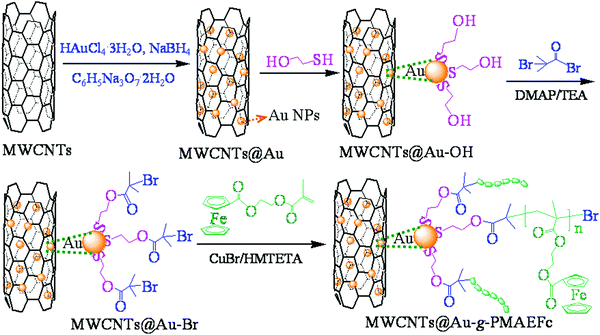
Novel multi-walled carbon nanotubes decorated with gold nanoparticles with poly(2-methacryloyloxyethyl ferrocenecarboxylate) grafted on to form organic–inorganic nanohybrids: preparation, characterization, and electrochemical sensing applications†
Xue-Peng
Wei‡
a,
Rui-Qian
Zhang‡
a,
Le-Bin
Wang
b,
Yan-Ling
Luo
 *a,
Feng
Xu
*a,
Feng
Xu
 *a and
Ya-Shao
Chen
a
*a and
Ya-Shao
Chen
a
aKey Laboratory of Macromolecular Science of Shaanxi Province, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi’an 710062, P. R. China. E-mail: luoyanl@snnu.edu.cn; fengxu@snnu.edu.cn; Fax: +86 29 81530727
bSchool of Materials Chemistry, Xiangtan University, Xiangtan 411105, P. R. China
First published on 24th November 2018
Abstract
With the increasing development of science and technology, food analysis and detection techniques are continouosly being updated, consummated, and are booming. To meet the demands for high efficiency, speediness, and accuracy in food detection, new rapid detection technology needs to be developed for ensuring food safety and human heath. For this purpose, multi-walled carbon nanotubes decorated with gold nanoparticles-grafted poly(2-methacryloyloxyethyl ferrocenecarboxylate) (MWCNTs@Au-g-PMAEFc) nanohybrids were prepared through ATRP of MAEFc, with the MWCNTs@Au nanocomposites prepared via an in situ reduction route. The chemical structure and composition of the nanohybrids were characterized by 1H NMR, FTIR, Raman, XRD, TGA, UV-vis, and XPS techniques. The dispersion behavior and morphologies were investigated by UV-vis, TEM, and SEM observations. The electrochemical properties were studied by cyclic voltammetry (CV) and differential pulse voltammetry (DPV). The experimental results indicated that the nanohybrid-modified electrodes possessed clearly improved electrochemical properties and good reversibility of the redox process, which could be tuned by altering the composition proportions, including the mass ratios of MWCNTs to Au NPs, and the graft percentage of PMAEFc chain segments. The resultant modified electrode sensors could efficiently detect trace poisonous and harmful pesticide residues, and presented a good linear relationship between the concentration and the peak currents, giving a low limit of detection of about 2.7 × 10−8 mol L−1. The developed nanohybrids could therefore be fabricated into electrochemical sensors to detect trace pesticide for ensuring food safety.
1. Introduction
Food is the substance basis for human survival and development, providing human with the energy and nutrients needed to sustain life, to grow and develop, and to perform various activities. However, with the development of modern industry and agriculture, the living environment of humans has become polluted and destroyed, and the contamination of food by poisonous and harmful substances has gradually intensified, which can endanger human health and life safety.1–3 Organophosphorus pesticides (OPPs) are a kind of typical poisonous and harmful substances, of which trichlorfon is one of the most common OPPs. Their accumulation in the environment and food can cause direct or potential harm to human health. Trichlorfon is moderately toxic by ingestion or dermal absorption. When inhaled, the first effects are usually respiratory harm, including a runny nose, coughing, chest discomfort, difficult or short breath, and wheezing. It also leads to impaired memory and concentration, disorientation, severe depressions, irritability, confusion, headache, speech difficulties, delayed reaction times, nightmares, sleepwalking, and drowsiness or insomnia. It may have carcinogenicity, teratogenicity, and mutagenicity. To ensure food safety and the human health, it is generally demanded that the amount of food additives and pesticide residues in food should be controlled at the most inefficient levels. In 2006, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) confirmed maximum residue limits (MRLs) of trichlorfon for cows's milk of 50 μg kg−1, and the residual limit standard MRL in other fruits and vegetables was set as 0.5 mg kg−1.4 Consequently, the rapid, prompt, and accurate detection of the residual amount of health-relevant compounds in foods or agricultural products is very important for ensuring food safety and human health.At present, the food detection analysis techniques include chemical analysis (CA), infrared spectroscopy, and various chromatographic techniques, including thin layer chromatography (TCL), gas chromatography (GC), high-performance liquid chromatography (HPLC).4,5 NMR-spectroscopy was also reported for the nontargeted screening and simultaneous quantification of melamine.6 Compared with CA and TCL, although GC and HPLG possess high sensitivities, veracity, and accuracy, they generally need expensive equipment, a tedious pre-processing process, and high professional operation skills. In addition, the complicated sample matrix background, time-consuming analysis, low analyte concentrations, and confined qualitative ability of analytical instruments are responsible for the detection difficulties of trace pesticides and food additives. Consequently, they are not suitable for on-spot rapid determination and popularization. The development and applications of molecular imprinting, electrochemical, and biosensing technologies are compensating for the shortcomings of these techniques.7–9 In particular, electrochemical sensors based on nanocomposites have the advantages of a simple design, low cost, tunable electrochemical properties, high sensitivity, and rapid response, thus they have been widely used in the detection of OPPs residues.10 Therefore, the design of novel nanocomposite modified electrodes that can enhance the properties of the electrochemical sensors will be of great importance for development of the sensors and the follow-up detection of hazardous substances. In spite of this, there exist some difficulties in the food safety detection; in most cases, the limit of detection, reproducibility, and stability need to be improved so as to efficiently and effectively detect trace residues.
Gold nanoparticles (Au NPs) have a special quantum surface effect, excellent stability and biocompatibility, unique optical, electrical, magnetic, and catalytic properties, and high electrocatalytic activities, which thus have been widely used in the field of electrochemical sensing, biological analysis, biomedical testing, catalysis, anticancer drugs, drug delivery, sensors, antioxidants, larvicides, antimicrobials, nanofluids, and agriculture.11–13 In particular, the rapid detection technique based on Au NPs immune markers has increasingly been applied in modern food analysis and in detection, having the advantages of simplicity, celerity, high sensitivity, and specificity, low cost and a small amount of samples. Carbon nanotubes (CNTs)-based electrochemical sensors have been widely used in the detection of metal ions, pesticides, and other pollutants because CNTs exhibit eminent physical, mechanical, electrochemical, and optical properties, rapid electronic transport properties, large specific surface areas, and strong absorption performances etc.14–16 The decoration of Au NPs on the surface of CNTs will construct a new class of nanocomposites with multifunctional properties, including excellent conductivity, appealing optics, catalysis, and electrochemical redox characteristics, especially the electrocatalytic activity based on a cooperative effect when compared to either material alone.17–19 This combination also contributes to the improvement of dispersity of the CNTs by a strong non-covalent interaction between CNTs and Au NPs,20 and can therefore be potentially used in optical, catalytic, sensor, solar cells, and most importantly in biosensor applications. On the other hand, ferrocene-based polymers as a new class of functional polymers possess unique electron transfer traits, redox electrochemical properties, and semiconducting, photophysical, catalytic, photoelectronic and electronical properties,21,22 and thus are widely used as semiconducting materials, phase separation materials, catalysts, redox responsive drug carriers, biosensors, magnetic ceramic precursors and smart surface.23,24 Especially in recent years, Wong's research groups have synthesized nanopatterned face-centered tetragonal Fe–CoPt and Fe–Pt phase NPs by utilizing metallopolymer precursors containing ferrocene as templates which, upon pyrolysis or photolysis, generate NPs with narrow size distribution, precisely controllable composition and density per unit area.25–27 These materials can potentially be used as a high-density magnetic data storage media, bit-patterned magnetic recording media, spintronic “switching” devices, nanogranules in gap structures, and magnetic sensing heads.26–30 The most recent progress in ferrocene derivatives was related to the realization of the lithographic patterning of ferromagnetic FePt NPs from a single-source bimetallic precursor containing a hemiphasmidic structure, which offers a promising route to develop bit-patterned media for next-generation magnetic data-recording systems.31 These studies motivated us to further graft ferrocene-containing polymers with unique properties onto the CNTs@AuNPs nanomaterials, thus constructing a novel organic–inorganic nanohybrid with improved electrical conductivity and electron-transfer properties, especially better electrochemical responses based on a synergistic effect. This will open up an exciting new horizon in the science and technology of CNTs, electrochemical sensors, and biosensors, and provide significant technical innovations for food safety detection.
In this context, we aimed to modify MWCNTs with Au NPs through an in situ reduction reaction based on strong non-covalent supramolecular interactions or electrostatic attraction interactions, and then to design and synthesize novel MWCNTs@Au graft ferrocene-based polymer poly(2-methacryloyloxyethyl ferrocenecarboxylate) (MWCNTs@Au-g-PMAEFc) organic–inorganic nanohybrids through atom transfer radical polymerization (ATRP). The MWCNTs@Au-g-PMAEFc was fabricated into electrochemical sensing films, and the effect of the content of Au NPs and the graft percentage of PMAEFc on the electrochemical properties was investigated. To the best of our knowledge, there is no relevant literature reporting similar work. We expect that the sensing materials with higher charge-transfer rates and better electrocatalytic activities based on a synergistic effect could be obtained to detect the residues of organophosphorous pesticides and food additives in vegetables and fruits, etc., thus providing health assurance for food safety.
