Thermally activated delayed fluorescence emitters with low concentration sensitivity for highly efficient organic light emitting devices†
Xiao-Chun
Fan
a,
Kai
Wang
 *a,
Cai-Jun
Zheng
*a,
Cai-Jun
Zheng
 *b,
Gao-Le
Dai
*b,
Gao-Le
Dai
 a,
Yi-Zhong
Shi
a,
Yan-Qing
Li
a,
Jia
Yu
a,
Xue-Mei
Ou
a and
Xiao-Hong
Zhang
a,
Yi-Zhong
Shi
a,
Yan-Qing
Li
a,
Jia
Yu
a,
Xue-Mei
Ou
a and
Xiao-Hong
Zhang
 *a
*a
aInstitute of Functional Nano & Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University, Suzhou, Jiangsu 215123, P. R. China. E-mail: xiaohong_zhang@suda.edu.cn; wkai@suda.edu.cn
bSchool of Optoelectronic Science and Engineering, University of Electronic Science and Technology of China, Chengdu, Sichuan 610054, P. R. China. E-mail: zhengcaijun@uestc.edu.cn
First published on 27th June 2019
Abstract
Thermally activated delayed fluorescence (TADF) emitters normally suffer from serious concentration sensitivity, realizing peak electroluminescence efficiencies with precise control of the doping concentrations, which may hinder their practical applications in organic light emitting devices (OLEDs). In this work, we developed TADF emitter 2′,7′-di(10H-phenoxazin-10-yl)spiro[fluorene-9,9′-thioxanthene]10′,10′-dioxide (SPFS-PXZ) based on conventional TADF emitter 10,10′-(sulfonylbis(4,1-phenylene))bis(10H-phenoxazine) (DPS-PXZ). By introducing a fluorene group, the molecular rigidity and steric hindrance of SPFS-PXZ are improved greatly, which contributes to suppressing the strong π–π stacking tendency between phenoxazine segments and reduces intermolecular interactions. In turn, SPFS-PXZ successfully exhibits low concentration sensitivity in OLEDs, and keeps high maximum external quantum efficiencies (EQEs) over 20.8% with a small EQE fluctuation of 2.1% in a wide doping concentration range from 10 wt% to 50 wt%. These results prove that SPFS-PXZ is an ideal model to develop efficient TADF emitters with low concentration sensitivity.
Introduction
Organic light emitting devices (OLEDs) always draw wide attention due to their great potential in displays and lighting.1,2 In devices, only 25% of excitons are electro-generated in the singlet state according to the spin statistics rule, thus the 75% of triplet excitons will be wasted for traditional fluorescent emitters.3 Although phosphorescent emitters can utilize both singlet and triplet excitons, the high cost of noble metals limits their practical application.4–6 As a result, pure organic thermally activated delayed fluorescence (TADF) emitters are considered to be the best candidates as the emitters of OLEDs, since they can theoretically achieve 100% internal quantum efficiency via an efficient reverse intersystem crossing (RISC) process from the lowest triplet state (T1) to the lowest singlet state (S1) at room temperature.7To achieve an efficient RISC process, the singlet–triplet splittings (ΔESTs) between the S1 and T1 states must be limited to extremely small values, which can only be realized by minimizing the overlap between the highest occupied molecular orbital (HOMO) and the lowest occupied molecular orbital (LUMO).8 Therefore, linking electron donor (D) and electron acceptor (A) moieties with a twisted structure has become the most common and effective method to construct TADF emitters, as the HOMO and LUMO can be separately distributed in D and A moieties in these D–A frameworks. With various D and A segments, a large number of efficient TADF emitters have been developed in the past few years,9–38 and the optimized OLEDs based on these TADF emitters have realized extremely high maximum external quantum efficiencies (EQEs) over 30%, demonstrating their promising future. However, current TADF emitters normally suffer from serious concentration sensitivity in the OLEDs. With enough doping concentration to harvest all excitons on the host, most of the TADF emitters fall into trouble with significant concentration quenching. Therefore, the peak efficiencies of the OLEDs based on these TADF emitters can only be achieved with precise control of the doping concentrations, which are usually lower than 15 wt%.39 However, during practical manufacturing, uniform and reproducible control over doping concentrations is hardly achieved especially with low values, resulting in unstable performance and high cost of commercial products. Furthermore, according to Zhang et al.'s report, high doping concentrations of TADF emitters could also benefit in realizing more balanced charge transport and a wider charge recombination zone, and thus significantly improve the stability of the devices.40 Therefore, it is highly desired to develop TADF emitters with low concentration sensitivity, keeping peak efficiencies within a wide doping concentration range.
