A one-pot and modular self-assembly strategy for high-performance organized enzyme cascade bioplatforms based on dual-functionalized protein–PtNP@mesoporous iron oxide hybrid†
Yan
Liu
 *,
Yuling
Qin
,
Yuanlin
Zheng
,
Yong
Qin
,
Mengjun
Cheng
and
Rong
Guo
*,
Yuling
Qin
,
Yuanlin
Zheng
,
Yong
Qin
,
Mengjun
Cheng
and
Rong
Guo
 *
*
School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, 225002, Jiangsu, P. R. China. E-mail: yanliu@yzu.edu.cn; guorong@yzu.edu.cn; Fax: +86-514-87971802; Tel: +86-514-87971802
First published on 22nd November 2018
Abstract
Inspired by the delicate structure and prominent efficiency of natural multiple-enzyme systems, combining nanotechnologies such as nanomaterials, self-assemblies, and enzyme mimics is fascinating for the development of next-generation high-performance organized enzyme cascade bioplatforms. In our facile and convenient design, a dual-functionalized β-casein-Pt nanoparticles@mesoporous-Fe3O4 (CM-PtNP@m-Fe3O4) hybrid acts as both a nanozyme with outstanding peroxidase-like activity and a scaffold to immobilize and stabilize a natural oxidase, resulting in a high-performance organized enzyme cascade bioplatform for a one-pot assembly procedure. Owing to special physicochemical surface properties, the multipoint attachment of various interactions between natural enzymes and protein/inorganic hybrids leads to efficient immobilization of the enzyme with retained activity. The proposed cascade bioplatform provides superior cholesterol sensing, including simplicity (one-step detection), reusable enzymes (peroxidase mimic and oxidase), and excellent sensitivity (detection limit, 0.05 μM). To our knowledge, the bioplatform presented in this work shows the highest sensitivity for cholesterol detection among all reported colorimetric methods based on nanozymes. Therefore, the highly rationally designed protein/inorganic hybrid and dual-functional strategy used in this study will provide a facile one-pot and effective high-performance organized enzyme cascade bioplatform with potential applications in biosensing, biotransformation, decontamination, and biofuel.
Introduction
In a natural system, enzyme cascade reactions are efficient methods for improving the catalytic performance of enzymes.1–3 Owing to their extraordinarily high efficiency, enzyme cascade reactions with promising applications in biocatalysis and biosensing are of great interest both academically and industrially.4–6 Cooperating enzymes are often confined in the same compartment to perform a cascade reaction efficiently by mainly providing a high local concentration of enzymes and substrates, and efficient mass transfer. Currently, the construction and application of organized enzyme cascade systems with synergistic and complementary functions remains at the preliminary stage, because some drawbacks, including high cost, low stability, and inactivation, can be amplified when natural enzymes are used to fabricate an enzyme cascade system.7–9Recently, nanomaterial-based artificial enzymes (nanozymes) have attracted increasing attention and shown potential in biological, environmental, food, and medical applications owing to advantages such as low cost, stability, and tunable catalytic activities.10–15 Integrating nanozymes and natural enzymes into appropriate scaffolds has attracted much attention as an effective approach to developing enzyme cascade platforms.16–20 However, many methods require scaffold preparation or complicated surface linkages between the scaffold and natural enzyme, which inevitably affects the enzyme activity and leads to the cascade system having low efficiency. Furthermore, hindered substrate access and diffusion within the scaffold can also lead to low efficiency in the cascade system.
The direct combination of nanozymes and natural enzymes to fabricate enzyme cascade systems without a scaffold via self-assembly is convenient and facile. However, the adsorption of desired enzymes directly onto naked nanomaterial surfaces might result in denaturation or a loss in bioactivity.21–24 Furthermore, the nonspecific attachment of biomolecules might lead to a loss in nanozyme activity.25,26 Therefore, a key challenge in fabricating enzyme cascade systems through the direct binding of natural enzymes to nanozymes is retaining the activity of both natural enzymes and nanozymes. To this end, tailoring the physicochemical properties of nanozymes, including size, shape, component, and surface chemistry, allows interactions between the nanozyme and natural enzyme to be adjusted, providing the possibility of retaining both their activities.
Among various nanozymes, Pt nanozyme has attracted sustained attention and has been shown to be effective and useful in biosensing applications owing to its superior activity and excellent biocompatibility.27–29 However, cascade systems concerning Pt nanozymes have usually involved multiple separate processes.30–34 Evidently, multistep analysis and unrecoverable enzymes lead to complicated detection processes and enzyme waste (both of natural oxidase and Pt nanozyme). Although organized cascade systems based on Pt/Fe3O4 hybrid peroxidase nanozyme and glucose oxidase have been developed,35 the peroxidase activity of the nanozyme and efficiency of the integrated enzyme cascade system has not been studied. In previous work, the binding process of the natural enzyme to the hybrid has been complex and slow, which might lead to the bound nature oxidase having reduced activity. Therefore, the meticulous design of Pt nanozymes with extremely high enzyme-like activity and the ability to immobilize a natural enzyme to fabricate high-performance organized enzyme cascade systems is challenging.
Owing to their unique magnetic properties and relatively good biocompatibility, iron oxide nanozymes have received widespread attention since they were found to possess intrinsic peroxidase mimic activity by the Yan group.10 Compared with Fe3O4 nanoparticles (NPs), mesoporous Fe3O4 nanospheres (m-Fe3O4) have received considerable attention owing to their large surface areas, high saturation magnetization, and abundant active surface sites.36,37 Furthermore, magnetic nanomaterials are superior for enzyme immobilization owing to easy separation by magnetic fields. As an effective approach, combining the individual properties of each component will contribute to the multifunctionality and appealing properties of Pt NP/m-Fe3O4 hybrid nanozymes.38,39 However, PtNP/m-Fe3O4 hybrid-based integrated cascade platforms have remained unexplored.
Significantly, the hydrophilic–hydrophobic properties of nanozyme surfaces are heavily related to the mass transfer and enzyme activity of the enzyme cascade system.40,41 Therefore, the careful design of nanozymes with balanced hydrophilic/hydrophobic properties is required. In addition to bearing many functional groups, such as thiol, disulfide, amino, carboxylic, and imidazole groups, proteins are complex amphiphilic biopolymers with hydrophobic and hydrophilic patches on their surfaces. Desired features and functions can be achieved by introducing amphiphilic proteins into inorganic nanozymes, providing an opportunity for molecular level regulation.42–44 This will contribute to the development of nanozyme-based integrated cascade platforms with extremely high activity and selectivity.