2. Experimental section
2.1 Materials and reagents
Gold acid chloride trihydrate or hydrogen tetrachloroaurate(III) trihydrate (HAuCl4·3H2O, Au ≥ 48%) was provided by Sinopharm Chemical Reagent Co., Ltd, China, and prepared as a 1% aqueous solution before use. MWCNTs (95%, particle diameter 10–20 nm, and length 10–30 μm) were purchased from Chengdu Institute of Organic Chemistry, CAS, and ground to powders in a mortar in advance and then dried in an oven at 120 °C before use. Trisodium citrate dihydrate (TCD, C6H5Na3O7·2H2O, 99%) and sodium borohydride (NaBH4, 98%) were supplied by Sinopharm Chemical Reagent Co., Ltd, China, and used directly. 2-Mercaptoethanol (ME, HOCH2CH2SH, 99%) and 4-dimethylaminopyridine (DMAP, 98%) were obtained from J & K Scientific Ltd, Beijing, China, and used as received. 2-Bromoisobutyryl bromide (BIB, 98%) and 1,1,4,7,10,10-hexamethyltriethylenetetramine (HMTETA, 97%) were purchased from Aladdin without further purification before use. Triethylamine (TEA, 98%), dichloromethane (DCM, 98%), and N,N-dimethylformamide (DMF, 99.5%) were purchased from Sinopharm Chemical Reagent Co., Ltd, China, and refluxed and distilled over CaH2 to remove water before use. Ferrocenecarboxylic acid (FCA, 98%), 2-hydroxyethyl methacrylate (HEMA, 97%), and N,N-dicyclohexylcarbodiimide (DCC, 99%) were purchased from Aldrich Industrial Corp. (Shanghai, China), and used without further purification. Cuprous bromide (CuBr, 99%) was offered by Meryer Chemical Technology Co., Ltd, Shanghai, China, and purified as per previous work.322.2 Preparation procedure
The preparation of MWCNTs@Au-g-PMAEFc nanohybrids was carried out in a five-step reaction, as shown in Scheme 1. First, MWCNTs were modified so as to enlarge the micropores on the tube wall of the MWCNTs and to improve the loading efficiency of Au NPs on the surface of MWCNTs.14,15 Then, Au nanoparticles were decorated on the modified MWCNTs through an in situ reduction method at a mass ratio of MWCNTs to Au of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (w/w),16,33 affording MWCNTs@Au nanocomposites (mean yield: 72%). Unless otherwise specified, the amount of Au was calculated according to the mass percentage of Au in HAuCl4·3H2O (ca. 48%) thereinafter. Details are given in the ESI.†
4 (w/w),16,33 affording MWCNTs@Au nanocomposites (mean yield: 72%). Unless otherwise specified, the amount of Au was calculated according to the mass percentage of Au in HAuCl4·3H2O (ca. 48%) thereinafter. Details are given in the ESI.†
In the third step, the ATRP initiator precursor MWCNTs@Au-OH with hydroxyl groups on the surface was prepared through the coordination interaction between Au NPs and –SH groups in ME at a mole ratio of ME![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au of 6
Au of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Taking MWCNTs
1. Taking MWCNTs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au = 1
Au = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) for example, 0.05 g MWCNTs@Au (Au NPs: 0.105 mmol) was dispersed in 50 ml deionized water and ultrasonicated for about 30–60 min. After that, the calculated amount of ME (0.044 ml, 0.63 mmol) was added into the solution. The reaction solution was stirred at room temperature for 24 h, and then centrifuged. The solid was repeatedly rinsed with absolute ethyl alcohol to remove the unreacted ME. The crude product was dried under vacuum at 80 °C for 24 h, offering MWCNTs@Au-OH nanocomposites (yield: 78%; theoretical amount of ME: 5.56 mmol g−1). Other nanocomposites with various compositions could be prepared in the same way as the above description.
1 (w/w) for example, 0.05 g MWCNTs@Au (Au NPs: 0.105 mmol) was dispersed in 50 ml deionized water and ultrasonicated for about 30–60 min. After that, the calculated amount of ME (0.044 ml, 0.63 mmol) was added into the solution. The reaction solution was stirred at room temperature for 24 h, and then centrifuged. The solid was repeatedly rinsed with absolute ethyl alcohol to remove the unreacted ME. The crude product was dried under vacuum at 80 °C for 24 h, offering MWCNTs@Au-OH nanocomposites (yield: 78%; theoretical amount of ME: 5.56 mmol g−1). Other nanocomposites with various compositions could be prepared in the same way as the above description.
ATRP initiator MWCNTs@Au-Br was prepared through an esterification route as per the mole ratio of MWCNTs@Au-OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BIB
BIB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TEA of 1
TEA of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. Specifically, 0.05 g MWCNTs@Au-OH (MWCNTs
4. Specifically, 0.05 g MWCNTs@Au-OH (MWCNTs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au = 1
Au = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w), ME: 0.278 mmol) was dispersed in 50 ml dried DCM in a 100 ml three-neck flask, which was then sealed for ultrasonication for 30 min. Afterward, the mixture solution was cooled in an ice bath and purged with N2 for 30 min. When the reaction temperature was reduced to 0 °C, 137.5 μl (1.112 mmol) BIB dissolved in 5 ml DCM in advance was drop-wise added into the reaction system with a constant pressure funnel under the protection of N2 within 30 min. After, the mixture solution was kept at 0 °C for 30 min, then 154.1 μl TEA (1.112 mmol) dissolved in 5 ml DCM was drop-wise added into the reaction flask with a constant pressure funnel within 30 min, and then N2 was closed off. The reaction proceeded at 30 °C for 24 h with stirring. The mixture solution was centrifuged, and the solid was repeatedly rinsed with DCM to remove the unreacted BIB and TEA, and then dried at 60 °C for 24 h, giving the resultant MWCNTs@Au-Br (yield: 69%; theoretical amount of BIB: 3.39 mmol g−1).
1 (w/w), ME: 0.278 mmol) was dispersed in 50 ml dried DCM in a 100 ml three-neck flask, which was then sealed for ultrasonication for 30 min. Afterward, the mixture solution was cooled in an ice bath and purged with N2 for 30 min. When the reaction temperature was reduced to 0 °C, 137.5 μl (1.112 mmol) BIB dissolved in 5 ml DCM in advance was drop-wise added into the reaction system with a constant pressure funnel under the protection of N2 within 30 min. After, the mixture solution was kept at 0 °C for 30 min, then 154.1 μl TEA (1.112 mmol) dissolved in 5 ml DCM was drop-wise added into the reaction flask with a constant pressure funnel within 30 min, and then N2 was closed off. The reaction proceeded at 30 °C for 24 h with stirring. The mixture solution was centrifuged, and the solid was repeatedly rinsed with DCM to remove the unreacted BIB and TEA, and then dried at 60 °C for 24 h, giving the resultant MWCNTs@Au-Br (yield: 69%; theoretical amount of BIB: 3.39 mmol g−1).
To prepare MWCNTs@Au-g-PMAEFc organic–inorganic nanohybrids, 2-methacryloyloxyethyl ferrocenecarboxylate (MAEFc) was first prepared, as shown in Fig. S1(a–c) in ESI.† Subsequently, MWCNTs@Au-g-PMAEFc nanohybrids were prepared through the ATRP of MAEFc at mole ratios of [MWCNTs@Au-Br]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [MAEFc]
[MAEFc]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuBr]
[CuBr]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [HMTETA] of 1
[HMTETA] of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 1
5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150
150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.34,35 Typically, for [MWCNTs@Au-Br]
5.34,35 Typically, for [MWCNTs@Au-Br]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [MAEFc]
[MAEFc]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuBr]
[CuBr]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [HMTETA] of 1
[HMTETA] of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, a certain amount of MWCNTs@Au-Br (0.05 g, BIB: 0.1695 mmol for MWCNTs
5, a certain amount of MWCNTs@Au-Br (0.05 g, BIB: 0.1695 mmol for MWCNTs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au = 1
Au = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w)) and MAEFc (2.8985 g, 8.475 mmol) were added to a 25 ml dried Schlenk flask and then 4 ml desiccative DMF was introduced. The reaction flask was frozen, vacuumized, and then suffused with N2, and then a certain amount of HMTETA (230.5 μl, 0.8475 mmol) was injected into the flask under N2 atmosphere via a micro-syringe, followed by an immediate freeze–pump–thaw–nitrogen filling process. The purified CuBr powder (0.1459 g, 1.017 mmol) was promptly added to the system under N2 flow, followed by repeating the above freeze–pump–thaw–nitrogen filling operation. After, cooling and reflux units were connected with the reaction system, the polymerization proceeded at 90 °C for 24 h, with persistent stirring. The resulting solution was centrifuged, and repeatedly rinsed with DMF to get rid of the unreacted MAEFc and HMTETA, and then repeatedly flushed with absolute ethyl alcohol to remove CuBr. The product was dried in a vacuum oven at 80 °C for 24 h, giving MWCNTs@Au-g-PMAEFc organic–inorganic nanohybrids (yield: 57%).
1 (w/w)) and MAEFc (2.8985 g, 8.475 mmol) were added to a 25 ml dried Schlenk flask and then 4 ml desiccative DMF was introduced. The reaction flask was frozen, vacuumized, and then suffused with N2, and then a certain amount of HMTETA (230.5 μl, 0.8475 mmol) was injected into the flask under N2 atmosphere via a micro-syringe, followed by an immediate freeze–pump–thaw–nitrogen filling process. The purified CuBr powder (0.1459 g, 1.017 mmol) was promptly added to the system under N2 flow, followed by repeating the above freeze–pump–thaw–nitrogen filling operation. After, cooling and reflux units were connected with the reaction system, the polymerization proceeded at 90 °C for 24 h, with persistent stirring. The resulting solution was centrifuged, and repeatedly rinsed with DMF to get rid of the unreacted MAEFc and HMTETA, and then repeatedly flushed with absolute ethyl alcohol to remove CuBr. The product was dried in a vacuum oven at 80 °C for 24 h, giving MWCNTs@Au-g-PMAEFc organic–inorganic nanohybrids (yield: 57%).