As reported by Takuma Yasuda et al. in 2017, concentration quenching of TADF molecules was dominated by electron-exchange interactions for long-lived triplet excitons, and was quite sensitive with the intermolecular distances.23 Since lots of employed D and A moieties in TADF emitters, like carbazole, phenothiazine (PXZ), and anthraquinone, have planar structures to realize highly twisted structures, their derivatives suffer from strong π–π stacking tendency between these planar segments, resulting in serious concentration quenching behaviors. In this work, taking the reported TADF emitter 10,10′-(sulfonyl bis(4,1-phenylene))bis(10H-phenoxazine) (DPS-PXZ) as the original, we designed and synthesized optimized TADF emitter 2′,7′-di(10H-phenoxazin-10-yl)spiro[fluorene-9,9′-thioxanthene]10′,10′-dioxide (SPFS-PXZ) as shown in Fig. 1.22,40,41 Due to strong π–π stacking tendency between PXZ segments, DPS-PXZ exhibits high concentration-sensitivity. By introducing a fluorene group into the sulfonyldibenzene (DPS) moiety, the rigidity of the modified A component spiro[fluorene-9,9′-thioxanthene]10′,10′-dioxide (SPFS) is evidently enhanced, which will restrict the molecular relaxation of SPFS-PXZ and improve its photoluminescence quantum yield (PLQY). Moreover, compared with DPS-PXZ, intermolecular steric hindrance of SPFS-PXZ is significantly improved, resulting in suppressed concentration quenching and much lower concentration sensitivity. As expected, the optimized OLED based on SPFS-PXZ realizes excellent performance with a maximum EQE, current efficiency (CE) and power efficiency (PE) of 22.9%, 64.9 cd A−1 and 65.7 lm W−1, respectively, which is superior to the corresponding DPS-PXZ-based devices. More importantly, with doping concentration gradually increasing from 10 wt% to 50 wt%, SPFS-PXZ-based devices exhibit stable EQEs with a small fluctuation of 2.1%. These results prove that SPFS-PXZ is the ideal model to develop efficient TADF emitters with low concentration sensitivity.
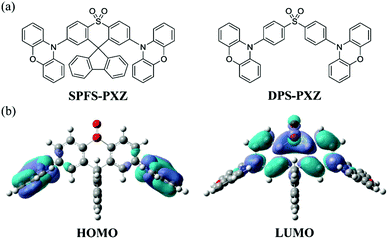 | ||
| Fig. 1 (a) Molecular structure of SPFS-PXZ and DPS-PXZ. (b) HOMO and LUMO spatial distributions of SPFS-PXZ. | ||
Results and discussion
Synthesis and characterization
SPFS-PXZ was obtained using a simple and efficient synthetic route with a high yield as shown in Scheme 1. Intermediate 3 was prepared via the carbonyl addition reaction involving n-butyllithium (n-BuLi) with 2,7-dibromo-9H-thioxanthen-9-one (1) and 2-bromo-1,1′-biphenyl (2). Then intermediate 3 was further subjected to dehydration ring-closure reactions and oxidation reactions to obtain 2′,7′-dibromospiro[fluorene-9,9′-thioxanthene]10′,10′-dioxide (5). Finally, the target compound SPFS-PXZ was synthesized via the Buchwald–Hartwig reaction involving 5 and PXZ. Nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry were fully characterized to confirm the chemical structures of the intermediates and the target compound.Thermal properties
The thermodynamic analysis was characterized by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) measurements. From TGA and DSC curves (shown in Fig. S1, ESI†), SPFS-PXZ exhibits high thermal stability with a decomposition temperature (Td, defined as the temperature of 5% weight loss) of 445 °C, slightly higher than that of DPS-PXZ. Moreover, in the measurement range from 25 to 200 °C, no distinct glass transition can be observed from the DSC curve. This result guarantees its high thermal stability for OLED applications.Theoretical calculations and electrochemical properties
