, and stable catalysts for OER.Experimental section
All the materials were of analytical grade and used without further purification.Synthesis of ZnCo MOFs
In the present synthesis process, 0.707 g Zn (NO3)2·6H2O and 1.386 g Co (NO3)2·6H2O are mixed in 40 mL methanol. The solution is added to 40 mL methanol solution containing 1 g 2-methylimidazole under stirring. After 5 minutes, the well-mixed solution is aged at the temperature of 25 °C for 24 h. Finally, the obtained ZnCo MOFs are washed with ethanol 3 times. Finally, the samples are dried at 70 °C for 12 h.Synthesis of ZnCo–Fe MOF-derived nanocages with ultrathin nanosheets
20 mg of the above ZnCo MOFs is dispersed in 1 mL ethanol as solution A. Further, 20 mg of iron(II) sulfate is quickly added to 5 mL of deionized (DI) water to get a transparent solution, while adding 5 mL ethanol as the solution B. Then, solution A is mixed with solution B and aged for 15 min at room temperature under N2 protection in a centrifuge tube with a capacity of 50 mL. After being centrifuged and washed with ethanol for several times, the green precipitate was dried in vacuum oven for 12 h. Finally, it is transferred to an oven for annealing at 300 °C under Ar2 for 1 h.Characterizations
Field emission scanning electron microscopy (FESEM) was performed on ZEISS-Merlin. Transmission electron microscopy (TEM) and energy dispersive X-ray spectroscopy (EDS) were tested on FEI Tecnai G2 F30 Super-Twin TEM. X-ray diffraction (XRD) curves were collected on Rigaku at 40 kv and 40 mA, and X-ray photoelectron spectroscopy (XPS) curves were recorded on a PHI Quantera SXM (ULVAC-PHI) instrument to determine the compositions and valence states of the elements in the samples. Brunauer–Emmett–Teller (BET) surface areas were calculated from nitrogen sorption isotherms by using Bei Shi De (3H-2000PM2).Electrochemical measurements
All the electrochemical measurements were tested on a CHI 660E electrochemical work station with a standard three-electrode cell. Hg/HgO electrode and graphite rod were selected as the reference and counter electrodes, respectively. A glassy carbon electrode (GCE, 0.196 cm2 area) used as the support for the working electrode. 0.05 mL Nafion (5 wt%) was dispersed in the mixed solution containing 0.6 mL ethanol and 0.35 mL H2O, followed by sonicating for 30 min to form ink. Then, 10 μL of the ink was dropped onto the surface of GCE with a diameter of 5 mm and dried at room temperature. The loading mass of the catalyst on GC was 255 μg cm−2. Rotating disk electrode (RDE) voltammograms were measured in an O2 (99.999%) saturated 1.0 M KOH solution. The potential was measured against Hg/HgO and converted to reversible hydrogen electrode (RHE) by Nernst equation (E(RHE) = E Hg/HgO + 0.0591pH + 0.098). The overpotential (η) was calculated according to the following equation: η = E(RHE) − 1.23 V. Cyclic Voltammograms (CV) were measured at a scan rate of 5 mVs−1, and the thermodynamic potential for the oxygen electrode reactions was determined using the two potentials at which the current crossed zero. The Tafel slope of log(i)–E curves for the OER was deduced from equation, η = a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(i). Electrochemical impedance spectroscopy was tested over the frequency range of 0.1 to 106 Hz at the AC voltage amplitude of either 0 or 1.5 V. All the data of electrochemistry were presented without any iR correction.
log(i). Electrochemical impedance spectroscopy was tested over the frequency range of 0.1 to 106 Hz at the AC voltage amplitude of either 0 or 1.5 V. All the data of electrochemistry were presented without any iR correction.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51776094 and 51406075), the Guangdong-Hong Kong joint innovation project (Grant No. 2016A050503012), the Guangdong Natural Science Funds for Distinguished Young Scholars (Grant No. 2015A030306044), the Training Program for Outstanding Young Teachers at Higher Education Institutions of Guangdong Province (Grant YQ2015151), the National Key Research and Development Project funding from the Ministry of Science and Technology of China (Grants No. 2016YFA0202400 and 2016YFA0202404), and the Peacock Team Project funding from the Shenzhen Science and Technology Innovation Committee (Grant No. KQTD2015033110182370). The starting grants from Southern University of Science and Technology are also acknowledged.Notes and references
- C. C. Mccrory, S. Jung, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2013, 135, 16977–16987 CrossRef CAS PubMed.