2.3 Characterization and determination
Fourier-transform infrared spectra (FTIR) of the samples were recorded on an EQUINX55 Fourier transformation infrared spectrophotometer (Brucker Corp., Germany) by KBr pellets. 1H NMR analyses were performed on a 400 MHz Bruker Avance III superconducting Fourier digital NMR spectrometer (Bruker Corp., Germany) using CDCl3 as the solvent and tetramethylsilane (TMS) as the internal standard at 25 °C. The Raman spectra were recorded on an ALMEGA dispersive Raman spectrometer (ALMEGA-TM, Therm Nicolet Corp., USA) using Ar+ laser with an excitation wavelength of 532 nm. X-ray diffraction (XRD) studies were performed on a D/Max-2550 VB+/PC full-automatic X-ray diffractometer (Rigaku Corp., Japan) employing Cu radiation (λ = 0.15418 nm) at a voltage of 40 kV, current of 30 mA, and scanning rate of 10° min−1. Thermal gravimetric analysis (TGA) was carried out on a Q1000DSC + LNCS + FACS Q600SDT thermoanalyzer system (TA Corp, USA) at a heating rate of 10 °C min−1 under a nitrogen atmosphere (flow rate: 40 ml min−1) at a heating rate of 10 °C min−1 from room temperature to 800 °C. X-Ray photoelectron spectroscopy (XPS) analysis was conducted to measure the elemental composition and contents of the prepared samples on a multifunctional imaging X-ray photoelectron spectrometer (XPS, AXIS ULTRA, Kratos Analytical Ltd (A wholly-owned subsidiary of Shimadzu Corp.), Japan) using a monochromatic Al-Kα X-ray source (1486.6 eV) at a voltage of 15 kV.UV-vis absorption spectra were recorded on a UV-3900/3900H UV-vis spectrophotometer (Hitachi, Japan) to examine the dispersion behavior of the prepared nanohybrids in ethanol solution, and to confirm the formation of the nanohybrids. The wavelength for measurement was 325 nm. A SU-8020 cold-field emission scanning electron microscope (FESEM, Hitachi High-Technologies Corp, Tokyo, Japan) was adopted to observe the surface morphologies of the MWCNTs@Au and MWCNTs@Au-g-PMAEFc nanohybrids and to estimate the system composition. The whole operation was conducted at an operating voltage of 1.0–15 kV. A transmission electron microscope (TEM, JEM H-600, Electron Corp., Japan) was used to inspect the morphologies, sizes, and size distribution of the samples, with an accelerating potential of 200 kV. Before observation, the samples were ground into powders, and were dispersed in chloroform under ultrasonication for 30–60 min. One drop of the sample dispersion was uniformly dripped on carbon-based supporting films, and another drop was added after natural drying. An energy-dispersive X-ray spectrometer (EDX) attached to TEM was used to detect the elemental compositions.
2.4 Preparation of the modified electrodes and electrochemical determination
To determine the electrochemical properties of the MWCNTs@Au-g-PMAEFc nanohybrids, the modified electrode films were first prepared as follows. The MWCNTs@Au-g-PMAEFc nanohybrids were dispersed in CHCl3 under ultrasonication for 30–60 min to form a uniform suspension solution, and then the suspension solution of 6 μl was dripped onto the surface of the treated naked glass carbon electrodes (GCEs). The modified electrodes were placed at room temperature for solvent evaporation. To obtain a better film-forming ability, 6 μl of a 1% Nafion solution was dripped onto the surface of the modified GCE and dried in air. After that, the redox properties of the nanohybrid samples were measured in PBS solution of pH = 7 by cyclic voltammetry (CV). The residual amount of trichlorfon and the linear relationship between the peak current and the concentration of trichlorfon were detected by differential pulse voltammetry (DPV) using 0.2 M PBS solution containing a certain concentration of trichlorfon organophosphorous insecticides as electrolytes.The CV and DPV experiments were performed on a CHI660E electrochemical workstation (Chenhua Instrument Co., Ltd, Shanghai, China), with a conventional three-electrode system consisting of bare and modified glassy carbon electrodes (GCEs) (4.0 mm in diameter) as a working electrode, a saturated calomel electrode as a reference electrode, and a platinum wire electrode as a counter electrode. The detection of the bare GCE was conducted in an oxidation–reduction probe solution (ORPS) consisting of K3Fe(CN)6/K4Fe(CN)6/KCl in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (pH = 7.0) as supporting electrolytes.36 The DPV measurements were performed at an amplitude of 25 mV; a pulse width and pulse period of 0.05 and 0.05 s, respectively; a scan rate of 15 mV s−1; an accumulation potential of 1.15 V; an accumulation time of 100 s, and a potential increment of 0.001 V.
100 (pH = 7.0) as supporting electrolytes.36 The DPV measurements were performed at an amplitude of 25 mV; a pulse width and pulse period of 0.05 and 0.05 s, respectively; a scan rate of 15 mV s−1; an accumulation potential of 1.15 V; an accumulation time of 100 s, and a potential increment of 0.001 V.
3. Results and discussion
3.1 Characterization of the MWCNTs@Au-g-PMAEFc nanohybrids
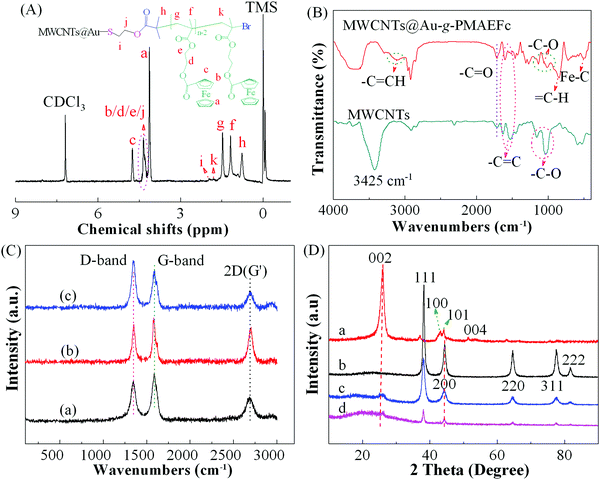
1H NMR is an important tool for analyzing the chemical composition and structure of copolymers, and was used here to confirm the preparation of the MWCNTs@Au-g-PMAEFc organic–inorganic nanohybrids. As shown in Fig. 1(A), the nanohybrid produces characteristic chemical shifts at 4.76, 4.35, and 4.13 ppm, assigned to the hydrogen proton shift signals in dicyclopentadienyl rings (Cp). The shift peaks at about 4.35 ppm are attributed to the overlapping of the methylene proton signals near the main chains (2H, m, Cp–OCOCH2–![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2OCO–) and close to Cp (2H, m, Cp–OCO
2OCO–) and close to Cp (2H, m, Cp–OCO![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2–CH2OCO–), and the 2H shift peak in the structure –SCH2–
2–CH2OCO–), and the 2H shift peak in the structure –SCH2–![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2O–. These results are in line with those of the monomer ferrocenes in Fig. S1 (ESI†). In comparison with the MAEFc, which exhibits split hydrogen proton signals on the terminal vinyl groups (2H, d, –OOC(CH3)
2O–. These results are in line with those of the monomer ferrocenes in Fig. S1 (ESI†). In comparison with the MAEFc, which exhibits split hydrogen proton signals on the terminal vinyl groups (2H, d, –OOC(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2) at 5.64 and 6.21 ppm, the two shift peaks disappear in the 1H NMR of the nanohybrid; meanwhile new signal peaks are produced at 1.48 (2H, s, –
2) at 5.64 and 6.21 ppm, the two shift peaks disappear in the 1H NMR of the nanohybrid; meanwhile new signal peaks are produced at 1.48 (2H, s, –![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2 in the main chains) and 1.19 (3H, s, –
2 in the main chains) and 1.19 (3H, s, –![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3 in the side chains of PMAEFc). The change of these shifted peaks is due to the transformation of double bonds into saturated single bonds, testifying the polymerization of MAEFc through ATRP.37–39 In addition, the shift signals at 1.96, 1.81, and 0.77 ppm are ascribed to the methylene close to the S atoms (2H, –S–
3 in the side chains of PMAEFc). The change of these shifted peaks is due to the transformation of double bonds into saturated single bonds, testifying the polymerization of MAEFc through ATRP.37–39 In addition, the shift signals at 1.96, 1.81, and 0.77 ppm are ascribed to the methylene close to the S atoms (2H, –S–![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH2–OOC–), the methylene near the terminal Br atom (2H, –
2CH2–OOC–), the methylene near the terminal Br atom (2H, –![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2C(CH3)–Br), and the methyl proton signals between the main chains and –OCO groups (6H, –OCOC(
2C(CH3)–Br), and the methyl proton signals between the main chains and –OCO groups (6H, –OCOC(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2(MAEFc)Br), respectively. By means of the integral area ratios of the shift peak at 1.96 or 0.77 ppm to 4.76 ppm, the grafting percentage is estimated to be about 8.1% and the percent conversion of monomer is about 16.2% in the case of [MWCNTs@Au-Br]
3)2(MAEFc)Br), respectively. By means of the integral area ratios of the shift peak at 1.96 or 0.77 ppm to 4.76 ppm, the grafting percentage is estimated to be about 8.1% and the percent conversion of monomer is about 16.2% in the case of [MWCNTs@Au-Br]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [MAEFc] of 1
[MAEFc] of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (mole ratios), here named MWCNTs@Au-g-PMAEFc-1. Similarly, the nanohybrid for [MWCNTs@Au-Br]
5 (mole ratios), here named MWCNTs@Au-g-PMAEFc-1. Similarly, the nanohybrid for [MWCNTs@Au-Br]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [MAEFc] of 1
[MAEFc] of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 has a graft percentage of 38.0% and percent conversion of 25.3% by NMR, and here is named MWCNTs@Au-g-PMAEFc-2.
150 has a graft percentage of 38.0% and percent conversion of 25.3% by NMR, and here is named MWCNTs@Au-g-PMAEFc-2.