To give an in-depth explanation on the spatial distributions of frontier molecular orbitals (FMOs), time-dependent density function theory (TD-DFT) calculations were carried out on SPFS-PXZ in the ground state (S0) using the B3LYP/6-31g(d) basis set in the Gaussian 09 program package. As shown in Fig. 1, benefitting from the nearly perpendicular dihedral angles between PXZ and SPFS moieties, an obvious FMO separation is achieved with the HOMO mainly locating on PXZ moieties and the LUMO mainly locating on the SPFS moiety. Specifically, since the spiral carbon atom breaks the conjugation between DPS and fluorene, there is barely any LUMO distribution observed on the fluorene group, suggesting that the additional fluorene group has no influence on the FMO distributions. As expected, ΔEST of SPFS-PXZ is calculated to be 0.02 eV by using the optimal HF exchange method (listed in Table S1, ESI†).41 Such a small ΔEST value would ensure an effective RISC process, thus resulting in TADF characteristics. Moreover, compared with DPS-PXZ, SPFS-PXZ has a more rigid and sterically hindered molecular structure due to the additional restriction from the fluorene group. Thus, it can be expected that the non-radiative process of SPFS-PXZ will be further suppressed, resulting in a higher PLQY than DPS-PXZ.The electrochemical property of SPFS-PXZ was investigated by performing cyclic voltammetry (CV) measurements. As shown in Fig. S3 (ESI†), from the onset positions of oxidation and reduction curves with respect to that of ferrocene, the HOMO and LUMO energy levels of SPFS-PXZ were estimated to be −5.39 and −2.79 eV, respectively. As listed in Table 1, the HOMO value of SPFS-PXZ is identical to that of DPS-PXZ, which can be ascribed to the same employed D moiety, whereas its LUMO energy level is slightly shallower than that of DPS-PXZ (−2.88 eV), which should be attributed to the low electron-donating ability of the additional fluorene group. Accordingly, the electrochemical band gaps of SPFS-PXZ and DPS-PXZ are calculated to be 2.60 and 2.51 eV, respectively.
| Compound | λ abs [nm] | λ em [nm] | S1c/T1c [eV] | HOMO/LUMOd [eV] | ΔESTe [eV] | PLQYf [%] |
|---|---|---|---|---|---|---|
| a Maximum wavelength of the UV-vis absorption spectrum. b Maximum wavelength of the fluorescence spectrum measured in toluene at room temperature. c The energy levels of S1 and T1 estimated from the onset position of the fluorescence and phosphorescence spectra of doping in mCP films at 77 K. d Measured in DMF at room temperature. e Calculated from the equation: ΔEST = S1 − T1. f The photoluminescence quantum yield of SPFS-PXZ and DPS-PXZ doped in mCP films. g 10 wt%. h 30 wt%. i 50 wt%. | ||||||
| SPFS-PXZ | 390 | 492 | 2.79/2.78 | −5.39/−2.79 | 0.013 | 55.9g/72.3h/60.3i |
| DPS-PXZ | 390 | 507 | 2.89/2.73 | −5.39/−2.88 | 0.081 | 51.5g/40.9h/32.3i |
Photophysical properties
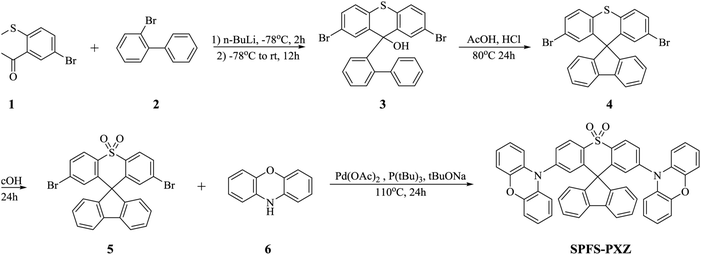
The photophysical properties of SPFS-PXZ were then fully characterized. Fig. 2a shows the UV-Vis absorption spectrum of SPFS-PXZ in toluene at room temperature. Due to the similar skeleton, SPFS-PXZ exhibits a similar absorption range and shape to DPS-PXZ (shown in Fig. S4, ESI†). The absorption below 340 nm could be attributed to the π–π* transition of the high-lying local excited state, while the structureless absorption band around 390 nm should be assigned to the intramolecular charge-transfer (ICT) transition from the PXZ moiety to the SPFS moiety. As shown in Fig. 2b, in toluene at room temperature, SPFS-PXZ shows bluish-green emission with a peak at 492 nm, which is slightly shorter than that of DPS-PXZ (λmax = 507 nm) and consistent with its slightly broader bandgap estimated