- D. Y. Kuo, J. K. Kawasaki, J. N. Nelson, J. Kloppenburg, G. Hautier, K. M. Shen, D. G. Schlom and S. Jin, J. Am. Chem. Soc., 2017, 139, 3473–3479 CrossRef CAS PubMed.
- E. Antolini, ACS Catal., 2014, 4, 1426–1440 CrossRef CAS.
- R. Zhang, P. A. Russo, A. G. Buzanich, T. Jeon and N. Pinna, Adv. Funct. Mater., 2017, 27, 1703158 CrossRef.
- S. Zheng, X. Li, B. Yan, Q. Hu, Y. Xu, X. Xiao, H. Xue and H. Pang, Adv. Energy Mater., 2017, 7, 1602733 CrossRef.
- M. Kuang, Q. Wang, H. Ge, P. Han, Z. Gu, A. M. Al-Enizi and G. Zheng, ACS Energy Lett., 2017, 2, 2498–2505 CrossRef CAS.
- X. Zhong, L. Zhang, J. Tang, J. Chai, J. Xu, L. Cao, M. Yang, M. Yang, W. Kong, S. Wang, H. Cheng, Z. Lu, C. Cheng, B. Xu and H. Pan, J. Mater. Chem. A, 2017, 5, 17954–17962 RSC.
- J. Miao, Z. Lang, X. Zhang, W. Kong, O. Peng, Y. Yang, S. Wang, J. Cheng, T. He, A. Amini, Q. Wu, Z. Zheng, Z. Tang and C. Cheng, Adv. Funct. Mater., 2019, 29, 1805893 CrossRef.
- K. Rui, G. Zhao, Y. Chen, Y. Lin, Q. Zhou, J. Chen, J. Zhu, W. Sun, W. Huang and S. X. Dou, Adv. Funct. Mater., 2018, 28, 1801554 CrossRef.
- S. Mao, Z. Wen, T. Huang, Y. Hou and J. Chen, Energy Environ. Sci., 2014, 7, 609–616 RSC.
- Z. Xue, Y. Li, Y. Zhang, W. Geng, B. Jia, J. Tang, S. Bao, H.-P. Wang, Y. Fan, Z.-w. Wei, Z. Zhang, Z. Ke, G. Li and C.-Y. Su, Adv. Energy Mater., 2018, 8, 1801564 CrossRef.
- L. Yu, J. F. Yang, B. Y. Guan, Y. Lu and X. W. D. Lou, Angew. Chem., Int. Ed., 2018, 8, 178–182 CrossRef.
- E. M. Miner and M. Dincă, Nat. Energy, 2016, 1, 16186 CrossRef.
- H. Xu, J. Cao, C. Shan, B. Wang, P. Xi, W. Liu and Y. Tang, Angew. Chem., Int. Ed., 2018, 57, 8654–8658 CrossRef CAS PubMed.
- J. Cravillon, R. Nayuk, S. Springer, A. Feldhoff, K. Huber and M. Wiebcke, Chem. Mater., 2011, 23, 2130–2141 CrossRef CAS.
- J. Zhao, X. Quan, S. Chen, Y. Liu and H. Yu, ACS Appl. Mater. Interfaces, 2017, 9, 28685 CrossRef CAS PubMed.
- J. Zhou, Y. Dou, A. Zhou, R. M. Guo, M. J. Zhao and J. R. Li, Adv. Energy Mater., 2017, 7, 1602643 CrossRef.
- D. Zhou, Z. Cai, X. Lei, W. Tian, Y. Bi, Y. Jia, N. Han, T. Gao, Q. Zhang and Y. Kuang, Adv. Energy Mater., 2017, 1701905 Search PubMed.
- W. Wang, Y. Liu, J. Li, J. Luo, L. Fu and S. Chen, J. Mater. Chem. A, 2018, 6, 14299–14306 RSC.
- G. Rajeshkhanna, S. Kandula, K. R. Shrestha, N. H. Kim and J. H. Lee, Small, 2018, 1803638 CrossRef PubMed.
- X. Zhang, Q. Liu, X. Shi, A. M. Asiri and X. Sun, Inorg. Chem. Front., 2018, 5, 1405–1408 RSC.
- B. You, N. Jiang, M. Sheng, S. Gul, J. Yano and Y. Sun, Chem. Mater., 2015, 27, 7636–7642 CrossRef CAS.