Fig. 1(B) illustrates the FTIR spectra of the MWCNTs and the resultant nanohybrids. The original pristine MWCNTs generate characteristic absorption peaks at 2915 and 2845 cm−1, attributed to the sp3 C–H and sp2 C–H stretching vibrations originating from the defects on the MWCNTs at the sidewalls;40 this conveys there are abundant reaction sites. The vibration bands at 1535 and 1630 cm−1 are assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of the hexagonal or benzenoid (νC
C stretching of the hexagonal or benzenoid (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) structure of the MWCNTs, indicating the graphite structure of the MWCNTs.41,42 The vibration bands at 3425, 1715, and 1025–1160 cm−1 are ascribed to the –OH, –C
C) structure of the MWCNTs, indicating the graphite structure of the MWCNTs.41,42 The vibration bands at 3425, 1715, and 1025–1160 cm−1 are ascribed to the –OH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–O stretch modes, respectively, which may be due to the moisture absorbed from air by MWCNTs and the residues of carboxylic groups during preparation. For the MWCNTs@Au-g-PMAEFc nanohybrid, several new vibration bands emerge at 3120, 850, and 479–515 cm−1, which correspond to the
O, and C–O stretch modes, respectively, which may be due to the moisture absorbed from air by MWCNTs and the residues of carboxylic groups during preparation. For the MWCNTs@Au-g-PMAEFc nanohybrid, several new vibration bands emerge at 3120, 850, and 479–515 cm−1, which correspond to the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H stretch, the
C–H stretch, the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H bending, and Fe–C or Cp–Fe stretch modes in ferrocene rings, respectively. The bands at 2850–2960, 1716, and 1015–1140 are ascribed to the C–H, C
C–H bending, and Fe–C or Cp–Fe stretch modes in ferrocene rings, respectively. The bands at 2850–2960, 1716, and 1015–1140 are ascribed to the C–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–O stretch modes, respectively. However, the peak intensity at 3100–3200 cm−1 is weakened in comparison with monomer MAEFc in Fig. S1(c) of the ESI† because the terminal double bonds turn into saturated single bonds after ATRP polymerization. In addition, the characteristic stretch vibration band at 1605 cm−1 is attributed to the benzenoid (νC
O, and C–O stretch modes, respectively. However, the peak intensity at 3100–3200 cm−1 is weakened in comparison with monomer MAEFc in Fig. S1(c) of the ESI† because the terminal double bonds turn into saturated single bonds after ATRP polymerization. In addition, the characteristic stretch vibration band at 1605 cm−1 is attributed to the benzenoid (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) structure in MWCNTs’ backbones, confirming the preparation of MWCNTs@Au-g-PMAEFc organic–inorganic nanohybrids.
C) structure in MWCNTs’ backbones, confirming the preparation of MWCNTs@Au-g-PMAEFc organic–inorganic nanohybrids.
Raman spectra were measured to further confirm the preparation of the nanohybrids, as displayed in Fig. 1(C). It can be clearly seen that the MWCNTs exhibit three typical vibration modes, including a D line at about 1346 cm−1, and G band at ca. 1587 cm−1, which correspond to the vibrations of amorphous carbon defects and disorder-induced sp3-hybridized carbon atoms, and of sp2-hybridized carbon atoms (E2g stretching mode) in a graphitic layer, respectively.43 The peak at 2690 cm−1 is assigned to the 2D frequency peak of graphene (the second-order Raman vibration peak of D* mode, also called the G′ peak).44,45 The relative peak intensity ratio of the D to G band (ID![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IG) was calculated to be about 0.85, suggesting that some defects exist in MWCNTs;42,46 which could be expected to make the Au NPs more favorably load on the surface of MWCNTs. In the case of MWCNTs@Au and MWCNTs@Au-g-PMAEFc, the three vibration bands ascribed to MWCNTs remain the same. The intensities of D peaks are, however, significantly increased, and the ID
IG) was calculated to be about 0.85, suggesting that some defects exist in MWCNTs;42,46 which could be expected to make the Au NPs more favorably load on the surface of MWCNTs. In the case of MWCNTs@Au and MWCNTs@Au-g-PMAEFc, the three vibration bands ascribed to MWCNTs remain the same. The intensities of D peaks are, however, significantly increased, and the ID![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IG intensity ratios increased up to 0.94 and 1.08, respectively. This indicates that Au NPs are loaded onto the surface or defect dots of MWCNTs, forming non-covalently bonded MWCNTs@Au nanocomposites, and that PMAEFc is covalently grafted onto the surface of MWCNTs@Au and/or MWCNTs.
IG intensity ratios increased up to 0.94 and 1.08, respectively. This indicates that Au NPs are loaded onto the surface or defect dots of MWCNTs, forming non-covalently bonded MWCNTs@Au nanocomposites, and that PMAEFc is covalently grafted onto the surface of MWCNTs@Au and/or MWCNTs.
XRD analysis was carried out to obtain information on the crystalline phase structure of the prepared nanohybrids, as shown in Fig. 1(D). The as-grown MWCNTs generate a sharp and strong diffraction peak at 2θ of ca. 25.9°, corresponding to the (002) diffraction plane of the graphite structure. Several other characteristic diffraction peaks appear at 43.0°, 44.1°, and 51.4°, attributed to the (100), (101), and (004) diffraction planes, respectively.47 In the XRD spectra of Au NPs, several main diffraction peaks emerge at about 38.2°, 44.4°, 64.6°, 77.5°, and 81.7°, which correspond to the (111), (200), (220), (311), and (222) phases, respectively.48,49 The average crystal size was calculated to be about 11.5 nm through the Scherrer formula based on the (111) diffraction plane showing strong diffraction. In the XRD patterns of MWCNTs@Au, the characteristic diffraction peaks of both MWCNTs and Au NPs can be observed; appearing at about 25.8° and 44.2° (MWCNTs) and 38.1°, 44.2°, 64.5°, 77.6°, and 81.7° (Au NPs), respectively. However, the peak intensities are weakened due to the non-covalent interaction between MWCNTs and Au NPs, as stated before. In the presence of MWCNTs@Au-g-PMAEFc, a wide diffuse scattering peak emerges at 2θ of 14.1°–34.0° assigned to PMAEFc moieties in addition to the characteristic diffraction peaks of MWCNTs and Au NPs. Since a large number of PMAEFc moieties are grafted on the surface of the MWCNTs@Au nanocomposites, the peak intensities are weaker than their precursors.
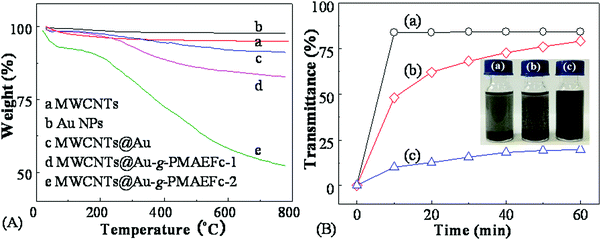
TGA analysis was performed to investigate the thermal decomposition behavior of MWCNTs@Au-g-PMAEFc nanohybrids, as illustrated in Fig. 2(A). It is clearly noticed that both MWCNTs and Au NPs are thermostable, and mass losses of only about 5.0% and 2.1% are produced separately until 800 °C. As such, it is understandable that MWCNTs@Au nanocomposites also have good thermostability with only approximately 8.7% mass loss up to 800 °C. A slight increase in the mass loss is maybe caused by the absorbed moisture, dust, and thermo-labile contaminants or functional groups during the preparation process.50 The thermal events of MWCNTs@Au-g-PMAEFc can be divided into two stages: a mass loss of ca. 3.1 for MWCNTs@Au-g-PMAEFc-1 and 10.2% for MWCNTs@Au-g-PMAEFc-2 until 200 °C, which is ascribed to the volatilization of the residual solvents and reagents, etc. The follow-up weight loss is attributed to the decomposition of the side-chain carbonyl groups and the skeleton in the nanohybrids. Until 800 °C, the mass loss reaches about 17.2% for MWCNTs@Au-g-PMAEFc-1 and 47.8% for MWCNTs@Au-g-PMAEFc-2. Considering that the theoretical content of Fe in PMAEFc is about 17%, it is estimated that the graft percentage of PMAEFc on the surface of MWCNTs@Au or MECNTs is about 7.0% and 33.2% for feed ratios of MWCNTs@Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MAEFc of 1
MAEFc of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and 1
50 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150, respectively. These values are slightly lower than those by 1H NMR.
150, respectively. These values are slightly lower than those by 1H NMR.
3.2 Dispersion behavior and morphologies
To evaluate the effect of the ATRP graft polymerization of ferrocene derivates MAEFc on the dispersion behavior of MWCNTs, the change of the transmittance with the centrifugation time in chloroform at room temperature and a centrifugation rate of 4000 rpm was compared for the MWCNTs, MWCNTs@Au, and MWCNTs@Au-g-PMAEFc nanohybrids, as shown in Fig. 2(B) and the corresponding insets. It can be clearly noticed that the original pristine MWCNTs rapidly subside under centrifugation, and the transmittance reach about 84% after centrifugation for 10 min, presenting poor dispersivity. The dispersion behavior of MWCNTs@Au nanocomposites is somewhat improved. The transmittance is only about 48% after centrifugation for 10 min, and gradually increases up to ca. 79% after 60 min. Only a part of the sediments can be seen at the bottom of the container. The main reason for this can probably be ascribed to the combination of Au NPs with good dispersity on the surface of MWCNTs via non-covalent interactions, which improves the dispersion behavior of the MWCNTs. Nonetheless, the MWCNTs still have a high surface free energy, and the improvement effect is limited. By contrast, the MWCNTs@Au-g-PMAEFc nanohybrids possess good dispersibility in CHCl3. The transmittance is only about 10% after centrifugation for 10 min, and only ca. 20% after 60 min, and almost no subsidence occurs because the soluble PMAEFc molecular chains prohibit the MWCNTs or MWCNTs@Au from aggregation and sedimentation. This signifies that the grafting of PMAEFc on the surface of MWCNTs@Au nanocomposites can significantly improve the dispersity of the MWCNTs.TEM was used to observe the morphologies, sizes, and size distribution of the prepared MWCNTs@Au-g-PMAEFc, as depicted in Fig. 3. The original pristine MWCNTs were randomly intertwined together due to the van der Waals forces between the MWCNTs, with a tubular diameter of 12–22 nm and a mean diameter of 17 nm, as specified by the manufacturer. Au NPs assume homogeneous globular mophologies, with a uniform particle size distribution, with most of them ranging from about 7 to 15 nm, with an average size of ca. 12 nm. After the in situ reduction, it could be observed that the spherical Au NPs were not evenly deposited and decorated on the tube walls of the MWCNTs, as demonstrated in Fig. 3(c). On this occasion, the defect points on the surface of MWCNTs were exactly the growth points of the Au NPs, leading to the growth of a mass of Au NPs grains on the ektexines of MWCNTs.51 Moreover, since the MWCNTs possess strong adsorptivity, large micropores, large specific areas, particularly the π-electrons on the surface, residual –COOH groups during preparation, and moisture absorbed from air (as proved by FTIR), Au3+ cations are able to attach onto the surface of the MWCNTs via electrostatic attraction interactions or strong non-covalent supramolecular interactions.52,53 After the Au3+ ions are reduced, Au NPs are tightly decorated onto the MWCNTs; whereas the heterogeneity of distribution for Au NPs is probably due to the unevenness of the oxygen moieties distribution. Anyway, they are indeed decorated on the surface of MWCNTs, and meanwhile not discovered elsewhere. To further verify the growth of Au NPs on the surface of MWCNTs, the element composition was analyzed using an energy-dispersive X-ray (EDX) spectrometer, as depicted in Fig. S2 of the ESI.† The observed sample was made of gold and carbon elements ascribed to MWCNTs and Au NPs. This manifests how the MWCNTs@Au nanocomposites were prepared via an in situ reduction way. In the TEM image of the MWCNTs@Au-g-PMAEFc nanohybrids in Fig. 3(d), the tubular topologies of MWCNTs can still be observed, but the boundaries between the inside and outside tubes become blurred, and the diameter of the tubes is significantly widened, which is an indicator of MWCNTs coated or covered by polymer matrices. Further observation reveals that there are some spherical grains that are uniformly decorated or scattered on the surface of the MWCNTs or MWCNTs coating with PMAEFc, which is proof of the existence of Au NPs in PMAEFc moieties covering the MWCNTs. EDX was also used to confirm the chemical composition of the nanohybrid sample, as illustrated in Fig. S3 (ESI†). The appearance of the elements C, O, and Fe indicates the formation of PMAEFc covering layers on the surface of MWCNTs, while the element Au is ascribed to the Au NPs. The mass percentages of C, O, Fe, and Au were representatively 78.92%, 10.84%, 2.31%, and 7.93%, which construct the MWCNTs@Au-g-PMAEFc nanohybrid. Considering the strong non-covalent binding of Au NPs with MWCNTs, the grafting PMAEFc moieties also bond with the MWCNTs via strong non-covalent supramolecular interactions.53–55 Therefore, the separation between MWCNTs and the functional polymers does not exist, by and large. Moreover, we did not observe any precipitates at the bottom of the container with the naked eye or by UV-vis transmittance measurements during a considerably long time period.