by the CV measurement.To further confirm its ICT characteristic, fluorescence spectra of SPFS-PXZ were measured in different solvents from initial low polar toluene to high polar dichloromethane (DCM) and N,N-dimethylformamide (DMF). As shown in Fig. 2b, fluorescence spectra undergo a significant red-shift from an initial peak at 492 nm in toluene to 524 nm in DCM and 563 nm in DMF, respectively, indicating significant ICT characteristics of SPFS-PXZ. As summarized in Table S2 (ESI†), the Stokes shift and FWHM of SPFS-PXZ are smaller than the corresponding parameters of DPS-PXZ, suggesting that the solvatochromic effect of SPFS-PXZ was relatively suppressed, which should be attributed to the more rigid structure of SPFS-PXZ further suppressing the molecular relaxation process. Fig. 2a also illustrates the fluorescence and phosphorescence spectra of 30 wt% SPFS-PXZ doped in 1,3-di(9H-carbazol-9-yl)benzene (mCP) films at 77 K. From the onset positions of the fluorescence (λon = 445 nm) and phosphorescence (λon = 447 nm) spectra, the energy levels of S1 and T1 states are calculated to be 2.789 and 2.776 eV respectively. Thus, an extremely small ΔEST of 0.013 eV is estimated for SPFS-PXZ, well consistent with our theoretical prediction, suggesting its potential TADF feature.
In the previous report, DPS-PXZ is confirmed to exhibit an aggregation-induced emission (AIE) phenomenon. Herein, to explore the influence of introducing steric hindrance on the AIE feature, we measured the emission behaviors of SPFX-PXZ in tetrahydrofuran (THF)/water mixtures with different water fractions. As shown in Fig. S5 (ESI†), a significant AIE phenomenon was observed, suggesting that SPFX-PXZ is also an AIE TADF emitter, which is beneficial to realize a high PLQY under high doping concentration.
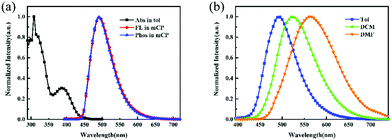
The transition PL decays of SPFS-PXZ were carried out at room temperature. As shown in Fig. 3, SPFS-PXZ-doped mCP films with doping concentrations from 10 wt% to 50 wt% all exhibit two distinct lifetimes with a prompt component at a nanosecond order of magnitude and a delay component at a microsecond order of magnitude, corresponding to the prompt fluorescence and the delayed fluorescence, respectively. The existence of the delayed fluorescence confirms the TADF characteristic of SPFS-PXZ. Moreover, with increased doping concentration, the SPFS-PXZ-doped films only show a slight change in transition PL decay curves; while for DPS-PXZ-doped ones, the decrease becomes more evident. These results confirmed that the introduction of the fluorene group in SPFS-PXZ further suppresses concentration quenching evidently, beneficial to realizing stable exciton utilization in a wide doping concentration range.
To further confirm their different concentration sensitivity, the PLQYs of SPFS-PXZ and DPS-PXZ doped mCP films were measured with various doping concentrations at room temperature. As summarized in Table 1, with doping concentrations from 10 wt% to 30 wt% and 50 wt%, SPFS-PXZ-doped films show high PLQYs of 55.9%, 72.3% and 60.3%, respectively, while under the same conditions, DPS-PXZ-doped films exhibit a sharp PLQY decline from 51.5% to 40.9% and 32.3%. The PLQY values of SPFS-PXZ are all evidently higher than that of DPS-PXZ, which should be ascribed to the reduced molecular relaxation. More importantly, compared with DPS-PXZ, the concentration sensitivity of SPFS-PXZ is significantly suppressed, because the large steric hindrance of the fluorene group breaks the strong π–π interactions of PXZ segments and reduces intermolecular interaction between SPFS-PXZ molecules.