- S. Zhao, Y. Wang, J. Dong, C.-T. He, H. Yin, P. An, K. Zhao, X. Zhang, C. Gao, L. Zhang, J. Lv, J. Wang, J. Zhang, A. M. Khattak, N. A. Khan, Z. Wei, J. Zhang, S. Liu, H. Zhao and Z. Tang, Nat. Energy, 2016, 1, 16184 CrossRef CAS.
- X. J. Zhao, X. L. Fang, W. U. Binghui, L. S. Zheng and N. F. Zheng, Sci. China: Chem., 2014, 57, 141–146 CrossRef CAS.
- P. Zhang, B. Y. Guan, L. Yu and X. W. D. Lou, Angew. Chem., Int. Ed., 2017, 56, 7141–7145 CrossRef CAS PubMed.
- X. Yang, J. Chen, J. Hu, S. Zhao, J. Zhao and X. Luo, Catal. Sci. Technol., 2018, 8, 573–579 RSC.
- C. Li, J. Balamurugan, N. H. Kim and J. H. Lee, Adv. Energy Mater., 2017, 8, 1702014 CrossRef.
- T. Xue, X. Wang and J. M. Lee, J. Power Sources, 2012, 201, 382–386 CrossRef CAS.
- C. Xiao, Y. Li, X. Lu and C. Zhao, Adv. Funct. Mater., 2016, 26, 3515–3523 CrossRef CAS.
- Z. Luo, C. Li, D. Zhang, T. Wang and J. Gong, Chem. Commun., 2016, 52, 9013–9015 RSC.
- S. Han, S. Liu, S. Yin, L. Chen and Z. He, Electrochim. Acta, 2016, 210, 942–949 CrossRef CAS.
- L. Han, C. Dong, C. Zhang, Y. Gao, J. Zhang, H. Gao, Y. Wang and Z. Zhang, Nanoscale, 2017, 9, 16467–16475 RSC.
- L. Yu, H. Zhou, J. Sun, F. Qin, D. Luo, L. Xie, F. Yu, J. Bao, Y. Li, Y. Yu, S. Chen and Z. Ren, Nano Energy, 2017, 41, 327–336 CrossRef CAS.
- X. Han, C. Yu, S. Zhou, C. Zhao, H. Huang, J. Yang, Z. Liu, J. Zhao and J. Qiu, Adv. Energy Mater., 2017, 7, 1602148 CrossRef.
- T. Wang, Z. Kou, S. Mu, J. Liu, D. He, I. S. Amiinu, W. Meng, K. Zhou, Z. Luo and S. Chaemchuen, Adv. Funct. Mater., 2017, 28, 1705048 CrossRef.
- D. Ding, K. Shen, X. Chen, H. Chen, J. Chen, T. Fan, R. Wu and Y. Li, ACS Catal., 2018, 8, 7879–7888 CrossRef CAS.
- W. Hong, M. Kitta and Q. Xu, Small Methods, 2018, 2, 1800214 CrossRef.
- W. Chen, Y. Zhang, G. Chen, R. Huang, Y. Zhou, Y. Wu, Y. Hu and K. Ostrikov, J. Mater. Chem. A, 2019, 7, 3090–3100 RSC.
- S. Li, S. Sirisomboonchai, A. Yoshida, X. An, X. Hao, A. Abudula and G. Guan, J. Mater. Chem. A, 2018, 6, 19221–19230 RSC.
- X. Wang, L. Yu, B. Y. Guan, S. Song and X. W. Lou, Adv. Mater., 2018, 30, 1801211 CrossRef PubMed.
- X. Song, M. Oh and M. S. Lah, Inorg. Chem., 2013, 52, 10869–10876 CrossRef CAS PubMed.
- Y. Li, B. Jia, Y. Fan, K. Zhu, G. Li and C.-Y. Su, Adv. Energy Mater., 2018, 8, 1702048 CrossRef.
- X.-F. Lu, L.-F. Gu, J.-W. Wang, J.-X. Wu, P.-Q. Liao and G.-R. Li, Adv. Mater., 2017, 29, 1604437 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: HRTEM, SAED images, Nyquist plots, N2 adsorption–desorption isotherms and pore size distribution, tables showing comparison of the electrochemical properties. See DOI: 10.1039/c9ta02017a |
| This journal is © The Royal Society of Chemistry 2019 |