Cholesterol detection is an ongoing concern in biomedical fields and has played a crucial role in improving public health. Among various technologies, colorimetric detection has attracted considerable attention because it provides a simple and convenient platform, and does not require sophisticated instruments, highly trained operators, or complex processes. However, to our knowledge, no investigations into the colorimetric detection of cholesterol using an integrated Pt nanozyme/nature oxidase cascade system have been performed.
Herein, for the first time, we adopted a structural-design approach to create an organized cascade platform based on a β-casein-Pt nanoparticles@mesoporous-Fe3O4 (CM-PtNP@m-Fe3O4) hybrid nanozyme (Scheme 1). The model protein used, β-casein, has a high content of acidic amino acid residues (such as glutamic acid and aspartic acid residues), is negatively charged at neutral pH, and contains a negatively charged hydrophilic N-terminal region and strongly hydrophobic C-terminal region.45,46 Specifically, compared with pure mesoporous Fe3O4 nanospheres and Pt nanoparticles, the synergistic effect of PtNP and m-Fe3O4 results in the hybrid having significantly enhanced peroxidase-mimicking activity. Furthermore, the meticulously designed CM-PtNP@m-Fe3O4 nanohybrid has a versatile hierarchical nanoporous structure and unique surface physicochemistry, which allows multipoint attachment through various interactions for immobilizing and stabilizing the natural oxidase and optimizing the enzymatic cascade reaction. This high performance enzyme cascade platform led to one-step cholesterol detection with excellent sensitivity and selectivity. These findings provide a new strategy and direction for the customization of protein/inorganic hybrids with ingenious hierarchical nanostructures and unique surface physicochemical properties, and a one-pot self-assembly method for fabricating high-performance enzyme cascade bioplatforms.
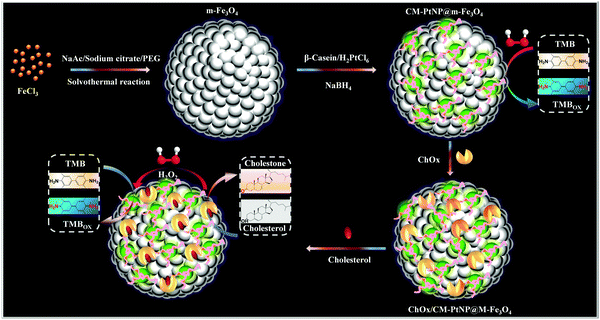 | ||
| Scheme 1 Schematic illustration of fabrication of the ChOx/CM-PtNP@m-Fe3O4 cascade platform, and the corresponding enzyme cascade reaction. | ||
Experimental section
Reagents and chemicals
β-Casein was purchased from Sigma (>99%). Chloroplatinic acid hexahydrate (H2PtCl6·6H2O, 99%), 3,3′,5,5′-tetramethylbenzidine (TMB), and casein enzyme hydrolysate were purchased from Sigma. Cholesterol oxidase from Microbe (5 U mg−1) was purchased from Worthington Biochemical Company. Ferric chloride (FeCl3), ethylene glycol, trisodium citrate (C6H5N3O7·2H2O), sodium acetate (CH3COONa), and 30% H2O2 were purchased from Shanghai Chemical Reagent Company (Shanghai, China). All other reagents and chemicals were of reagent grade and used directly in experiments. Deionized water (18.2 MΩ) was used throughout this study.Instrumentation and characterization
X-ray diffraction was conducted on a Bruker AXS D8 ADVANCE X-ray diffractometer. The composite morphology was examined by field-emission scanning electron microscopy (Zeiss Supra 55 VP FEG). Fourier transform infrared (FT-IR) spectra of samples were recorded in the range 400–4000 cm−1 using an FT-IR spectrometer (Nicolet-740), with samples prepared in pellet form using spectroscopic-grade KBr. X-ray photoelectron spectroscopy (XPS) was performed using an ESCALAB 250Xi spectrometer (Thermo Fisher Co., USA) with an Al X-ray source (1350 eV). An ultraviolet-visible (UV-vis) spectrophotometer (UV-2501, Shimadzu Corp., Japan) was used to record absorption spectra and measure absorbance.Preparation of m-Fe3O4 nanospheres, CM-PtNP@m-Fe3O4, and ChOx/CM-PtNP@m-Fe3O4 nanohybrid
m-Fe3O4 nanospheres were synthesized according to the solvothermal method with some modifications.47 FeCl3 (0.2 M) and C6H5N3O7·2H2O (32 mM) were dissolved in ethylene glycol (25 mL) and then CH3COONa (0.73 M) was added with stirring. The mixture was continually stirred at 1000 rpm for 120 min at 90 °C and transferred into a Teflon-lined stainless-steel autoclave. The autoclave was then heated at 200 °C for 12 h. The resulting Fe3O4 nanospheres were washed with ethanol and deionized water several times and then stored in deionized water at room temperature.The CM-PtNP@m-Fe3O4 nanohybrid was prepared by mixing chloroplatinic acid solution (400 μL, 3 mM) with β-casein solution (40 μL, 1 mg mL−1) in PBS buffer (2.76 mL, 10 mM) containing m-Fe3O4 (pH 7). After stirring for 3 h at 35 °C, ice-cold NaBH4 solution (400 μL, 10 mM) was added under stirring. After reaction completion (10 h), the hybrid was separated using a permanent magnet and washed with water three times.
ChOx (1 mL, 5 mg mL−1) was added to the as-prepared CM-PtNP@m-Fe3O4 nanohybrid solution (1 mL). The resulting mixture was incubated at ambient temperature under ultrasonication for 2 h. The ChOx/CM-PtNP@m-Fe3O4 nanohybrid was separated using a permanent magnet. To estimate the amount of ChOx adsorbed on the CM-PtNP@m-Fe3O4 nanohybrid, the supernatant (obtained from a solution containing CM-PtNP@m-Fe3O4 nanohybrid and ChOx) was measured by UV adsorption.