OLED devices
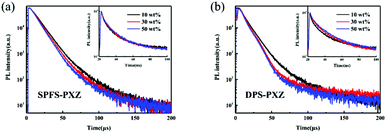
Finally, to compare the electroluminescence (EL) performance of SPFS-PXZ and DPS-PXZ, the OLEDs using SPFS-PXZ and DPS-PXZ as emitters were finally fabricated with an identical architecture of ITO/TAPC (30 nm)/TCTA (10 nm)/mCP: x wt% SPFS-PXZ or DPS-PXZ (20 nm)/TmPyPb (40 nm)/LiF (1 nm)/Al, where ITO (indium tin oxide), TAPC (4,4′-(cyclohexane-1,1-diyl)bis(N,N-di-p-tolylaniline)), TCTA (tris(4-(9H-carbazol-9-yl)phenyl)amine), TmPyPb (1,3,5-tri[(32pyridyl)-phen-3-yl]benzene), LiF and Al act as the anode, hole-injection, hole-transporting, electron-transporting, electron-injection and cathode layers, respectively. The energy level diagram and molecular structures of the used materials in the devices are shown in Fig. 4a and Fig. S9 (ESI†), respectively. According to the HOMO and LUMO energy levels of SPFS-PXZ, mCP was chosen as the optimal host matrix to balance the carrier recombination. And a series of devices with the doping concentration varying from 10 wt% to 50 wt% were fabricated to investigate the concentration sensitivity of SPFS-PXZ and DPS-PXZ in detail.Table 2 summarizes the key performance of SPFS-PXZ and DPS-PXZ based devices. All the devices exhibit low turn-on voltages (Von) of ∼3.0 V (defined voltage at 10 cd m−2). In particular, with the doping concentration gradually increasing from 10 wt% to 50 wt%, the Vons evidently decline for both emitters, which should be assigned to the direct charge trap of emitters. Meanwhile, as shown in Fig. 4b and Fig. S6 (ESI†), with doping ratios gradually increased, both EL spectra underwent a slight red-shift as well, from 504 to 516 nm for SPFS-PXZ and from 516 to 532 nm for DPS-PXZ, respectively. This is because SPFS-PXZ and DPS-PXZ molecules can also increase the polarity of the EML, thus causing solvatochromaticity like in solutions.
| Compound | Doping concentrationa [wt%] | V on [V] | Peak [nm] | Maximum CE/PE/EQEc [cd A−1/lm W−1/%] | CIE (x, y) |
|---|---|---|---|---|---|
| a Doping concentration in mCP. b Turn-on voltage defined at 10 cd m−2. c CE: current efficiency, PE: power efficiency, EQE: external quantum efficiency. | |||||
| SPFS-PXZ | 10 | 3.2 | 504 | 56.2/53.8/20.8 | (0.22, 0.47) |
| 30 | 2.9 | 512 | 66.8/68.7/22.9 | (0.25, 0.52) | |
| 50 | 2.8 | 516 | 62.5/63.3/21.0 | (0.27, 0.54) | |
| DPS-PXZ | 10 | 3.0 | 516 | 61.4/63.1/19.3 | (0.28, 0.56) |
| 30 | 2.7 | 524 | 58.0/60.7/17.2 | (0.31, 0.28) | |
| 50 | 2.6 | 532 | 40.9/41.4/12.2 | (0.35, 0.58) | |
Fig. 4c and Fig. S8 (ESI†) illustrate the luminance–EQE curves of these devices. The optimized OLED based on SPFS-PXZ exhibits the maximum EQE, CE and PE estimated up to 22.9%, 64.9 cd A−1 and 65.7 lm W−1, respectively, which are evidently higher than the optimized results of DPS-PXZ-doped devices (19.3%, 61.4 cd A−1 and 63.1 lm W−1), suggesting the higher exciton utilization of SPFS-PXZ. Moreover, at a luminance of 1000 cd m−2, the EQE still remained a high value of 21.5%, corresponding to an extremely small roll-off of only 6.1%, suggesting the superior exciton utilization under high current density. Intriguingly, the two emitters exhibit different variation trends with increased doping concentrations in the OLEDs. For DPS-PXZ, the best efficiency is achieved at a common doping concentration of 10 wt%, while with further increased doping concentration, DPS-PXZ-based devices suffer a sharp efficiency decline to