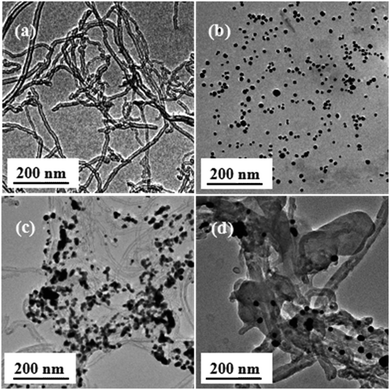 | ||
| Fig. 3 TEM images of (a) MWCNTs, (b) Au NPs, (c) MWCNTs@Au nanocomposites, and (d) MWCNTs@Au-g-PMAEFc nanohybrids. | ||
FESEM was further used to observe the morphological features of the MWCNTs, MWCNTs@Au, and MWCNTs@Au-g-PMAEFc, as depicted in Fig. 6. MWCNTs can be observed to assume a randomly-distributed, intertwined, and jumbled status, with a pipe diameter ranging from about 15 to 20 nm. The Au NPs present uniform spherical morphologies, and give a globular size of approximately 7–13 nm, as demonstrated in Fig. 4(b). These observations are consistent with the results estimated by TEM. In the case of MWCNTs@Au nanocomposites in Fig. 4(c), it can be clearly observed that Au NPs are uniformly decorated and loaded on the surface of MWCNTs along its length, by and large, and form rough surfaces, which is significantly different from the smooth surface of the original pristine MWCNTs. Our observations indicate that Au NPs are in situ deposited onto the MWCMTs. MWCNTs@Au-g-PMAEFc shows a unique morphological trait. A layer of thick polymers is observed to tightly wrap the MWCNTs@Au nanocomposites or MWCNTs, and the pipe diameter of MWCNTs is remarkably thickened. Although the MWCNTs@Au nanocomposites are almost covered with the polymer layer, some Au NPs can be clearly seen to be exposed on the polymer coating layer covering the MWCNTs@Au. A large fraction of Au NPs are wrapped in whole polymer layers. The MWCNTs@Au-g-PMAEFc nanohybrid with this morphology can be well dispersed, and is expected to produce good electrochemical properties via a synergistic effect among these components.
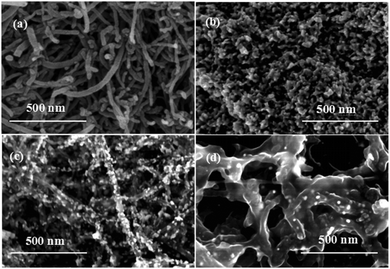 | ||
| Fig. 4 FESEM images of (a) MWCNTs, (b) Au NPs, (c) MWCNTs@Au nanocomposites, and (d) MWCNTs@Au-g-PMAEFc nanohybrids. | ||
3.3 UV-vis absorption spectra analyses
Fig. 5 shows the UV-vis absorption spectra of various materials in anhydrous ethanol. It can be noticed that the MWCNTs produce a weak absorption peak at ca. 264 nm, which keeps extending along the absorption edge of the entire visible spectrum.56 Au sols possess a strong absorption peak at about 525 nm, which is the characteristic plasmon resonance peak of Au NPs.57,58 For the MWCNTs@Au nanocomposites, the characteristic absorption peaks of MWCNTs and Au NPs appear at 270 and 540 nm, respectively. In comparison with bare MWCNTs and Au NPs, a slight red shift of the two absorption peaks discloses that there exists a certain interaction between the MWCNTs and Au NPs, which may be proof of the formation of MWCNTs@Au nanocomposites. Another possible explanation is that charge transfer exists between the Au NPs and –SH groups and the electron deficiency on the surface of Au NPs may have led to the red shift.59 The plasmon resonance peak intensities of Au NPs at 540 nm gradually increase with enhancing the amounts of Au NPs, as shown in Fig. 5(B). When the mass ratios of MWCNTs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au reach 1
Au reach 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the nanocomposite bears a maximal absorption peak intensity. This may be due to the fact that Au NPs have more loading (effect) on the surface of MWCNTs, which in turn gives rise to the increase in the UV absorption peak intensities.60 The UV-vis absorption spectra of the resultant nanohybrids in Fig. 5(A)d give the main characteristic peaks of both MWCNTs and Au NPs, and these are slightly red shifted to about 270 and 535 nm, respectively. In particular, two new absorption peaks emerge at 323 and 445 nm, which reflect the characteristic electronic spectra of ferrocenes. The generation of the two peaks is attributed to the symmetry prohibition transition of electrons from the non-bonding e2g and a1g levels, respectively, to the e1g* antibonding level; the 3d orbit of irons in this orbit exists in the form of reduction states in ferrocene groups. In comparison with the strong absorption peaks at 308 and 444 nm of the ferrocenyl monomer (derivative) MAEFc in Fig. 5(A)e, the peak intensities are somewhat reduced, and red shifts appear at different levels after the ATRP grafting polymerization reaction on the surface of the MWCNTs@Au. This signifies that certain covalent and noncovalent interactions exist among the three components MWCNTs, Au NPs, and PMAEFc, and that PMAEFc is actually grafted on the surface of MWCNTs@Au nanocomposites, forming MWCNTs@Au-g-PMAEFc nanohybrids.
4, the nanocomposite bears a maximal absorption peak intensity. This may be due to the fact that Au NPs have more loading (effect) on the surface of MWCNTs, which in turn gives rise to the increase in the UV absorption peak intensities.60 The UV-vis absorption spectra of the resultant nanohybrids in Fig. 5(A)d give the main characteristic peaks of both MWCNTs and Au NPs, and these are slightly red shifted to about 270 and 535 nm, respectively. In particular, two new absorption peaks emerge at 323 and 445 nm, which reflect the characteristic electronic spectra of ferrocenes. The generation of the two peaks is attributed to the symmetry prohibition transition of electrons from the non-bonding e2g and a1g levels, respectively, to the e1g* antibonding level; the 3d orbit of irons in this orbit exists in the form of reduction states in ferrocene groups. In comparison with the strong absorption peaks at 308 and 444 nm of the ferrocenyl monomer (derivative) MAEFc in Fig. 5(A)e, the peak intensities are somewhat reduced, and red shifts appear at different levels after the ATRP grafting polymerization reaction on the surface of the MWCNTs@Au. This signifies that certain covalent and noncovalent interactions exist among the three components MWCNTs, Au NPs, and PMAEFc, and that PMAEFc is actually grafted on the surface of MWCNTs@Au nanocomposites, forming MWCNTs@Au-g-PMAEFc nanohybrids.