Peroxidase-like activity of CM-PtNP@m-Fe3O4 nanohybrid
The peroxidase-like activity of the as-prepared CM-PtNP@m-Fe3O4 nanohybrid was investigated in the catalytic oxidation of peroxidase substrate TMB in the presence of H2O2. In a typical experiment, TMB (30 μL, 8.0 mM), CM-PtNP@m-Fe3O4 stock solution (10 μL), and H2O2 (60 μL, 4 M) were added into acetate buffer (2.9 mL, 0.2 M, pH 4.0) at 25 °C. The solution was transferred for UV-vis spectrophotometry after incubating for 15 min.Determination of H2O2
The CM-PtNP@m-Fe3O4 nanohybrid (20 μL) was introduced into acetate buffer (2880 μL, pH 4.0), followed by the addition of TMB solution (50 μL, 40 mM) and H2O2 (50 μL, various concentrations). The mixture was incubated at 25 °C for 20 min with continuous oscillation. The final reaction solution was used to perform absorption spectroscopy measurements.Cholesterol detection using ChOx/CM-PtNP@m-Fe3O4 nanohybrid
Cholesterol (30 μL, various concentrations) was added to acetate buffer (2.9 mL, 0.1 M, pH 4.0) containing the ChOx/CPtNP@m-Fe3O4 nanohybrid (20 μL) and TMB (50 μL, 48.0 mM). The resulting solution was incubated for 50 min at 40 °C. The ChOx/CPtNP@m-Fe3O4 nanohybrid was then isolated from the reaction solution using an external magnetic field. The final reaction solution was used to perform adsorption spectroscopy measurements.Determination of cholesterol in human serum samples
Human serum samples from a local hospital were used to measure the amount of cholesterol. TMB (50 μL, 48 mM), ChOx/CPtNP@m-Fe3O4 nanohybrid solution (20 μL), and serum sample (30 μL) were added into acetate buffer (2.9 mL, 0.2 M, pH 4.0) at 40 °C. The solution was transferred for UV-vis spectrophotometry after incubating for 50 min.Results and discussion
Characterization of CM-PtNP@m-Fe3O4 nanohybrid
The as-prepared CM-PtNP@m-Fe3O4 nanohybrid was first identified from the X-ray diffraction (XRD) pattern. For comparison, the standard position and relative intensities of Pt (JCPDS 04-0802) and Fe3O4 (JCPDS 19-0629) crystals have been provided in Fig. S1A and B (ESI†).48,49 In addition to the identified Fe3O4 peaks, the CM-PtNP@m-Fe3O4 nanohybrid showed all major peaks contributed by Pt, indicating that it was composed of Fe3O4 and Pt. Fig. 1A shows TEM images of the CM-PtNP@m-Fe3O4 nanohybrid, which displayed mesoporous spherical nanostructures formed by the assembly of small-sized nanospheres. The SEM image confirmed the mesoporous spherical assembled nanostructure of the hybrid (Fig. S2, ESI†). A similar structure was observed for the m-Fe3O4 nanosphere sample (Fig. S3, ESI†). This indicated that Pt nanoparticles of similar sizes were grown on the m-Fe3O4 nanosphere, which could not be distinguished from Fe3O4 nanoparticles in the TEM images. HRTEM images showed that the lattice fringes had lattice spacing distances of 0.24 and 0.33 nm (Fig. 1B), attributed to the (111) plane of Pt and (311) plane of Fe3O4 nanoparticles, respectively. The as-prepared CM-PtNP@m-Fe3O4 nanohybrids were also characterized by HAADF-STEM. The elemental mapping images showed Fe, Pt, O, N, and C elements within the nanostructure (Fig. 1D–H). Energy-dispersive X-ray spectroscopy (EDX) confirmed that the CM-PtNP@m-Fe3O4 nanohybrid was composed of C, O, N, Pt, and Fe without other impurities (Fig. S1C, ESI†). The ratio of Fe and Pt atoms in the hybrid was about 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6, as estimated from the EDX analysis. Notably, the very low Pt content in the hybrid reduced the use of Pt, which will enhance the application of Pt-based nanozymes in practice. The inset in Fig. 1A shows a digital image of the CM-PtNP@m-Fe3O4 nanohybrid in the absence and presence of a magnetic field in aqueous solution. The excellent magnetic response of the CM-PtNP@m-Fe3O4 nanohybrid allowed instantaneous separation using a magnet, because m-Fe3O4 in the CM-PtNP@m-Fe3O4 nanohybrid was composed of many primary Fe3O4 particles.50 The nanohybrid exhibited an excellent magnetic response, which contributed to the recycling of CM-PtNP@m-Fe3O4.
2.6, as estimated from the EDX analysis. Notably, the very low Pt content in the hybrid reduced the use of Pt, which will enhance the application of Pt-based nanozymes in practice. The inset in Fig. 1A shows a digital image of the CM-PtNP@m-Fe3O4 nanohybrid in the absence and presence of a magnetic field in aqueous solution. The excellent magnetic response of the CM-PtNP@m-Fe3O4 nanohybrid allowed instantaneous separation using a magnet, because m-Fe3O4 in the CM-PtNP@m-Fe3O4 nanohybrid was composed of many primary Fe3O4 particles.50 The nanohybrid exhibited an excellent magnetic response, which contributed to the recycling of CM-PtNP@m-Fe3O4.
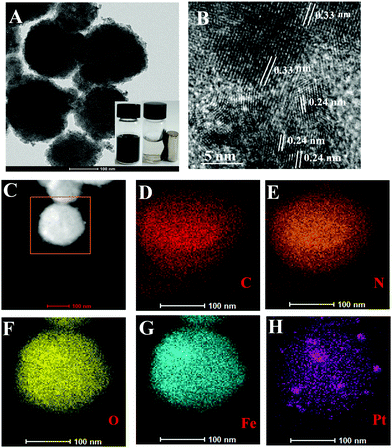 | ||
| Fig. 1 (A) TEM image and (B) HRTEM image of CM-PtNP@m-Fe3O4 nanohybrid. (C) HAADF-STEM image, and corresponding TEM elemental mappings of (D) C, (E) N, (F) O, (G) Fe, and (H) Pt signals. | ||
X-ray photoelectron spectroscopy (XPS) was used to further clarify the nanohybrid composition and surface information. The XPS spectrum showed the presence of C, O, N, Pt, and Fe (Fig. S4A, ESI†). In Fig. S4B (ESI†), two broad peaks at binding energies of around 710.9 and 724.5 eV are assigned to Fe 2p3/2 and Fe 2p1/2, respectively, which are characteristic of the Fe3O4 phase. As shown in Fig. S4C (ESI†), the Pt 4f7/2 electron spectrum of the CM-PtNP@m-Fe3O4 nanohybrid could be deconstructed into Pt0 and Pt2+ components with binding energies of 71.4 eV and 72.4 eV, respectively. Furthermore, the PtNPs peak (Pt2+ 4f7/2, 72.4 eV) shifted toward a lower binding energy (approx. 0.8 eV) compared with that of Pt2+ (73.2 eV), suggesting electron transfer from the carboxylic group of the proteins to the Pt surface. The existence of Pt2+ species might be due to coordination of Pt nanoparticles to –COO− groups in casein, which contains a high content of acid amino acid residues, such as aspartate and glutamate residues.
Fig. S5A (ESI†) shows the FTIR spectra of native β-casein and the CM-PtNP@m-Fe3O4 nanohybrid. The bands at 1447 and 1398 cm−1 due to the COO− groups of Asp and Glu residues 3334 had significantly changed in the CM-PtNP@m-Fe3O4 nanohybrid, demonstrating that Asp and Glu residues bind with metal via carboxyl groups. Furthermore, the band shift from 1645 to 1632 cm−1 indicated that an unordered structure transformed into the extended β-casein chain in the CM-PtNP@m-Fe3O4 nanohybrid. Furthermore, the appearance of a strong peak at 1052 cm−1, contributed by alkoxy stretching vibrations, demonstrated the interaction between metal and OH groups. For comparison, the FTIR spectra of CM/m-Fe3O4 and CM-PtNP are shown in Fig. S5B (ESI†). The spectrum of the CM-PtNP@m-Fe3O4 nanohybrid was different from those of both CM/m-Fe3O4 and CM-PtNP, which indicated that the protein might bind to m-Fe3O4 and CM-PtNP together. Functional groups in proteins, including –NH2, –COOH, and –OH, exhibit high affinities for metal ions. β-Casein contains about 200 amino acid residues, including a high content of acidic amino acid residues (such as glutamic acid and aspartic acid residues). All these functional groups, including –NH2, –COOH, and –OH (especially the high content of carboxyl groups), can bind to Fe3O4 and Pt nanoparticles through complexation.
The specific surface area and pore volume of the CM-PtNP/m-Fe3O4 nanohybrid were characterized using the nitrogen sorption technique, with a typical isotherm shown in Fig. S6 (ESI†). The Brunauer–Emmett–Teller (BET) specific surface area of the hybrid was measured as 79.3 m2 g−1, which was similar to that of many reported mesoporous Fe3O4 structures.51–53 The Barrett–Joyner–Halenda (BJH) average pore diameter calculated from the adsorption branch of the isotherms was 3.8 nm in the hybrid.
Peroxidase-like activity of CM-PtNP@m-Fe3O4 nanohybrid
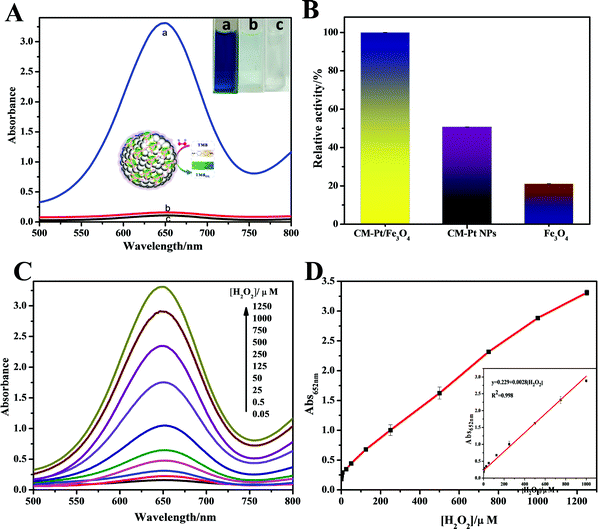
Among the most common chromogenic substrates of peroxidase, TMB was chosen to demonstrate the peroxidase-like activity of the CM-PtNP@m-Fe3O4 nanohybrid. As shown in Fig. 2A, the CM-PtNP@m-Fe3O4 nanohybrid could catalyze the oxidation of TMB by H2O2 to produce the typical blue color with maximum absorbance at 652 nm. Control experiments showed that the TMB–H2O2 system and the CM-PtNP@m-Fe3O4 nanohybrid–TMB system had no obvious color change, confirming the peroxidase-like activity of the CM-PtNP@m-Fe3O4 nanohybrid. For comparison, we studied the peroxidase activity of m-Fe3O4 nanospheres and CM-PtNPs (Fig. 2B). Significantly, CM-PtNP@m-Fe3O4 showed a much higher activity toward TMB than individual m-Fe3O4 nanospheres and CM-PtNPs, which indicated that the synergistic effect of PtNP and m-Fe3O4 efficiently improved the peroxidase-like activity of the hybrid.Similar to peroxidase and other nanomaterial-based peroxidase mimics, the catalytic activity of CM-PtNP@m-Fe3O4 was also dependent on pH, temperature, and substrate concentration. The optimal pH and temperature were pH 4.0 and 25 °C, respectively (Fig. S7A and B, ESI†). As shown in Fig. S7A (ESI†), the enzyme activity of CM-PtNP@m-Fe3O4 changed less than 20% between 15 to 35 °C, which indicated that the hybrid can be used more freely among a range of ambient temperatures. The apparent steady-state kinetic parameters were measured to assess the peroxidase activity of CM-PtNP@m-Fe3O4. In a certain substrate concentration range, typical Michaelis–Menten curves were obtained for both TMB and H2O2 (Fig. S8, ESI†). The Michaelis–Menten constant (Km) and maximum initial velocity (Vmax) were obtained using a Lineweaver–Burk plot, with the results shown in Table S1 (ESI†). The small apparent Km value of CM-PtNP@m-Fe3O4 with both TMB (0.257 mM) and H2O2 (0.036 mM) as substrates indicated that CM-PtNPs had a high affinity for both TMB and H2O2. Meanwhile, the Vmax values of the CM-PtNP@m-Fe3O4 nanohybrid with TMB and H2O2 as substrates were higher than those of the other catalysts, suggesting a higher peroxidase-like activity toward the catalytic reaction due to the synergistic effect of protein, PtNPs, and m-Fe3O4. Notably, caseins can be thought of as amphiphilic block copolymers consisting of blocks with high levels of hydrophobic or hydrophilic amino acid residues.45,46 Therefore, both hydrophobic interactions and electrostatic attraction between casein and TMB led to the high affinity of TMB for the hybrid. The high affinity of H2O2 for the hybrid was not due to its facile adsorption onto Fe3O4, but mainly the synergistic effects of every component in the hybrid (the protein, PtNPs, and Fe3O4). Furthermore, the microporous structure of the m-Fe3O4 NPs caused the reactant and product molecules to diffuse freely in and out the hybrid.27,50 These factors contributed to the high peroxidase-like activity of the CM-PtNP@m-Fe3O4 nanohybrids synergistically.
H2O2 is an important enzymatic intermediate produced by many enzyme–substrate reactions and substances used in various areas. As the CM-PtNP@m-Fe3O4 hybrid possesses outstanding peroxidase-like activity and a high affinity for H2O2, it can be used to quantitatively detect H2O2 concentrations using TMB as substrate. Fig. 2C shows the gradual increase in the absorbance at 652 nm of the TMB system with increasing H2O2 concentration. As shown in Fig. 2D, the linear range for H2O2 detection was 0.01–1000 μM, with a detection limit of 1 nM (signal-to-noise ratio = 3). Compared with other nanozymes in earlier studies (Table S2, ESI†), the H2O2 sensor using the CM-PtNP@m-Fe3O4 nanohybrid was much more sensitive and had a much wider linear range.
We further studied the stability and reusability of the CM-PtNP@m-Fe3O4 nanohybrid. The response sensitivity was more than 97% retained over one month and the catalytic activity was 90% maintained after five cycles, indicating the high stability of the as-prepared CM-PtNP@m-Fe3O4 nanohybrid (Fig. S9, ESI†). Repeated use of the hybrid nanostructure did not significantly alter its morphology (Fig. S10, ESI†), indicating the excellent structural stability of the hybrid nanostructure.
Fabrication of organized ChOx/CM-PtNP@m-Fe3O4 cascade bioplatform
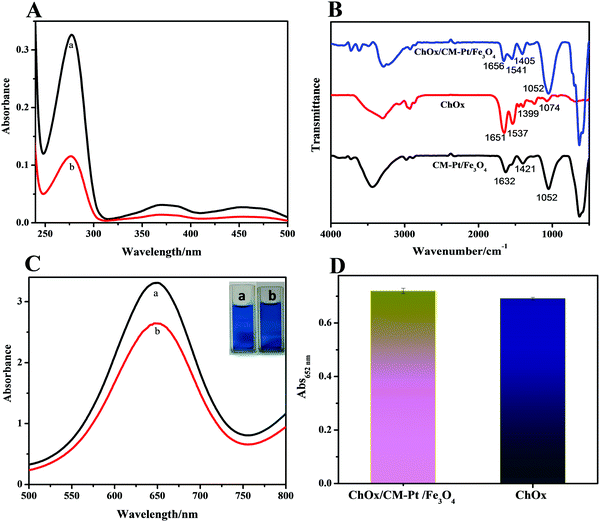
Cholesterol monitoring is among the most studied topics in biosensing, as determining the cholesterol concentration in blood is a considerably important factor in human health. Encouraged by the outstanding performance of the CM-PtNP@m-Fe3O4 nanohybrid, incorporating cholesterol oxidase (ChOx) into the hybrid was a facile and novel strategy for constructing an enzyme cascade reaction system for cholesterol detection with excellent sensitivity.Enzyme immobilization is a key factor affecting biosensor performance. The nanostructure and surface physicochemical features of the CM-PtNP@m-Fe3O4 nanohybrid could affect the immobilization of ChOx and its catalytic properties. When ChOx was incubated with the CM-PtNP@m-Fe3O4 nanohybrid in phosphate buffer solution, ChOx molecules were spontaneously entrapped in the CM-PtNP@m-Fe3O4 nanohybrid, as confirmed by UV-vis and FTIR spectra results (Fig. 3A and B). The enzyme-coated hybrids were purified from the excess enzyme via three-fold magnetic separation/redispersion. The complete removal of unbound (free) enzymes from the enzyme-coated nanohybrid was confirmed by the supernatant of the third purification step not showing a protein absorbance peak (280 nm for ChOx) in the UV-vis spectrum. The immobilization yield was about 60%, as determined by UV-vis spectroscopy, indicating that the hybrid exhibited significantly higher immobilization efficiency for ChOx. FTIR spectroscopy has been well established as the method of choice for analyzing protein secondary structure.54,55 As shown in Fig. 3B, compared with the FTIR spectra of individual ChOx and CM-PtNP@m-Fe3O4 nanohybrids, the spectrum of the ChOx/CM-PtNP@m-Fe3O4 nanohybrid indicated that ChOx had binded to the CM-PtNP@m-Fe3O4 nanohybrid. Furthermore, the peak intensity increased in the range 554–640 cm−1, attributed to Fe–O vibration, demonstrating the interaction between the enzyme and m-Fe3O4 in the CM-PtNP@m-Fe3O4 nanohybrid. Simultaneously, bands at 1453 and 1399 cm−1 attributed to COO− of Asp and Glu residues 3334 had changed significantly after the binding of ChOx to the CM-PtNP@m-Fe3O4 nanohybrid (Fig. 3B), which indicated that Asp and Glu residues of ChOx contributed to the natural enzyme binding to the CM-PtNP@m-Fe3O4 nanohybrid. Significantly, compared with the FTIR spectrum of ChOx, the band at 1656 cm−1 indicated that the α-helix structure of ChOx was retained in the hybrid. Therefore, interaction with the CM-PtNP@m-Fe3O4 nanohybrid did not lead to the compact α-helix structure being lost, which is important for preserving ChOx activity. Fig. S11 (ESI†) shows the XPS survey scan spectrum of the ChOx/CM-PtNP/m-Fe3O4 nanohybrid. Compared with the CM-PtNP/m-Fe3O4 nanohybrid XPS spectrum, the intensity of peaks assigned to C, O, and N (Fig. S11A, ESI†) had increased, and the Pt0 4f7/2 and Fe 2p peaks had shifted (Fig. S11B and C, ESI†), confirming binding of the oxidase to the hybrid. Compared with the SEM image of CM-PtNP/m-Fe3O4, the SEM image of the ChOx/CM-PtNP/m-Fe3O4 nanohybrid indicated the binding of oxidase to the hybrid (Fig. S12, ESI†).
The binding of polymers to nanoparticles is known to lead to reduced nanoparticle activity. Simultaneously, direct interactions between nanoparticles and enzyme can induce conformational changes and reduced activity in natural enzymes. Therefore, both the peroxidase and oxidase activities of the ChOx/CM-PtNP@m-Fe3O4 nanohybrid were measured. As shown in Fig. 3C, the peroxidase activity of the CM-PtNP@m-Fe3O4 nanohybrid was decreased to about 80% of the original value due to ChOx binding. As the CM-PtNP@m-Fe3O4 nanohybrid exhibited extremely high activity, the ChOx/CM-PtNP@m-Fe3O4 nanohybrid still possessed sufficiently high activity for fabrication of the following cascade system. The activity of ChOx in the ChOx/CM-PtNP@m-Fe3O4 nanohybrid was measured using a two-step method, namely, HRP to detect H2O2 generated from the oxidation of cholesterol by O2 in the presence of the ChOx/CM-PtNP@m-Fe3O4 nanohybrid. Compared with native ChOx at the same concentration, the activity of CM-PtNP@m-Fe3O4-bound ChOx was slightly higher (Fig. 3D), indicating that the hybrid provided a favored microenvironment to immobilize oxidase and preserve the oxidase activity.
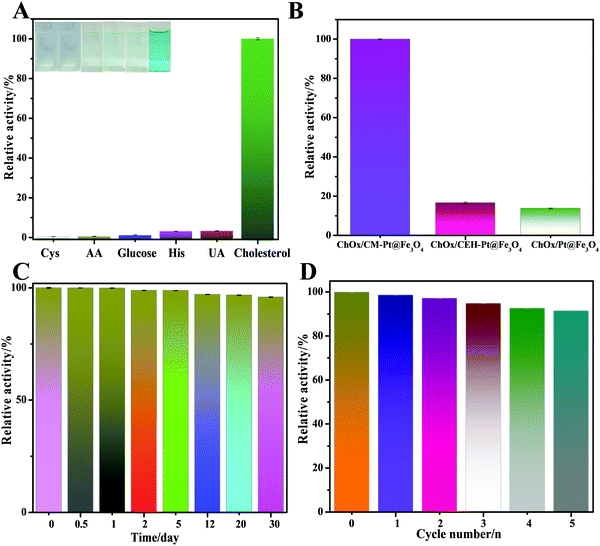
To confirm the role of amphiphilic protein in fabricating the cascade platform, control experiments were conducted. The catalytic efficiencies of bare PtNP@m-Fe3O4 nanohybrid and a casein enzymatic hydrolysate (CEH)-modified PtNP@m-Fe3O4 nanohybrid were assessed under the same experimental conditions (Fig. 5B). Casein hydrolysates are mixtures of small peptides that lack the amphiphilicity of a protein. As shown in Fig. 5B, the response of cholesterol to treatment with the bare PtNP@m-Fe3O4 and CEH/PtNP@m-Fe3O4 nanohybrid systems with ChOx was very low, which indicated that these two hybrids did not provide an environment suitable for immobilizing ChOx or preserving the ChOx activity. The UV-vis absorption spectra indicated that both PtNP@m-Fe3O4 and the CEH/PtNP@m-Fe3O4 nanohybrid could not immobilize ChOx (Fig. S13, ESI†). These results were in agreement with previous reports that natural enzymes are preferentially immobilized and retain their activity in microdomains with balanced hydrophilic/hydrophobic properties.56,57 Therefore, the presence of amphiphilic protein provides an optimal environment for immobilizing the natural enzyme (Scheme 1), which could potentially reduce unwanted direct interactions between inorganic particles and oxidase. This will also contribute to preserving the activity of the natural enzyme and the peroxidase-like activity of the hybrid.
Cholesterol detection using CM-PtNP@m-Fe3O4 nanohybrid-triggered cascade platform
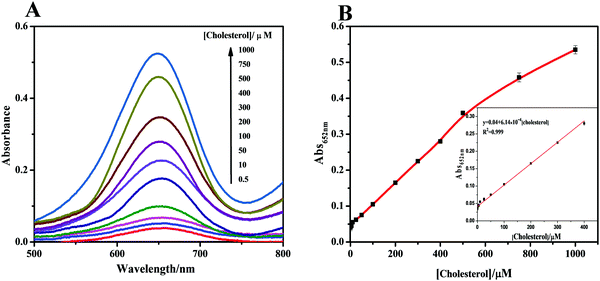
When the cholesterol solution was added, cascade catalysis of cholesterol oxidation and subsequent TMB oxidation occurred (Fig. 4A). Control experiments were performed in the absence of the hybrid, with no notable color change observed. This oxidation was promoted by increased cholesterol concentrations, which supplied more H2O2 for TMB oxidation catalyzed by the cascade system. To obtain optimal assay performance, the reaction temperature and pH were optimized (Fig. S14, ESI†). The optimal conditions were set as pH 4.0 and 40 °C. The formed cascade platform was used to detect cholesterol. The absorbance of the reaction products at 652 nm was proportional to the cholesterol concentration in the range 0.1–400 μM (Fig. 4B), with a linear correlation of 0.999. The estimated detection limit for cholesterol (S/N = 3) was 50 nM. The analytical results obtained using our method, including the linear range and cholesterol detection limit, show outstanding progress compared with previously reported detection limits of 5 μM17 (Table S3, ESI†). Compared with a previous Fe3O4 nanoparticle-based cascade platform,17 the CM-PtNP@m-Fe3O4 nanozyme-based cascade platform exhibited much higher sensitivity. This sensitivity was mainly due to: (i) the CM-PtNP@m-Fe3O4 nanozyme having outstanding peroxidase-like activity; (ii) the hybrid nanozyme serving as a scaffold to immobilize and stabilize natural oxidase with retained activity; (iii) the amphiphilic protein in the hybrid contributing to binding of the hydrophobic cholesterol substrate to the hybrid; and (iv) the diffusion-limited kinetics in the silicon dioxide scaffolds being eliminated and the hybrid contributing to efficient mass transfer.According to all aforementioned results and discussions, a schematic illustration of the enzyme mimic cascade system on the ChOx/CM-Pt NP@m-Fe3O4 nanohybrid is proposed in Scheme 1, which aids understanding of the high-efficiency generation of active radicals by mimicking an enzyme cascade pathway. The combination of the porous structure and the physicochemical surfaces properties of the CM-Pt NP@m-Fe3O4 nanohybrid provides an optimal environment for immobilizing and stabilizing ChOx, resulting in an oxidase/peroxidase bienzyme cascade system, as shown in Scheme 1. Benefiting from activation of the immobilized ChOx, cholesterol will be oxidized to form H2O2 as the oxygen-containing product. This H2O2 then acts as the substrate for the ChOx/CM-PtNP@m-Fe3O4 nanohybrid, which oxidizes TMB to a colored product, TMBox (λ = 650 nm). Here, the absorbance change at 650 nm (owing to the quantity of generated TMBox) is indirectly related to, and could be used to quantify, the cholesterol concentration. During the reaction process, the hydrophobic microdomain provided by casein molecules contributes to the binding of cholesterol to the CM-PtNP@m-Fe3O4 nanohybrid via hydrophobic interactions because cholesterol is hydrophobic, resulting in a high affinity of the substrate towards the cascade system. Furthermore, the nanoporous structure of the CM-PtNP@m-Fe3O4 nanohybrid promotes an increase in the reaction diffusion rate of reactants from the solution to the active site. The ChOx/CM-PtNP@m-Fe3O4 hybrid containing CM-PtNP@m-Fe3O4 as the artificial peroxidase and ChOx as the oxidase provides a new approach to constructing multiple enzyme systems mainly using a self-assembly method instead of chemical processes.