a maximum EQE of only 12.2% at a doping concentration of 50 wt%. Such a high concentration sensitivity is similar with most reported TADF emitters, which should be attributed to the strong π–π interactions of PXZ segments which leads to serious concentration quenching. Correspondingly, as large steric hindrance of an additional fluorene group suppresses the π–π interactions of PXZ units, and SPFS-PXZ reaches its optimized device performance at a high doping concentration of 30 wt%. Moreover, in a wide doping concentration range from 10 wt% to 50 wt%, SPFS-PXZ-based devices all possess high maximum EQEs of over 20.8%. Such a small EQE fluctuation of 2.1% is significantly smaller than DPS-PXZ, indicating the superior low concentration sensitivity of SPFS-PXZ.
Conclusions
In summary, to suppress annoying concentration quenching, we proposed a simple strategy by introducing extra steric hindrance to develop TADF emitters with low concentration sensitivity. Accordingly, optimized TADF emitter SPFS-PXZ was developed based on the reported TADF emitter DPS-PXZ. The additional fluorene framework restricts the molecular relaxation, resulting in an improved PLQY. Moreover, the large steric hindrance of the fluorene group can evidently suppress the strong π–π stacking tendency of PXZ segments for SPFS-PXZ, thus reducing concentration sensitivity. In the OLEDs, SPFS-PXZ exhibits excellent performance with peak EQE, CE, and PE of 22.9%, 64.9 cd A−1 and 65.7 lm W−1 and a small efficiency roll-off of only 6.1% at a high luminance of 1000 cd m−2. More importantly, in a wide doping concentration range from 10 wt% to 50 wt%, SPFS-PXZ-based OLEDs exhibit an extremely small EQE fluctuation of 2.1%, proving its superior low concentration sensitivity. We believed that such a practice could act as a simple strategy to develop TADF emitters with low concentration sensitivity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research & Development Program of China (grant number 2016YFB0401002), the National Natural Science Foundation of China (grant numbers 51533005, 51821002, and 51773029), the China Postdoctoral Science Foundation (grant no. 2018M642307 and 2016M590498), the Jiangsu Planned Projects for Postdoctoral Research Funds (grant number 1601241C), the Collaborative Innovation Center of Suzhou Nano Science & Technology, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the 111 Project, and the Joint International Research Laboratory of Carbon-Based Functional Materials and Devices.Notes and references
- C. W. Tang and S. A. VanSlyke, Appl. Phys. Lett., 1987, 51, 913–915 CrossRef CAS.
- M. A. Baldo, D. F. O'Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Nature, 1998, 395, 151–154 CrossRef CAS.
- M. A. Baldo, D. F. O’Brien, M. E. Thompson and S. R. Forrest, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 14422–14428 CrossRef CAS.
- C. Adachi, M. A. Baldo, M. E. Thompson and S. R. Forrest, J. Appl. Phys., 2001, 90, 5048–5051 CrossRef CAS.
- S. J. Su, H. Sasabe, Y. J. Pu, K. Nakayama and J. Kido, Adv. Mater., 2010, 22, 3311–3316 CrossRef CAS PubMed.
- C. W. Lee and J. Y. Lee, Adv. Mater., 2013, 25, 5450–5454 CrossRef CAS PubMed.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- S. Hirata, Y. Sakai, K. Masui, H. Tanaka, S. Y. Lee, H. Nomura, N. Nakamura, M. Yasumatsu, H. Nakanotani, Q. Zhang, K. Shizu, H. Miyazaki and C. Adachi, Nat. Mater., 2015, 14, 330–336 CrossRef CAS PubMed.