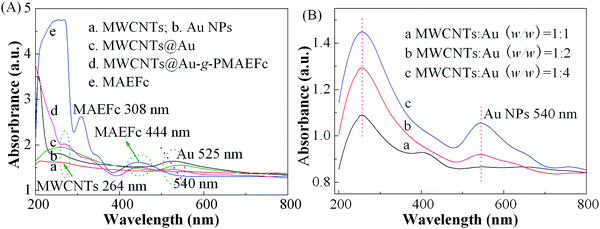 | ||
| Fig. 5 UV-vis absorption spectra of (A) various materials and (B) MWCNTs@Au with various mass ratios of MWCNTs to Au NPs in anhydrous ethanol (concentration: 0.01 mg ml−1). | ||
3.4 XPS analysis
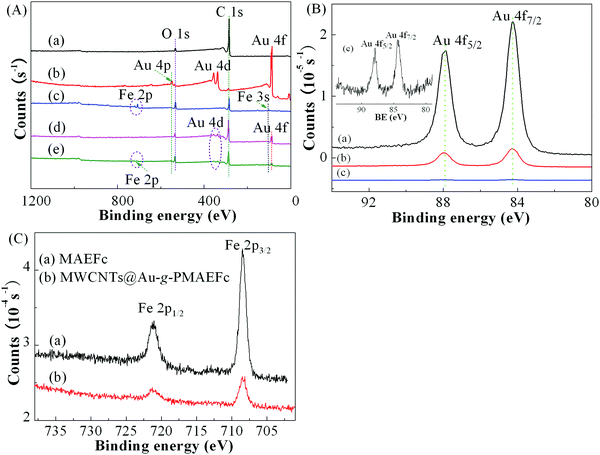
To further investigate the change of chemical composition and surface functional groups of the resultant nanohybrids, and to verify the grafting of PMAEFc on the surface of MWCNTs, XPS measurements were conducted, as depicted in Fig. 6. It can be noticed from the wide-survey XPS spectra in Fig. 6(A) that the MWCNTs produce two photoelectronic signal peaks at binding energies (BEs) of about 284.9 and 531.6 eV, which are assigned to C 1s and O 1s signals, and their atomic ratios are 97.4% and 2.6%, respectively. Au NPs give several characteristic signal peaks at BEs of 87.7, 337.2, 356.5, and 550.4 eV attributed to Au 4f, Au 4d5/2, Au 4d3/2, and Au 4p photoelectrons, respectively, of which Au 4f possesses the highest atomic ratios and signal peak intensities at 87.7 eV.61 The Au 4f core-level spectrum in Fig. 6(B) shows that two split signal peaks emerge at BEs of ca. 84.3 and 87.9 eV, corresponding to the photoelectron peaks of Au 4f7/2 and Au 4f5/2 orbits in Au0 NPs in the metal state. After Au NPs grow on the surface of MWCNTs, as shown in Fig. 6(A)d, new signal peaks appear at BEs of around 88.3, 337.2, 356.9, and 550.4 eV in addition to the C 1s and O 1s peaks, ascribed to Au 4f, Au 4d5/2, Au 4d3/2, and Au 4p photoelectrons, respectively. In comparison with the signal peaks of Au NPs, the intensities of the photoelectron signal peaks of Au elements in MWCNTs@Au are distinctly weakened, as illustrated in Fig. 6A(d) and B(b); this is due to the growth of Au atoms on the surface of MWCNTs and the resulting strong electron interactions between the nanotubes and Au atoms. These findings confirm that the Au NPs have successfully covered the surface of the MWCNTs, forming MWCNTs@Au nanocomposites. In Fig. 6A(e), new signal peaks are further detected at BEs of 54.7, 103.6, and 711.2–721.3 eV, separately attributed to Fe 3p, Fe 3s, and Fe 2p photoelectrons; this is in agreement with the XPS spectra of the monomer MAEFc in Fig. 6A(c).62–64 In addition, the signal peaks from C 1s, O 1s, Au 4f, Au 4d5/2, Au 4d3/2, and Au 4p electrons can also be clearly identified from Fig. 6A(e), but the signal intensities of Au 4f and Fe 2p electrons in MWCNTs@Au-g-PMAEFc recede in comparison with the Au NPs and MAEFc. In particular, it can be noticed from the high-resolution XPS spectra of Au 4f in Fig. 6(B)c that the signal peaks of Au NPs in the final nanohybrids are even weaker than those in the MWCNTs@Au in Fig. 6(B)b; but it can still be seen from the inset of Fig. 6(B) that the Au 4f7/2 and Au 4f5/2 signal peaks in MWCNTs@Au-g-PMAEFc emerge at BEs of ca. 84.3 and 87.9 eV, respectively. This weakening of the signal peak intensities may be caused by the inclusion of Au NPs in a majority of PMAEFc. The Fe 2p core-level XPS spectrum is depicted in Fig. 6(C). The two peaks at BEs of 708.4 and 721.3 eV correspond to the photoelectronic energy spectra of Fe 2p3/2 and Fe 2p1/2 orbits,62–64 and the signal intensities of Fe 2p electrons in MWCNTs@Au-g-PMAEFc are obviously lower than those in the monomer MAEFc. These results indicate that the PMAEFc moieties have successfully been grafted onto the surface of MWCNTs or MWCNTs@Au, offering the resultant organic–inorganic nanohybrids. The insights into the chemical states or electronic structural information of surface atoms by O 1s and C 1s core-level XPS spectra further corroborate the above deduction, as shown in Fig. S4 of the ESI.†3.5 Electrochemical behavior
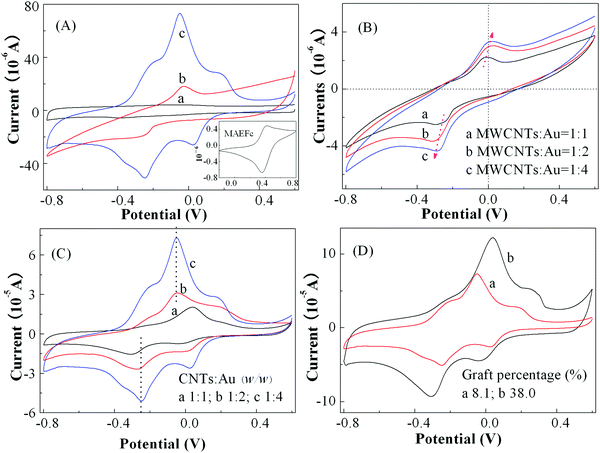
To understand the electrochemical response behavior of the prepared nanohybrids, the voltage–current characteristics of various materials were measured in PBS of pH = 7 with a concentration of 0.2 M as electrolyte solutions, and the results are demonstrated in Fig. 7(A). It can be noticed that no obvious redox signal was detected for the original pristine MWCNTs; whereas MWCNTs@Au nanocomposites with the feed ratio of MWCNTs to HAuCl4·3H2O of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 generated an obvious redox current at the anode voltage (Ep,a) of −0.028 V and cathode voltage Ep,c of −0.28 V. The redox reversibility was, however, poor because of a larger peak separation or potential difference between the anodes and cathodes (ΔEp = Ep,a − Ep,c) of about 252 mV and the poorer symmetry of the redox curves. In contrast, in the CV curves of MWCNTs@Au-g-PMAEFc (MWCNTs
4 generated an obvious redox current at the anode voltage (Ep,a) of −0.028 V and cathode voltage Ep,c of −0.28 V. The redox reversibility was, however, poor because of a larger peak separation or potential difference between the anodes and cathodes (ΔEp = Ep,a − Ep,c) of about 252 mV and the poorer symmetry of the redox curves. In contrast, in the CV curves of MWCNTs@Au-g-PMAEFc (MWCNTs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au = 1
Au = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and graft percentage of about 39.1%), the redox peak currents arise not only at Ep,c = −0.25 V and Ep,a = −0.05 V attributed to Au NPs (ΔEp = 200 mV), but also at Ep,c = 0.03 V and Ep,a = 0.18 V (ΔEp = 150 mV) assigned to the PMAEFc moieties as well. In addition, the new redox electrochemical process appears at approximately −0.21 V of Ep,a for a pre-wave and −0.38 V of Ep,c for a post-wave. These data suggest that there probably exists an interaction between the Au NPs and PMAEFc moieties. It could be further noticed that the peak currents of Au NPs are increased 3 or 4 times over those of MWCNTs@Au. Considering the drop of the peak potential differences of Au NPs from 252 to 200 mV after the PMAEFc polymers are grafted onto the surface of MWCNTs@Au, the reversibility of the redox processes is improved for Au NPs. In contrast, the increase in ΔE of PMAEFc segments from 60 to 150 mV in comparison with that of the monomer (Ep,c = 0.36 V, Ep,a = 0.42 V, and ΔE is 60 mV) leads to a decreased redox reversibility for the PMAEFc moieties. Nonetheless, the redox electrochemical processes for the PMAEFc moieties remain quasi-reversible. In addition, the anode and cathode peak current ratio (Ip,a/Ip,c) was found to be close to integer, 1.07 for the PMAEFc moieties and 0.91 for Au NPs, further corroborating the quasi-reversibility of the redox electrochemical process. This change can be ascribed to intense covalent interactions existing between PMAEFc and MWCNTs@Au after the surface grafting of PMAEFc.65 This interaction also makes the redox potentials of the ferrocene segments decrease, and thus oxidation–reduction is more likely to take place in the PMAEFc moieties. In conclusion, the nanohybrids have clearly enhanced electrochemical properties and dislay a quasi-reversible redox electrochemical process to become a fine modified electrode material.
4, and graft percentage of about 39.1%), the redox peak currents arise not only at Ep,c = −0.25 V and Ep,a = −0.05 V attributed to Au NPs (ΔEp = 200 mV), but also at Ep,c = 0.03 V and Ep,a = 0.18 V (ΔEp = 150 mV) assigned to the PMAEFc moieties as well. In addition, the new redox electrochemical process appears at approximately −0.21 V of Ep,a for a pre-wave and −0.38 V of Ep,c for a post-wave. These data suggest that there probably exists an interaction between the Au NPs and PMAEFc moieties. It could be further noticed that the peak currents of Au NPs are increased 3 or 4 times over those of MWCNTs@Au. Considering the drop of the peak potential differences of Au NPs from 252 to 200 mV after the PMAEFc polymers are grafted onto the surface of MWCNTs@Au, the reversibility of the redox processes is improved for Au NPs. In contrast, the increase in ΔE of PMAEFc segments from 60 to 150 mV in comparison with that of the monomer (Ep,c = 0.36 V, Ep,a = 0.42 V, and ΔE is 60 mV) leads to a decreased redox reversibility for the PMAEFc moieties. Nonetheless, the redox electrochemical processes for the PMAEFc moieties remain quasi-reversible. In addition, the anode and cathode peak current ratio (Ip,a/Ip,c) was found to be close to integer, 1.07 for the PMAEFc moieties and 0.91 for Au NPs, further corroborating the quasi-reversibility of the redox electrochemical process. This change can be ascribed to intense covalent interactions existing between PMAEFc and MWCNTs@Au after the surface grafting of PMAEFc.65 This interaction also makes the redox potentials of the ferrocene segments decrease, and thus oxidation–reduction is more likely to take place in the PMAEFc moieties. In conclusion, the nanohybrids have clearly enhanced electrochemical properties and dislay a quasi-reversible redox electrochemical process to become a fine modified electrode material.
In consideration of the poor reversibility of the electrochemical process of the aforementioned MWCNTs@Au nanocomposites, the electrochemical properties were modulated by changing the composition ratios of MWCNTs to Au NPs to acquire better redox characteristics, and the results are shown in Fig. 7(B). It can be clearly seen that all the MWCNTs@Au nanocomposites with various component ratios exhibit obvious redox electrochemical properties. With increasing the mass ratios of MWCNTs to HAuCl4·3H2O or the contents of Au NPs, the redox peaks are more obvious, and the peak currents are stronger, offering better electrochemical responsiveness; this is consistent with the increase in the peak intensities in the UV-vis absorption spectra. However, as the contents of Au NPs increase, the potentials of the oxidation peaks and reduction peaks shift for the anodes and cathodes, respectively, and the ΔEp value is increased from about 245 to 303 mV, and the Ip,a/Ip,c ratios decrease from 0.9 to 0.83 and 0.77; this makes the redox process harder, and the reversibility becomes poorer. Clearly, to obtain MWCNTs@Au with superior comprehensive properties, it is necessary to reasonably modulate the composition proportions of the two constituents or the contents of the Au NPs.