To assess the selectivity of the developed method for cholesterol determination, we investigated the influence of ascorbic acid, cysteine, glucose, histidine, and uric acid on double the concentration of cholesterol. The results of selectivity testing are shown in Fig. 5A. Notably, none of these compounds caused any obvious interference, as also confirmed by the solution colors shown in the inset of Fig. 5A. These results clearly demonstrated the excellent selectivity of the colorimetric bioassay toward cholesterol.
We further studied the stability and reusability of the ChOx/CM-PtNP@m-Fe3O4 nanohybrid (Fig. 5C and D). The as-prepared biosensor was stored in the refrigerator for 30 days at 4 °C and the response sensitivity was more than 96% retained after one month, illustrating that the ChOx/CM-PtNP@m-Fe3O4 cascade platform possessed excellent stability. Furthermore, the catalytic activity maintained 92% of the original value after five cycles, demonstrating the excellent reproducibility of the as-prepared ChOx/CM-PtNP@m-Fe3O4 cascade platform.
The feasibility of the biosensor for cholesterol detection was tested by analyzing different real blood serum samples. The serum samples were injected into the cell instead of cholesterol without any pretreatment. The cholesterol contents of the biosensor were estimated from the calibration curve. As shown in Table S4 (ESI†), the results were in good agreement with the values obtained from a local hospital. Therefore, the biosensor is a reliable and accurate tool for cholesterol determination in real samples.
Conclusions
A meticulously designed protein–PtNP@m-Fe3O4 nanohybrid has a versatile hierarchical nanoporous structure and unique surface physicochemical properties for optimizing the enzymatic cascade reaction. The hybrid provides a favored microenvironment that can immobilize oxidase and preserve the oxidase activity, which resulted in significantly enhanced peroxidase-like activity. The nanoporous structure and the hydrophobic microdomain of the CM-PtNP@m-Fe3O4 hybrid contributes to the high substrate affinity and ensures the product of the first reaction can be consumed by the second reaction in a timely fashion, thus activating the cascade reaction. The CM-PtNP@m-Fe3O4 nanohybrid-triggered cascade platform exhibited excellent sensitivity with a wide linear range of 0.1–400 μM and a detection limit of 0.05 μM for cholesterol. This strategy provides a universal and easily operated route for using amphiphilic protein-functionalized nanozymes to immobilize natural enzymes for efficient enzyme cascade platform.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Nature Science Foundations of China (21573190), Nature Science Key Basic Research of Jiangsu Province for Higher Education (15KJA150009), and PAPD.References
- G. Delaittre, C. Reynhout, J. L. M. Cornelissen and J. M. Nolte, Chem. – Eur. J., 2009, 15, 12600–12603 CrossRef CAS PubMed.
- D. M. Vriezema, P. M. Garcia, O. N. Sancho, N. S. Hatzakis, S. M. Kuiper, R. J. Nolte, A. E. Rowan and J. C. van Hest, Angew. Chem., 2010, 119, 7522–7526 CrossRef.
- S. M. Mckenna, S. Leimkühler, S. Herter, N. J. Turner and A. J. Carnell, Green Chem., 2015, 17, 3271–3275 RSC.
- S. K. Kuk, R. K. Singh, D. H. Nam, R. Singh, J. Lee and C. B. Park, Angew. Chem., Int. Ed., 2017, 56, 3827–3832 CrossRef CAS PubMed.
- J. Muschiol, C. Peters, N. Oberleitner, M. D. Mihovilovic, U. T. Bornscheuer and F. Rudroff, Chem. Commun., 2015, 51, 5798–5811 RSC.
- S. Wu, Y. Chen, Y. Xu, A. Li, Q. Xu, A. Glieder and Z. Li, ACS Catal., 2014, 4, 409–420 CrossRef CAS.
- T. Farrugia, A. W. Perriman, K. P. Sharma and S. Mann, Chem. Commun., 2017, 53, 2094–2097 RSC.
- X. Zhao, H. Palacci, V. Yadav, M. M. Spiering, M. K. Gilson, P. J. Butler, H. Hess, S. J. Benkovic and A. Sen, Nat. Chem., 2018, 10, 311–317 CrossRef CAS PubMed.
- J. Fu, M. Liu, Y. Liu, N. W. Woodbury and H. Yan, J. Am. Chem. Soc., 2012, 134, 5516–5519 CrossRef CAS.
- L. Gao, J. Zhuang, L. Nie, J. Zhang and Y. Zhang, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS.
- H. Wei and E. Wang, Chem. Soc. Rev., 2013, 42, 6060–6093 RSC.
- Y. Hu, H. Cheng, X. Zhao, J. Wu, F. Muhammad, S. Lin, J. He, L. Zhou, C. Zhang, Y. Deng, P. Wang, Z. Zhou, S. Nie and H. Wei, ACS Nano, 2017, 11, 5558–5566 CrossRef CAS.
- P. Weerathunge, R. Ramanathan, R. Shukla, T. K. Sharma and V. Bansal, Anal. Chem., 2014, 86, 11937–11941 CrossRef CAS.
- X. Qu, H. Sun, Y. Zhou and J. Ren, Angew. Chem., Int. Ed., 2018, 57, 2–16 CrossRef.
- V. K. Singh, P. K. Yadav, S. Chandra, D. Bano, M. Talatb and S. H. Hasan, J. Mater. Chem. B, 2018, 6, 5256–5268 RSC.
- H. Cheng, L. Zhang, J. He, W. Guo, Z. Zhou, X. Zhang, S. Nie and H. Wei, Anal. Chem., 2016, 88, 5489–5497 CrossRef CAS.
- M. I. Kim, J. Shim, T. Li, J. Lee and H. G. Park, Chem. – Eur. J., 2011, 17, 10700–10707 CrossRef CAS.
- M. I. Kim, Y. Ye, B. Y. Won, S. Shin, J. Lee and H. G. Park, Adv. Funct. Mater., 2011, 21, 2868–2875 CrossRef CAS.
- H. Liang, B. Liu, Q. Yuan and J. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 15615–15622 CrossRef CAS.
- M. Huo, L. Wang, Y. Chen and J. Shi, Nat. Commun., 2017, 8, 357–368 CrossRef.
- Q. Shao, Y. Qian, P. Wu, H. Zhang and C. Cai, Colloids Surf., B, 2013, 109, 115–120 CrossRef CAS.
- E. Tellechea, K. J. Wilson, E. Bravo and K. Hamadschifferli, Langmuir, 2012, 28, 5190–5200 CrossRef CAS.
- A. A. Vertegel, R. W. Siegel and J. S. Dordick, Langmuir, 2004, 20, 6800–6807 CrossRef CAS.
- J. Wang, U. B. Jensen, G. V. Jensen, S. Shipovskov and V. S. Balakrishnan, Nano Lett., 2011, 11, 4985–4991 CrossRef CAS.
- Y. Liu, Y. Xiang, D. Ding and R. Guo, RSC Adv., 2016, 6, 112435 RSC.
- F. Q. Yu, Y. Z. Huang, A. J. Cole and V. C. Yang, Biomaterials, 2009, 30, 4716–4722 CrossRef CAS.
- L. J. Schussel and J. E. Atwater, Enzyme Microb. Technol., 1996, 18, 229–235 CrossRef CAS.
- H. Deng, X. Lin, Y. Liu, K. Li, Q. Zhuang, H. Peng, A. Liu, X. Xia and W. Chen, Nanoscale, 2017, 9, 10292–10300 RSC.
- G. Wu, S. He, H. Peng, H. Deng, A. Liu, X. Lin, X. Xia and W. Chen, Anal. Chem., 2014, 86, 10955–10960 CrossRef CAS.
- Z. Li, X. Yang, Y. Yang, Y. Tan, Y. He, M. Liu, X. Liu and Q. Yuan, Chem. – Eur. J., 2018, 24, 409–415 CrossRef CAS.
- Y. He, X. Li, X. Xu, J. Pan and X. Niu, J. Mater. Chem. B, 2018, 6, 5750–5755 RSC.
- L. Jin, Z. Meng, Y. Zhang, S. Cai, Z. Zhang, C. Li, L. Shang and Y. Shen, ACS Appl. Mater. Interfaces, 2017, 9, 10027–10033 CrossRef CAS.
- W. He, J. Cai, H. Zhang, L. Zhang, X. Zhang, J. Li and J. Yin, ACS Appl. Nano Mater., 2018, 1, 222–231 CrossRef CAS.
- W. Shi, H. Fan, S. Ai and L. Zhu, Sens. Actuators, B, 2015, 221, 1515–1522 CrossRef CAS.
- Y. Zhuo, Y. Li, J. Han and N. Liao, Sens. Actuators, B, 2014, 193, 461–466 CrossRef.
- Y. Zhang, Z. Su, B. Li, L. Zhang, D. Fan and H. Ma, ACS Appl. Mater. Interfaces, 2016, 8, 12344–12351 CrossRef CAS.
- J. Xu and Y. Zhu, ACS Appl. Mater. Interfaces, 2012, 4, 4752–4757 CrossRef CAS.
- Y. Zhang, F. Wang, C. Liu, Z. Wang, L. Kang, Y. Huang, K. Dong, J. Ren and X. Qu, ACS Nano, 2018, 12, 651–661 CrossRef CAS.
- S. Zhang, H. Li, Z. Wang, J. Liu, H. Zhang, B. Wang and Z. Yang, Nanoscale, 2015, 7, 8495–8502 RSC.
- B. S. Sandanaraj, D. R. Vutukuri, J. M. Simard, A. Klaikherd, R. Hong, V. M. Rotello and S. Thayumanavan, J. Am. Chem. Soc., 2005, 127, 10693–10698 CrossRef CAS.
- F. Jia, Y. Zhang, B. Narasimhan and S. K. Mallapragada, Langmuir, 2012, 28, 17389–17395 CrossRef CAS PubMed.
- Y. Chang, Z. Zhang, J. Hao, W. Yang and J. Tang, Sens. Actuators, B, 2016, 232, 692–697 CrossRef CAS.
- W. Li, B. Chen, H. Zhang, Y. Sun, J. Wang, J. Zhang and Y. Fu, Biosens. Bioelectron., 2015, 66, 251–258 CrossRef CAS PubMed.
- J. Fan, J. Yin, B. Ning, X. Wu, Y. Hu, M. Ferrari, G. J. Anderson, J. Wei, Y. Zhao and G. Nie, Biomaterials, 2011, 32, 1611–1618 CrossRef CAS PubMed.
- Y. Liu, L. Yang and R. Guo, Soft Matter, 2013, 9, 3671–3680 RSC.
- M. Panouillé, D. Durand and T. Nicolai, Biomacromolecules, 2005, 6, 3107–3111 CrossRef.
- J. Liu, Z. Sun, Y. Deng, Y. Zou, C. Li, X. Guo, L. Xiong, Y. Gao, F. Li and D. Zhao, Angew. Chem., Int. Ed., 2009, 48, 5875–5879 CrossRef CAS.
- O. U. Rahman, S. C. Mohapatra and S. Ahmad, Mater. Chem. Phys., 2012, 132, 196–202 CrossRef CAS.
- Y. Xiao, S. Wan, X. Zhang, J. Hu, Z. Wei and L. Wan, Chem. Commun., 2012, 48, 10331–10333 RSC.
- M. Zhu and G. Diao, J. Phys. Chem. C, 2011, 15, 18923–18934 CrossRef.
- Y. Zhu, W. Zhao, H. Chen and J. Shi, J. Phys. Chem. C, 2007, 111, 5281–5285 CrossRef CAS.
- X. Liu, Q. Hu, Z. Fang, Q. Wu and Q. Xie, Langmuir, 2009, 25, 7244–7248 CrossRef CAS.
- L. Zhong, J. Hu, H. Liang, A. Cao, W. Song and L. Wan, Adv. Mater., 2006, 18, 2426–2431 CrossRef CAS.
- V. Renugopalakrishnan, G. Chandrakasan, S. Moore, T. B. Hutson, C. V. Berney and R. S. Bhatnagar, Macromolecules, 1989, 22, 4121–4124 CrossRef CAS.
- G. Liu, J. Li, K. Shi, S. Wang, J. Chen, Y. Liu and Q. Huang, J. Agric. Food Chem., 2009, 57, 4552–4558 CrossRef CAS.
- N. Suthiwangcharoen and R. Nagarajan, Biomacromolecules, 2014, 15, 1142–1152 CrossRef CAS.
- R. X. Xu, T. Pawelczyk, T. Xia and S. C. Brown, Biochemistry, 1997, 36, 10709–10717 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8tb02162g |
| This journal is © The Royal Society of Chemistry 2019 |