- Q. Zhang, J. Li, K. Shizu, S. Huang, S. Hirata, H. Miyazaki and C. Adachi, J. Am. Chem. Soc., 2012, 134, 14706–14709 CrossRef CAS PubMed.
- X. K. Liu, Z. Chen, C. J. Zheng, C. L. Liu, C. S. Lee, F. Li, X. M. Ou and X. H. Zhang, Adv. Mater., 2015, 27, 2378–2383 CrossRef CAS PubMed.
- J. X. Chen, K. Wang, C. J. Zheng, M. Zhang, Y. Z. Shi, S. L. Tao, H. Lin, W. Liu, W. W. Tao, X. M. Ou and X. H. Zhang, Adv. Sci., 2018, 5, 1800436 CrossRef PubMed.
- F. B. Dias, K. N. Bourdakos, V. Jankus, K. C. Moss, K. T. Kamtekar, V. Bhalla, J. Santos, M. R. Bryce and A. P. Monkman, Adv. Mater., 2013, 25, 3707–3714 CrossRef CAS PubMed.
- L. Gan, K. Gao, X. Cai, D. Chen and S.-J. Su, J. Phys. Chem. Lett., 2018, 9, 4725–4731 CrossRef CAS PubMed.
- M. Li, C.-F. Chen, M. Li, C.-F. Chen, S.-H. Li, M.-K. Fung, D. Zhang, M. Cai and L. Duan, Angew. Chem., Int. Ed., 2018, 57, 2889–2893 CrossRef CAS PubMed.
- X.-L. Li, G. Xie, M. Liu, D. Chen, X. Cai, J. Peng, Y. Cao and S.-J. Su, Adv. Mater., 2016, 28, 4614–4619 CrossRef CAS PubMed.
- W. Liu, J.-X. Chen, C.-J. Zheng, K. Wang, D.-Y. Chen, F. Li, Y.-P. Dong, C.-S. Lee, X.-M. Ou and X.-H. Zhang, Adv. Funct. Mater., 2016, 26, 2002–2008 CrossRef CAS.
- X. K. Liu, Z. Chen, C. J. Zheng, M. Chen, W. Liu, X. H. Zhang and C. S. Lee, Adv. Mater., 2015, 27, 2025–2030 CrossRef CAS PubMed.
- Y. Z. Shi, K. Wang, X. Li, G. L. Dai, W. Liu, K. Ke, M. Zhang, S. L. Tao, C. J. Zheng, X. M. Ou and X. H. Zhang, Angew. Chem., Int. Ed., 2018, 57, 9480–9484 CrossRef CAS PubMed.
- K. Wang, C. J. Zheng, W. Liu, K. Liang, Y. Z. Shi, S. L. Tao, C. S. Lee, X. M. Ou and X. H. Zhang, Adv. Mater., 2017, 29, 1701476 CrossRef PubMed.
- W. Zeng, H.-Y. Lai, W.-K. Lee, M. Jiao, Y.-J. Shiu, C. Zhong, S. Gong, T. Zhou, G. Xie, M. Sarma, K.-T. Wong, C.-C. Wu and C. Yang, Adv. Mater., 2018, 30, 1704961 CrossRef PubMed.
- D. Zhang, M. Cai, Y. Zhang, D. Zhang and L. Duan, ACS Appl. Mater. Interfaces, 2015, 7, 28693–28700 CrossRef CAS PubMed.
- S. Gan, W. Luo, B. He, L. Chen, H. Nie, R. Hu, A. Qin, Z. Zhao and B. Z. Tang, J. Mater. Chem. C, 2016, 4, 3705–3708 RSC.
- J. Lee, N. Aizawa, M. Numata, C. Adachi and T. Yasuda, Adv. Mater., 2017, 29, 1604856 CrossRef PubMed.
- W. L. Tsai, M. H. Huang, W. K. Lee, Y. J. Hsu, K. C. Pan, Y. H. Huang, H. C. Ting, M. Sarma, Y. Y. Ho, H. C. Hu, C. C. Chen, M. T. Lee, K. T. Wong and C. C. Wu, Chem. Commun., 2015, 51, 13662–13665 RSC.