The effect of the mass ratios of MWCNTs to Au NPs on the redox electrochemical properties of the nanohybrids was further investigated in Fig. 7(C). It can be noticed that with increasing the content of Au NPs, the symmetry of the redox peaks is increased, and the redox peak currents generated by Au NPs and the grafted ferroncene segments are enhanced, whereby the oxidation peak potentials decrease while the reduction peak potentials increase. This finding further reveals that the covalent interaction between PMAEFc and MWCNTs@Au makes the redox process easily occur, and consequently the nanohybrids bear better electrochemical responsiveness. The redox potentials and ΔEp values of the nanohybrids with various compositional ratios are summarized in Table S1 (ES1†). As the mass percentage of Au NPs is increased in the nanohybrids, the ΔEp values based on Au NPs and ferrocene-containing units gradually decrease, and the peak current ratios of anodes to cathodes Ip,a/Ip,c approach to integer 1, signifying that the prepared nanohybrids possess improved symmetry and reversibility. It is hence deduced that the nanohybrids with excellent electrochemical responsiveness and responsive reversibility can be achieved by modulating the component proportions. In particular, the nanohybrid with the mass ratio of MWCNTs to HAuCl4·3H2O of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 possesses optimized redox properties and electrochemical reversibility.
4 possesses optimized redox properties and electrochemical reversibility.
The effect of the graft percentage of PMAEFc chains on the electrochemical properties of the nanohybrids was examined in Fig. 7(D). In comparison with the oxidation potential of the monomer MAEFc (Epa,Fc = 0.420 V), the oxidation peak potentials of the ferrocene-containing polymers after the graft polymerization decrease to 0.18 V for a low graft percentage and 0.25 V for the graft percentage of 38.0%; this indicates that the ferrocene groups or moieties are more easily oxidized after the graft polymerization. As the chain lengths of the graft ferrocene monomer units increase, the oxidation peak potential Ep,a and the reduction peak potential Ep,c generated by the ferrocene units and Au NPs obviously increase, and shift toward the anodes and cathodes, leading to the enlargement of the peak potential difference from 150 and 195 mV for a low graft percentage to 290 and 350 mV for a high graft percentage. This may be due to the grafting and participation of more PMAEFc redox components, which decreases the rate of mass diffusion and the charge transfer between the active sites, making the redox process become difficult.66 Hence, in spite of these obviously increased redox peak currents with increasing the length of the graft ferrocene chains, the higher ΔEp values make the reversibility of the redox process reduce. Therefore, the nanohybrid with the graft percentage of 8.1% and the mass ratio of MWCNTs to Au NPs of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 was selected as the electrode modified material for use in the detection of trace poisonous and harmful substances in real samples in this study.
4 was selected as the electrode modified material for use in the detection of trace poisonous and harmful substances in real samples in this study.
3.6 Detection of trace poisonous and harmful substances
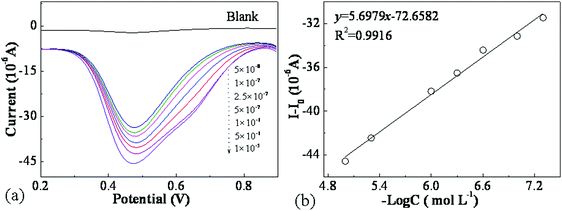
It was expected that the good electrochemical properties of the nanohybrids could be used to detect pesticide residues and/or the content of additives in food. To assess the applications of the prepared nanohybrids in the modified electrode sensors, trichlorphon residues in several fruits and vegetables were attempted to be detected by DPV under optimal test conditions. Before the determination, calibration curves of trichlorphon were prepared by detecting the peak current change with the concentrations of trichlorphon using DPV. All the experiments were carried out in 10 ml 0.2 mol L−1 PBS electrolyte solutions containing trichlorphon solutions with a wide concentration range from 1.0 × 10−5 to 5.0 × 10−8 mol L−1, and the results are shown in Fig. 8. It can be clearly observed that the anodic or oxidation peak current (Ip,a) absolute values increase linearly with the increase in the concentration of trichlorfon (C), and the linear calibration equation can be expressed as follows:Ip,a (μA) = 5.6979(−log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C) − 72.6582 (R2 = 0.9916) C) − 72.6582 (R2 = 0.9916) | (1) |
The limit of detection (LOD) is estimated using three times noise, i.e., signal/noise = 3 (S/N = 3):36
| LOD = KSb/r | (2) |
The repeatability of the MWCNTs@Au-g-PMAEFc nanohybrid films for the detection of trichlorfon was assessed by measuring 5 × 10−8 and 5 × 10−7 M trichlorfon response for six successive measurements in the same sample under identical experimental conditions, giving an intra-assay relative standard deviation (RSD) of about 0.67 and 1.21%, respectively. To simultaneously assess the sensor-to-sensor reproducibility, five modified electrodes were individually fabricated and then used to detect the DPV current response at trichlorfon concentration of 2.5 × 10−8 mol L−1, giving an inter-assay relative standard deviation (RSD) of about 1.36%. This suggests that this method has good reproducibility, and the sensor-to-sensor repeatability results are acceptable for the trace detection of trichlorfon. The long-term stability of the electrochemical sensor was evaluated by measuring the electrode response with 2.5 × 10−8 M trichlorfon in PBS of pH 7.0, and the current response of the sensor based on DPV was found to maintain about 95.3% of the initial value after storage for 15 days at 4 °C. The experimental results are displayed in Fig. S5 (ESI†).
To detect pesticide residues in real samples, apple, tomatoes, and cucumber samples of 1.5 g were cut into small pieces and put into a 50 ml beaker, and then immersed in PBS buffer solutions containing trichlorfon concentrations of 0.5 × 10−8, 1.0 × 10−8, 2.0 × 10−8, and 4.0 × 10−8 mol L−1 for 6 h. After the spiked samples were ultrasonicated in a 30 ml PBS solution of pH 7.0 for 1–2 h, they were filtrated through a 0.45 μm filter membrane to obtain extractions. The extractions were then transferred into a 50 ml volumetric flask, and diluted to the scale mark with PBS buffer solution, and then stored at 4 °C. The anodic peak current (Ip,a) of each sample was determined five times by DPV to give a mean value and then to estimate the residual amount in food, the percentage of recovery, and the relative standard deviations, as summarized in Table S2 (ESI†). It is noticed that the nanohybrid modified electrodes bear almost similar data between the measured values and the added amount of trichlorfon residues when they were used to detect a certain concentration of trichlorfon in apple, tomato, and cucumber sample solutions, offering a high recovery of 92.0–101.5%, 89.0–99.0%, and 87.0–102.5% with a low RSD value of 0.9–4.3%, 0.6–6.4%, and 1.6–5.1%, respectively. Consequently, the modified electrode sensor developed in this study was manifested to be a promising and reliable tool for the rapid, convenient, and efficient determination of trichlorfon residues in real samples, for example, food and fruits.
4. Conclusion
In summary, novel MWCNTs@Au-g-PMAEFc organic–inorganic nanohybrids were prepared via the ATRP of MAEFc using MWCNTs@Au-Br as an initiator, as confirmed by FT-IR, 1H NMR, Raman, XPS, XRD, UV-vis, and TGA, etc. UV-vis, EDX, SEM, and TEM findings revealed that the PMAEFc moieties were well grafted on the surface of MWCNTs or MWCNTs@Au, which significantly improved the dispersibility of the MWCNTs and the electrochemical properties via a synergistic effect among the components. The nanohybrids were used to fabricate electrochemical sensing thin films, and exhibited good electrochemical properties, including sensitivity, reversibility, and reproducibility, and this was dependent upon the system composition proportion of MWCNTs and Au NPs and the graft percentage of PMAEFc. The fabricated electrochemical modified electrode sensors were used to detect trace trichlorfon in food or fruits, with a wide linear range and a low detection limit at a 10−8 mol L−1 level. The developed MWCNTs@Au-g-PMAEFc organic–inorganic nanohybrid modified electrode sensors provide an accurate and reliable method for the analysis of food safety as they can be used to detect pesticide residues in real samples in a simple, rapid, and effective way.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National natural Science Foundation of China (grant 21273142, 21572123 and 21773149) and the Fundamental Research Funds for the Central Universities (GK201601003).References
- Z. Li, Y. Yu, Z. Li and T. Wu, Anal. Bioanal. Chem., 2015, 407, 2711 CrossRef CAS PubMed.
- S. Chowdhury and R. Balasubramanian, Adv. Colloid Interface Sci., 2014, 204, 35 CrossRef CAS PubMed.
- M. Battaglia, C. W. Cruywagen, T. Bertuzzi, A. Gallon, M. Moschini, G. Piva and F. Masoero, J. Dairy Sci., 2010, 93, 5338 CrossRef CAS PubMed.
- D. Meng, Y. Song, X. Fu and Y. Xu, Chin. Fishery Quality and Standards, 2014, 4, 49 Search PubMed.
- Y. Zha, C. Yang, L. Lin, T. Li and S. Cheng, Food Sci., 2011, 32, 278 CAS.
- D. W. Lachenmeier, E. Humpfer, F. Fang, B. Schütz, P. Dvortsak, C. Sproll and M. Spraul, J. Agric. Food Chem., 2009, 57, 7194 CrossRef CAS PubMed.
- R. Xiao, C. W. Wang, A. N. Zhu and F. Long, Biosens. Bioelectron., 2016, 79, 661 CrossRef CAS PubMed.
- K. B. Pradeep, S. Lakkavarapu, R. R. Kasarla and J. S. Bondili, RSC Adv., 2017, 7, 37898 RSC.
- L. Hem, S. Khay, J. H. Choi, E. D. Morgan, A. M. A. El-Aty and J. H. Shim, Toxicol. Res., 2010, 26, 149 CrossRef CAS PubMed.
- Q. Cao, H. Zhao, Y. J. He, N. Ding and J. Wang, Anal. Chim. Acta, 2010, 675, 24 CrossRef CAS PubMed.
- M. Vergara-Barberan, M. J. Lerma-Garcia, E. F. Simo-Alfonso and J. M. Herrero-Martinez, Anal. Chim. Acta, 2016, 917, 37 CrossRef CAS PubMed.
- C. Balalakshmi, K. Gopinath, M. Govindarajan, R. Lokesh, A. Arumugam, N. S. Alharbi, S. Kadaikunnan, J. M. Khaled and G. Benelli, J. Photochem. Photobiol., B, 2017, 173, 598 CrossRef CAS PubMed.