- J. Li, T. Nakagawa, Q. Zhang, H. Nomura, H. Miyazaki and C. Adachi, Adv. Mater., 2013, 25, 3319–3323 CrossRef CAS PubMed.
- L. S. Cui, S. B. Ruan, F. Bencheikh, R. Nagata, L. Zhang, K. Inada, H. Nakanotani, L. S. Liao and C. Adachi, Nat. Commun., 2017, 8, 2250 CrossRef PubMed.
- W. Liu, C. J. Zheng, K. Wang, M. Zhang, D. Y. Chen, S. L. Tao, F. Li, Y. P. Dong, C. S. Lee, X. M. Ou and X. H. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 32984–32991 CrossRef CAS PubMed.
- X. Li, Y. Z. Shi, K. Wang, M. Zhang, C. J. Zheng, D. M. Sun, G. L. Dai, X. C. Fan, D. Q. Wang, W. Liu, Y. Q. Li, J. Yu, X. M. Ou, C. Adachi and X. H. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 13472–13480 CrossRef CAS PubMed.
- W.-W. Tao, K. Wang, J.-X. Chen, Y.-Z. Shi, W. Liu, C.-J. Zheng, Y.-Q. Li, J. Yu, X.-M. Ou and X.-H. Zhang, J. Mater. Chem. C, 2019, 7, 4475–4483 RSC.
- L. Gan, Z. Xu, Z. Wang, B. Li, W. Li, X. Cai, K. Liu, Q. Liang and S. J. Su, Adv. Funct. Mater., 2019, 1808088 CrossRef.
- M. Cai, D. Zhang, J. Xu, X. Hong, C. Zhao, X. Song, Y. Qiu, H. Kaji and L. Duan, ACS Appl. Mater. Interfaces, 2019, 11, 1096–1108 CrossRef CAS PubMed.
- D.-d. Zhang, K. Suzuki, X.-z. Song, Y. Wada, S. Kubo, L. Duan and H. Kaji, ACS Appl. Mater. Interfaces, 2019, 11, 7192–7198 CrossRef CAS PubMed.
- W. Zeng, C. Zhong, S. Gong, T. Zhou, G. Xie, C. Yang, H.-Y. Lai, W.-K. Lee, M. Jiao, Y.-J. Shiu, C.-C. Wu, M. Sarma and K.-T. Wong, Adv. Mater., 2018, 30, 1704961 CrossRef PubMed.
- M. Bian, D. Zhang, Y. Wang, Y.-H. Chung, Y. Liu, H. Ting, L. Duan, Z. Chen, Z. Bian, Z. Liu and L. Xiao, Adv. Funct. Mater., 2018, 28, 1800429 CrossRef.
- B. Li, Z. Wang, S.-J. Su, F. Guo, Y. Cao and Y. Zhang, Adv. Opt. Mater., 2019, 1801496 CrossRef.
- W. Li, X. Cai, B. Li, L. Gan, Y. He, K. Liu, D. Chen, S.-J. Su and Y.-C. Wu, Angew. Chem., Int. Ed., 2019, 58, 582–586 CrossRef CAS PubMed.
- M. Huang, Y. Li, K. Wu, J. Luo, G. Xie, L. Li and C. Yang, Dyes Pigm., 2018, 153, 92–98 CrossRef CAS.
- X. Zhou, H. Yang, Z. Chen, S. Gong, Z. Lu and C. Yang, J. Mater. Chem. C, 2019, 7, 6607–6615 RSC.
- M. Y. Wong and E. Zysman-Colman, Adv. Mater., 2017, 29, 1605444 CrossRef.
- Q. Zhang, D. Tsang, H. Kuwabara, Y. Hatae, B. Li, T. Takahashi, S. Y. Lee, T. Yasuda and C. Adachi, Adv. Mater., 2015, 27, 2096–2100 CrossRef CAS PubMed.
- Q. Zhang, B. Li, S. Huang, H. Nomura, H. Tanaka and C. Adachi, Nat. Photonics, 2014, 8, 326–332 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Cyclic voltammetry, TGA and DSC thermograms, and EL performances. See DOI: 10.1039/c9tc02032b |
| This journal is © The Royal Society of Chemistry 2019 |