- B. Sebez, L. Su, B. Ogorevc, Y. Tong and X. Zhang, Electrochem. Commun., 2012, 25, 94 CrossRef CAS.
- A. T. Lawal, Mater. Res. Bull., 2016, 73, 308 CrossRef CAS.
- A. Q. Shi, J. Wang, X. W. Han, X. A. Fang and Y. Z. Zhang, Sens. Actuators, B, 2014, 200, 206 CrossRef CAS.
- C. Y. Neo, N. K. Gopalan and J. Y. Ouyang, J. Mater. Chem. A, 2014, 2, 9226 RSC.
- H. F. Xiao, Z. F. Zhang, M. L. Xu and X. K. Yang, Chin. J. Rare Met., 2012, 36, 921 CAS.
- H. F. Xiao, M. L. Xu, Z. F. Zhang and X. K. Yang, Mater. Heat Treat, 2012, 41, 40 Search PubMed.
- H. J. Choi, I. Y. Jeon, D. W. Chang and D. S. Yu, J. Phys. Chem. C, 2011, 115, 1746 CrossRef CAS.
- L. Zhang, Y. R. Liang, T. W. Zhang, S. C. Yang, Z. M. Yang and B. J. Ding, Mater. Rev., 2006, 20, 191 Search PubMed.
- V. Bellas and M. Rehahn, Angew. Chem., Int. Ed., 2007, 46, 5082 CrossRef CAS PubMed.
- G. R. Whittell and I. Manners, Adv. Mater., 2007, 19, 3439 CrossRef CAS.
- Y. J. Ma and W. F. Dong, Nat. Mater., 2006, 5, 724 CrossRef CAS PubMed.
- B. Y. Kim, E. L. Ratcliff, N. R. Armstrong, T. Kowalewski and J. Pyun, Langmuir, 2010, 26, 2083 CrossRef CAS PubMed.
- Z. Meng, G. Li, H. F. Wong, S. M. Ng, S. C. Yiu, C. L. Ho, C. W. Leung, I. Manners and W. Y. Wong, Nanoscale, 2017, 9, 731 RSC.
- Q. Dong, W. Qu, W. Liang, K. Guo, H. Xue, Y. Guo, Z. Meng, C. L. Ho, C. W. Leung and W. Y. Wong, Nanoscale, 2016, 8, 7068 RSC.
- Q. Dong, G. Li, C. L. Ho, M. Faisal, C. W. Leung, P. W. T. Pong, K. Liu, B. Z. Tang, I. Manners and W. Y. Wong, Adv. Mater., 2012, 24, 1034 CrossRef CAS PubMed.
- Q. Dong, Z. Meng, C. L. Ho, H. Guo, W. Yang, I. Manners, L. Xu and W. Y. Wong, Chem. Soc. Rev., 2018, 47, 4934 RSC.
- Z. Meng, G. Li, S. M. Ng, H. F. Wong, S. C. Yiu, C. L. Ho, C. W. Leung and W. Y. Wong, Polym. Chem., 2016, 7, 4467 RSC.
- K. Liu, C. L. Ho, S. Aouba, Y. Q. Zhao, Z. H. Lu, S. Petrov, N. Coombs, P. Dube, H. E. Ruda, W. Y. Wong and I. Manners, Angew. Chem., Int. Ed., 2008, 47, 1255 CrossRef CAS PubMed.
- Z. Meng, C. L. Ho, H. F. Wong, Z. Q. Yu, N. Zhu, G. Li, C. W. Leung and W. Y. Wong, Sci. China Mater., 2018 DOI:10.1007/s40843-018-9350-4.
- S. I. Yamamoto, J. Pietrasik and K. Matyjaszewski, Macromolecules, 2008, 41, 7013 CrossRef CAS.
- J. X. Li, B. Q. Zhu, X. J. Yao, Y. C. Zhang, Z. Zhu, S. Tu, S. S. Jia, R. D. Liu, H. Z. Kang and C. Y. J. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 16800 CrossRef CAS PubMed.
- H. D. Jin, L. Wang, H. J. Yu, R. B. Tong and W. D. Zhou, Prog. Chem., 2016, 28, 51 Search PubMed.
- F. Xu, H. Li, Y. L. Luo and W. Tang, ACS Appl. Mater. Interfaces, 2017, 9, 5181 CrossRef CAS PubMed.
- P. S. Dorraji and F. H. Jalali, Sens. Actuators, B, 2014, 200, 251 CrossRef CAS.
- L. C. Liu, L. L. Rui, Y. Gao and W. A. Zhang, Polym. Chem., 2015, 6, 1817 RSC.
- S. G. Zhai, J. Shang, D. Yang, S. Y. Wang, J. H. Hu, G. L. Lu and X. Y. Huang, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 811 CrossRef CAS.
- K. Li, J. Q. Zhang, S. Y. Kang, H. M. Shi and H. Y. Zhou, Chin. J. Appl. Chem., 2015, 32, 236 Search PubMed.
- A. S. Al-Hobaib, K. M. Al-Sheetan, M. R. Shaik and M. S. Al-Suhybani, Appl. Water Sci., 2017, 7, 4341 CrossRef CAS.
- C. Termvidchakorn, V. Itthibenchapong, S. Songtawee, B. Chamnankid, S. Namuangruk, K. Faungnawakij, T. Charinpanitkul, R. Khunchit, N. Hansupaluk, N. Sano and H. Hinode, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2017, 8, 035006 Search PubMed.
- H. Wang, J. Li, X. Zhang, Z. Ouyang, Q. Li, Z. Su and G. Wei, RSC Adv., 2013, 3, 9304 RSC.
- H. X. Wu, R. Tong, X. Q. Qiu, H. F. Yang, Y. H. Lin, R. F. Cai and S. X. Qian, Carbon, 2007, 45, 152 CrossRef CAS.
- H. J. Yoo, K. H. Kim, S. K. Yadav and J. W. Cho, Compos. Sci. Technol., 2012, 72, 1834 CrossRef CAS.
- B. Kumar, M. Castro and J. F. Feller, Sens. Actuators, B, 2012, 161, 621 CrossRef CAS.
- S. H. Keshel1, M. Entezari, M. R. Tavirani1, M. Ebrahimil and M. R. Tavirani, Gastroenterol. Hepatol. Bed. Bench., 2013, 6, S39 Search PubMed.
- J. Azizian, M. Entezari and H. Anaraki-Ardakani, J. Chem., 2013, 11, 1494 Search PubMed.
- X. H. Pang, J. X. Li, Y. B. Zhao, D. Wu, Y. Zhang, B. Du, H. M. Ma and Q. Wei, ACS Appl. Mater. Interfaces, 2015, 7, 19260 CrossRef CAS PubMed.
- M. L. Xu, G. T. Yang, X. K. Yang, X. Y. Cui and C. G. Min, Kunming Uni. Sci. Technol., 2014, 2, 2305 Search PubMed.
- H. X. Wu, X. Q. Qiu, R. F. Cai and S. X. Qian, Appl. Surf. Sci., 2007, 253, 5122 CrossRef CAS.
- L. H. Zou, Q. Zhang, L. P. Zeng and G. Q. Zhang, Chin. J. Environ. Eng., 2012, 6, 270 CAS.
- J. Lee, S. R. Ahmed, S. Oh, J. Kim, T. Suzuki, K. Parmar, S. S. Park, J. Lee and E. Y. Park, Biosens. Bioelectron., 2015, 64, 311 CrossRef CAS PubMed.
- A. T. Lawal, Mater. Res. Bull., 2016, 73, 308 CrossRef CAS.
- A. Q. Shi, J. Wang, X. Han, X. Fang and Y. Zhang, Sens. Actuators, B, 2014, 200, 206 CrossRef CAS.
- S. Mehmood, R. Ciancio, E. Carlino and A. S. Bhatti, Int. J. Nanomed., 2018, 13, 2093 CrossRef PubMed.
- H. B. Wu, S. Luo and D. H. Qin, New Chem. Mater., 2014, 42, 216 CAS.
- G. Li, C. G. Zeng and R. C. Jin, J. Phys. Chem. C, 2015, 119, 11143 CrossRef CAS.
- M. Gadogbe, Y. Zhou, S. H. Alahakoon, G. S. Perera, S. L. Zou, C. U. Pittman Jr. and D. M. Zhang, J. Phys. Chem. C, 2015, 119, 18414 CrossRef CAS.
- Y. Wei, R. Klajn, A. O. Pinchuk and B. A. Grzbowski, Small, 2008, 4, 1635 CrossRef CAS PubMed.
- P. Barathi and A. S. Kumar, Langmuir, 2013, 29, 10617 CrossRef CAS PubMed.
- A. Feiz and A. Bazgir, Catal. Commun., 2016, 73, 88 CrossRef CAS.
- Y. Peng, J. E. Lu, C. P. Deming, L. M. Chen, N. Wang, E. Y. Hirata and S. W. Chen, Electrochim. Acta, 2016, 211, 704 CrossRef CAS.
- A. Rabti, C. C. Mayorga-Martinez, L. Baptista-Pires, N. Raouafi and A. Merkoci, Anal. Chim. Acta, 2016, 926, 28 CrossRef CAS PubMed.
- O. S. Kang, J. P. Bruce, D. E. Herbert and M. S. Freund, ACS Appl. Mater. Interfaces, 2015, 7, 26959 CrossRef CAS PubMed.
- Z. Li, X. H. Zheng, Q. L. Sheng, Z. Y. Yang and J. B. Zheng, RSC Adv., 2016, 6, 11218 RSC.
- G. Ghimire, Y. Yi, M. A. Derylo, L. A. Baker and T. Ito, Langmuir, 2015, 31, 12307 CrossRef CAS.
- D. Li, X. Zhang, F. Kong, X. Qiao and Z. Xu, Food Anal. Method, 2017, 10, 1284 CrossRef.
- N. Xia and Y. Gao, Int. J. Electrochem. Sci., 2015, 10, 713 Search PubMed.
- R. Sundarmurugasan, M. B. Gumpu, B. L. Ramachandra, N. Nesakumar, S. Sethuraman, U. M. Krishnan and J. B. B. Rayappana, Sens. Actuators, B, 2016, 230, 306 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8tc05294h |
| ‡ Xue-Peng Wei and Rui-Qian Zhang contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |