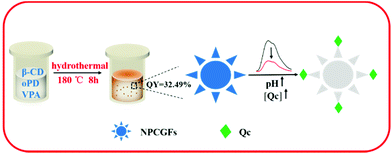
Fluorometric detection of pH and quercetin based on nitrogen and phosphorus co-doped highly luminescent graphene-analogous flakes†
Buhong
Gao
 a,
Yu
Sun
b,
Yingchun
Miao
a,
Li
Xu
a,
Yu
Sun
b,
Yingchun
Miao
a,
Li
Xu
 *b and
Zhongxia
Wang
*b and
Zhongxia
Wang
 *c
*c
aAdvanced Analysis & Testing Center, Nanjing Forestry University, Nanjing 210037, China. E-mail: gaobuhong@126.com
bCollege of Science, Nanjing Forestry University, Nanjing, 210037, China
cSchool of Chemistry and Chemical Engineering, Yancheng Institute of Technology, Yancheng 224051, China. E-mail: wangzx198411@163.com
First published on 6th November 2019
Abstract
Highly luminescent nitrogen and phosphorus co-doped graphene-analogous flakes (NPCGFs) were synthesized by a one-pot simple hydrothermal reaction using β-cyclodextrin (β-CD), vinylphosphoric acid (VPA), and o-phenylenediamine (oPD) as the precursors. VPA, as an important organic P-containing monomer, was selected as the phosphorus source to generate additional conjugated and effective binding sites on the surface of the NPCGFs. This synthetic strategy not only allows enhancement of structural rigidity, but also effectively eliminates surface traps of the NPCGFs, resulting in an improved fluorescence quantum yield (FL QY) of the NPCGFs. Additionally, oPD simultaneously acts as a nitrogen source and enables amino functionalisation of the NPCGF surface in the synthesis process. The NPCGFs (QY, 32.49%) are irregularly shaped with a typical diameter of approximately 54 nm and display strong fluorescence, with excitation/emission maxima of 360/445 nm. It was found that the NPCGFs can serve as a multifunctional FL probe for pH measurement and quercetin (Qc) detection. A linear relationship exists between the decrease in FL intensity and the concentration of Qc in the range from 0.35 to 30 μg mL−1 as well as the pH variation between 4.0 and 7.0. The probe was further applied to the determination of Qc in living cells.
Introduction
Carbon-based nanomaterials (CNMs) are highly desirable because of their high photostability and chemical stability, excellent biocompatibility, low cytotoxicity, and chemical inertness.1,2 However, the use of most pristine CNMs is restricted because of their low fluorescence (FL) efficiency and poor selectivity due to the high density of defect states. Very recently, CNMs containing heteroatoms (e.g., N, S, P, and B) have been actively pursued and considered as the most promising candidates for reducing surface defects and tailoring their intrinsic properties, such as electronic energy level and optical properties, thereby improving the FL efficiency and selectivity.3,4The P atom has been widely used for the preparation of P-doped CNMs because it has properties comparable to those of carbon atoms and also possesses multivalent electrons for bonding with carbon. For example, Yang et al. synthesized P-doped carbon dots with tunable emission using hydrothermal treatment of glucose in the presence of monopotassium phosphate.5 Xu et al. prepared N and P co-doped high-FL carbon dots using sodium citrate and diammonium phosphate as raw materials.6 Wang et al. synthesized N and P co-doped carbon polymers by a hydrothermal approach using urea and H3PO4 as the C–N and P sources, respectively.7 Recent research involving the P-doping of CNMs mainly with P from inorganic sources aroused our interest to further examine the corresponding performance of its counterpart by P-doping CNMs with P from organic sources. Thus far, research on the P-doping of CNMs using organic P sources is still rare. Hence, it is worthwhile to prepare new P-doped CNMs with high quantum yields and selectivity using different organic P monomers.
As the most active antioxidant of the flavonol family, quercetin (Qc) is widely present in vegetables, flowers, fruits, and especially in traditional Chinese herbs.8–11 In the past few years, there has been great interest in Qc because of its antioxidant properties affecting human DNA in vitro,12 and also for its antitumour and antiviral properties, and its ability to aid in adjusting the immune system.13,14 As a consequence, developing an effective analytical technique for selective and sensitive detection of trace amounts of Qc is essential in biochemistry, clinical medicine, and natural pharmaceutical chemistry. Thus far, a series of methods have been developed to detect Qc, including high-performance liquid chromatography,15 electrochemistry,16 and Raman spectroscopy.17 However, each of these methods is limited by severe disadvantages, such as time-consuming processes, complicated equipment, and high costs, which severely limit their wider applications. In contrast, FL analysis can provide a non-destructive, ultrasensitive, and simple detection platform that is free from complicated pretreatment processes. To date, some materials including gold nanoclusters,18 organosilane-functionalized carbon dots,19 and carbon nanoribbon polymers20 have been used as FL sensors for Qc detection. Despite these good examples, it is still very necessary to explore novel FL probes with low toxicity or that are non-toxic, and that also possess ease of synthesis with high photostability and quantum yield (QY) for sensitive and selective determination of Qc.
In the current study, as a novel method for doping CNMs, low-cost N and P co-doped fluorescent graphene-analogous flake (NPCGF) nanoprobes with excellent optical properties, high stability, and superior water dispersibility were successfully synthesized for the first time by hydrothermal treatment of β-cyclodextrin, vinylphosphoric acid, and o-phenylenediamine with high QY of approximately 32.49%. Surprisingly, the NPCGFs showed highly efficient FL quenching ability in the presence of Qc and H+ cation, indicating their potential application as a dual-readout assay platform. The principle of the proposed Qc and H+ cation-sensing concept is shown in Scheme 1. As far as we know, this is the first example of the construction of a FL assay platform for Qc and pH based on highly efficient NPCGFs. Additionally, NPCGFs with excellent selectivity and satisfactory recovery were further used to detect Qc in complex real samples and living cells.
Experimental section
Reagents and chemicals
β-Cyclodextrin (β-CD) and o-phenylenediamine (oPD) were purchased from Shanghai Sinopharm Chemical Reagents Co. Ltd (Shanghai, China). Quercetin (Qc) was purchased from Sigma-Aldrich. Vinylphosphoric acid (VPA) was obtained from Shanghai Macklin Biochemical Co. Ltd (Shanghai, China). All reagents were of analytical grade and were used as received without further purification. All solutions were prepared with double distilled water.Apparatus
The morphologies and energy-dispersive X-ray spectroscopy (EDX) of NPCGFs samples were characterized with a JEM-2100 transmission electron microscope (JEOL Ltd). Atomic force microscopy (AFM) images were obtained with a Dimension Edge system with tapping mode. X-ray diffraction (XRD) patterns were recorded with an Ultima IV X-ray diffractometer. The Raman spectrum was obtained on a DXR532 Raman microscope. X-ray photoelectron spectroscopy (XPS) characterizations were conducted using an AXIS Ultra DLD instrument (Kratos, UK). UV-Vis absorption spectra were recorded on a Lambda 950 spectrometer (PE, USA). FT-IR spectra were obtained using a VERTEX 80 V spectrometer (Bruker, Germany) at a resolution of 4 cm−1 in the range of 600–4000 cm−1. The fluorescence (FL) spectra were recorded on a LS55 luminescence spectrometer (PE, USA). The absolute QY and FL lifetime of NPCGFs were obtained using a FL-TCSPC fluorescence spectrophotometer with an excitation wavelength of 360 nm (Horiba Scientific, France). The FL images were recorded using a 710 confocal laser scanning microscope (Zeiss, Germany).Synthesis of nitrogen and phosphorus co-doped graphene-analogous flakes
N and P co-doped graphene-analogous flakes (NPCGFs) were prepared using the following procedures: 0.1 g of β-CD, 0.1 g of oPD, and 0.05 g VPA were added to 5 mL of ultrapure water. After ultrasonic treatment for 10 min, the mixture was transferred into a 25 mL Teflon-lined autoclave and heated at 180 °C for 8 h. The resultant brown solution was naturally cooled to room temperature, and then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min to concentrate the NPCGFs. The NPCGFs exhibited blue photoluminescence under UV light (365 nm).
000 rpm for 15 min to concentrate the NPCGFs. The NPCGFs exhibited blue photoluminescence under UV light (365 nm).
Detection procedure for pH and Qc
Fluorescent measurements were conducted at an excitation wavelength of 360 nm, with excitation and emission slit widths of 15 nm and 3 nm, respectively. For pH detection, the prepared NPCGFs were added to 700 μL phosphate-buffered saline (PBS) solution (100 mM) with different pH values, and the FL spectra were recorded. For Qc detection, in a typical run, 7 μL of the stock solution of NPCGFs was added to 70 μL of PBS solution (100 mM, pH 7.4), followed by the addition of different amounts of Qc. The mixture was then diluted with ultrapure water to 700 μL and thoroughly mixed by vortexing. After incubation for 5 min at room temperature, the solution was transferred for fluorescence measurement.Selectivity investigation
In the selectivity experiment, structural analogues of Qc including rutin, catechol, morin, and quinol, possible interfering compounds such as histidine and cysteine, as well as metal ions (Fe3+, Pb2+, Hg2+, Mg2+, Cu2+, Fe2+, Co2+, and Ag+) were extensively investigated as coexisting substances. The concentration of each analogue was set as 3-fold that of Qc, but the concentration of Fe3+, Pb2+, Hg2+, Mg2+, Cu2+, Fe2+, Co2+, and Ag+ was set as 200-fold that of Qc, and histidine and cysteine were used at a concentration that was 5-fold that of Qc. The same detection conditions mentioned above were applied for the investigation.Cell imaging
Human cervical carcinoma (HeLa) cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin streptomycin at 37 °C under 5% CO2. The cells were pre-cultured with 2 mL of media, and then mixed with 2 mL of NPCGFs at a final concentration of 40 μg mL−1. They were incubated for 4 h, and then washed three times with PBS buffer to remove the residual NPCGFs before cell imaging. Next, different concentrations of Qc were added and the cells were incubated for another 30 min at 37 °C. Finally, the cells were washed three times with PBS buffer to remove the remaining Qc, and cell images were captured using a confocal laser scanning microscope (LSM).Results and discussion
Synthesis and characterization of NPCGFs
Heteroatoms (e.g., N, P, F, B, or S) can effectively tailor the intrinsic properties of CNMs by forming a new surface state to trap excited electrons and increase density.21,22 Therefore, the types of precursors containing doped heteroatoms might be the main reason to determine the optical properties of synthetic CNMs. Among many doped heteroatoms, the important light N and P atoms, having comparable atomic sizes and valence electrons for bonding with carbon atoms, have been widely used for the preparation of CNMs co-doped with N and P. However, until now, considerable research efforts have been focused on the preparation of novel FL nanoprobes consisting of CNMs co-doped with N and P using inorganic P precursors (Table S1†). Research on the preparation of CNMs co-doped with N and P using organic P precursors is still rare. Intuitively, co-doping with N and organic P-containing precursors can alter the optical characteristics of the prepared CNMs and offer more active sites, thus resulting in new phenomena and unique properties.In order to understand the mechanism governing the formation of the NPCGFs, control experiments were conducted, and the products were tested by FL spectra. Based on our previous work, we revealed that the CNMs from pure β-CD emitted nearly no fluorescence. The FL intensity was slightly increased by analysing both comonomers of β-CD and VPA (Fig. S1,† blue line). Interestingly, the FL intensity of NPCGFs at 445 nm clearly increases because of the comonomers of β-CD, VPA, and oPD (Fig. S1,† black line). In order to further reveal the role of oPD, two phenylenediamines isomers, (m-phenylenediamine (mPD) and p-phenylenediamine (pPD), were replaced with oPD to prepare CNMs under the same reaction conditions as those of NPCGFs.
It was found that the synthesized CNMs from mPD and pPD exhibited weak emission at 445 nm compared to the NPCGFs. Moreover, the maximum emission peaks of the products from pPD and mPD were 522 nm with excitation of 440 nm (Fig. S1,† green line), and 536 nm with the excitation of 380 nm (Fig. S1,† red line), respectively. Subsequently, phenylenediamine analogue (o-dihydroxybenzene) was also used to study the effect of chemical composition on the FL of CNMs. The obtained spectrum was significantly different from that of the NPCGFs (Fig. S1,† purple line), which could be due to the synergetic effect of the simultaneous existence of comonomers of β-CD, VPA, and oPD for prepared NPCGFs. The chemical reaction between the different active sites during the synthesis process may have resulted in the different unique arrangement of molecular structure, and ultimately caused the difference in the optical property.
We further verified whether C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in VPA affected the FL feature of the CNMs. Compared with the NPCGFs, the CNMs obtained from phosphoric acid as the phosphorous source have a relative low FL intensity (Fig. S1,† gray line). The reason for this is because the introduction of C
C bonds in VPA affected the FL feature of the CNMs. Compared with the NPCGFs, the CNMs obtained from phosphoric acid as the phosphorous source have a relative low FL intensity (Fig. S1,† gray line). The reason for this is because the introduction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in VPA increased the number of active sites during the reaction, resulting in a stronger structural rigidity of the NPCGFs. All the above results suggested that the relative positions and chemical composition of substituent groups were the principal factors in tuning the FL properties of the CNMs.
C bonds in VPA increased the number of active sites during the reaction, resulting in a stronger structural rigidity of the NPCGFs. All the above results suggested that the relative positions and chemical composition of substituent groups were the principal factors in tuning the FL properties of the CNMs.
Finally, the mass ratio between β-CD and oPD was investigated in parallel reactions, and a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was found to be optimal for the strongest FL (Table S2†). The TEM images (Fig. 1a and b) clearly reveal that the NPCGFs are graphene-analogous structures with irregular shapes and a size distribution between 40 and 70 nm; the average size is approximately 54 nm, which was calculated by measuring several hundred particles. In addition, these nanoparticles are homogeneously dispersed without apparent aggregation. The SAED pattern (Fig. 1c) clearly shows no well-resolved lattice fringes, indicating the amorphous nature of the NPCGFs.23 As shown in Fig. 1d, the EDX spectrum shows four peaks corresponding to C, N, O, and P, respectively. The AFM image (Fig. 1e) reveals a typical topographic height of 0.5–1.5 nm (Fig. 1f), as derived from the topographical height profile (section analysis), demonstrating that the construction of some of the NPCGFs may include a multi-layered structure.
1 was found to be optimal for the strongest FL (Table S2†). The TEM images (Fig. 1a and b) clearly reveal that the NPCGFs are graphene-analogous structures with irregular shapes and a size distribution between 40 and 70 nm; the average size is approximately 54 nm, which was calculated by measuring several hundred particles. In addition, these nanoparticles are homogeneously dispersed without apparent aggregation. The SAED pattern (Fig. 1c) clearly shows no well-resolved lattice fringes, indicating the amorphous nature of the NPCGFs.23 As shown in Fig. 1d, the EDX spectrum shows four peaks corresponding to C, N, O, and P, respectively. The AFM image (Fig. 1e) reveals a typical topographic height of 0.5–1.5 nm (Fig. 1f), as derived from the topographical height profile (section analysis), demonstrating that the construction of some of the NPCGFs may include a multi-layered structure.
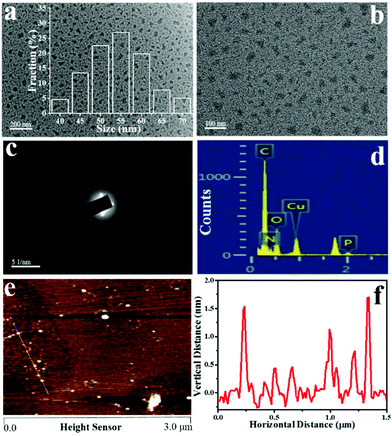 | ||
| Fig. 1 (a, b) TEM images with different magnifications, (c) SAED pattern, (d) EDS spectrum, (e) AFM image, and (f) height distributions of the NPCGFs. | ||
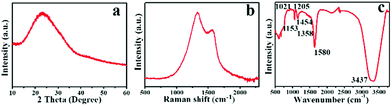
The XRD profile (Fig. 2a) exhibits a broad peak at 2θ = 23.96°, corresponding to a disordered graphitic structure.24 The Raman spectrum (Fig. 2b) contains characteristically wide D and G bands around 1360 and 1590 cm−1, which are typical for amorphous carbons or disordered graphite, respectively. The intensity ratio of the D and G bands is 1.35, indicating the very low degree of graphitization of the resulting hydrothermal carbon material,4 which further demonstrates that the NPCGFs obtained via the hydrothermal carbonization of β-CD in the presence of VPA and oPD are amorphous. This finding is in agreement with the SAED pattern and XRD analysis.
XPS and FT-IR spectroscopy were further performed to study the functional groups and the chemical composition of the NPCGFs. The survey spectrum (Fig. 3a) displays four strong signals from C 1s at 285 eV, O 1s at 531 eV, N 1s at 400 eV, and P 2p at 133 eV, with atomic percentages of 60.53%, 20.35%, 10.49%, and 8.63%, respectively. The high-resolution of C 1s (Fig. 3b) can be well deconvoluted into five surface composites, corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at a binding energy of 284.09 eV, C–C at 284.83 eV, C–N/C–P at 285.87 eV, C–O at 286.73 eV, and C
C at a binding energy of 284.09 eV, C–C at 284.83 eV, C–N/C–P at 285.87 eV, C–O at 286.73 eV, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 287.39 eV.6 The O 1s spectrum (Fig. 3c) shows three peaks at 531.56 eV, 532.17 eV, and 533.59 eV, which were attributed to C
O at 287.39 eV.6 The O 1s spectrum (Fig. 3c) shows three peaks at 531.56 eV, 532.17 eV, and 533.59 eV, which were attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–OH, and C–O–C groups, respectively.25Fig. 3d shows the XPS spectrum of N 1s peaks at 399.10 eV and 400.64 eV, which were attributed to pyrrolic-like N and N–C/N–H, respectively.26 As shown in Fig. 3e, the high resolution of P2p exhibits three peaks at 132.11 eV, 132.84 eV, and 133.75 eV, which were associated with the P–O, P
O, C–OH, and C–O–C groups, respectively.25Fig. 3d shows the XPS spectrum of N 1s peaks at 399.10 eV and 400.64 eV, which were attributed to pyrrolic-like N and N–C/N–H, respectively.26 As shown in Fig. 3e, the high resolution of P2p exhibits three peaks at 132.11 eV, 132.84 eV, and 133.75 eV, which were associated with the P–O, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and P–C/P–N binding energies, respectively.27
O, and P–C/P–N binding energies, respectively.27
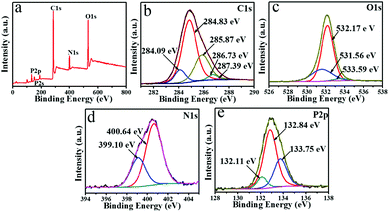 | ||
| Fig. 3 The (a) XPS survey showing the (b) C 1s, (c) O 1s, (d) N 1s, and (e) P 2p spectra of the prepared NPCGFs. | ||
The FT-IR spectrum also exhibits the characteristic absorption bands of the stretching vibration of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1205 cm−1 and P–O at 1021 cm−1 (Fig. 2c), which indicated that the functional monomer (VPA) was successfully incorporated into the NPCGFs.7 The peak at 1635 cm−1 was associated with vibrational absorption of C
O at 1205 cm−1 and P–O at 1021 cm−1 (Fig. 2c), which indicated that the functional monomer (VPA) was successfully incorporated into the NPCGFs.7 The peak at 1635 cm−1 was associated with vibrational absorption of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and the band at 1454 cm−1 refers to the C–N bond.28 The peaks at 1385 cm−1 and 1153 cm−1 were attributed to the C–O stretching vibration and C–O–C stretching vibration.27 The absorption bands of O–H and N–H appeared at 3437 cm−1 and 1580 cm−1, respectively. All these absorption peaks indicate that the NPCGFs were rich in hydroxyl, amine, and phosphate groups on the framework. The above data, including the XPS and FT-IR results, indicated the successful incorporation of P and N into the NPCGF framework.
O, and the band at 1454 cm−1 refers to the C–N bond.28 The peaks at 1385 cm−1 and 1153 cm−1 were attributed to the C–O stretching vibration and C–O–C stretching vibration.27 The absorption bands of O–H and N–H appeared at 3437 cm−1 and 1580 cm−1, respectively. All these absorption peaks indicate that the NPCGFs were rich in hydroxyl, amine, and phosphate groups on the framework. The above data, including the XPS and FT-IR results, indicated the successful incorporation of P and N into the NPCGF framework.
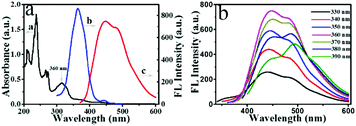
The UV-Vis absorption and FL spectra were characterized to further explore the optical properties of the prepared NPCGFs. The UV-Vis absorption spectrum of the NPCGFs (Fig. 4a, curve a) shows several absorption maxima/bands at approximately 240 nm, 266 nm, 273 nm, 315 nm, 360 nm, and 425 nm. The peaks at 240 nm and 266 nm corresponded to the π–π* transition of the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and n–π transition of C–N, while the peak at 273 nm was attributed to the n–π* transition of the aromatic π system C
C bond and n–π transition of C–N, while the peak at 273 nm was attributed to the n–π* transition of the aromatic π system C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and all these do not typically generate FL.29 The absorption bands at 315 nm, 360 nm, and 425 nm, in the lower-energy region, could be attributed to the trapping of the excited state energy by the surface states, which results in strong fluorescence.30,31 Furthermore, the maximum excitation and emission peaks of the NPCGFs were located at 360 nm and 445 nm (Fig. 4a, curves b and c), respectively, with a stock shift of 85 nm. Similar to the characteristics of CNMs, NPCGFs also exhibited an excitation-dependent emission (Fig. 4b), indicating the multicolour properties of the NPCGFs. The above feature might be attributed to different surface states and sizes of the NPCGFs.27 The absolute quantum yield (QY) of the NPCGFs was 32.49% using water as the solvent (Fig. S2†).
O, and all these do not typically generate FL.29 The absorption bands at 315 nm, 360 nm, and 425 nm, in the lower-energy region, could be attributed to the trapping of the excited state energy by the surface states, which results in strong fluorescence.30,31 Furthermore, the maximum excitation and emission peaks of the NPCGFs were located at 360 nm and 445 nm (Fig. 4a, curves b and c), respectively, with a stock shift of 85 nm. Similar to the characteristics of CNMs, NPCGFs also exhibited an excitation-dependent emission (Fig. 4b), indicating the multicolour properties of the NPCGFs. The above feature might be attributed to different surface states and sizes of the NPCGFs.27 The absolute quantum yield (QY) of the NPCGFs was 32.49% using water as the solvent (Fig. S2†).
The NPCGFs as a pH nanosensor
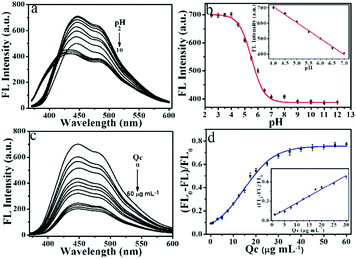
The effect of the irradiation, ionic strength, and temperature on the FL property were studied. There was no obvious variation under continuous UV irradiation (Fig. S3†), at different NaCl concentrations (Fig. S4†) and different temperatures (Table S3†), which suggests the excellent photostability of the NPCGFs. Interestingly, it was found that the NPCGFs are pH-sensitive. As shown in Fig. 5a and b, the FL intensity of the NPCGFs rapidly decreased as the pH increased from 4.0 to 7.0, and nearly remained unchanged under alkaline conditions. This pH-dependent FL may result from protonation and deprotonation of the elementary speciation of nitrogen atoms (pyrrole-like N and amino N) on the surface of the NPCGFs, especially amino N.32 Under acid conditions, the H+ cations and the lone pair of electrons on the nitrogen atoms are attracted together, forming a coordination bond. Hence, we deduced that H+ cations binding with nitrogen atoms (pyridine-like N, amino N, and pyrrole-like N) in the NPCGFs caused the significant FL spectral response to pH. On account of this mechanism, a pH detection sensor was fabricated based on the NPCGFs.Sensitive detection of Qc
To demonstrate a potential application, the NPCGFs were also employed as a FL probe for Qc determination. As shown in Fig. 5c, the FL intensity of NPCGFs decreased with increasing concentration of Qc, indicating that the sensing system was sensitive to the Qc concentration. As shown in Fig. 5d, the FL quenching Stern–Volmer plot does not fit a linear Stern–Volmer equation (eqn (1)):| FL0/FL = 1 + Kc, | (1) |
Until now, many methods have been adopted to detect Qc that show excellent sensitivity and a low limit of detection (LOD), but there are still some limitations consisting of a time-consuming process, multi-step sample preparation, toxic drugs, and/or specialised skills. Fortunately, the NPCGF probe has the advantage of being non-toxic to the environment, and the fabricated sensor also satisfies the requirement of one site and excellent selectivity (Table S4†).
The fluorescence quenching mechanism
Qc, as an unusual quencher, can quench the FL of carbon-based materials through electron or energy transfer.33 Also, it is well-known that Qc is a polyhydroxy-substituted aromatic derivative that can activate a benzene ring, and perhaps serve as a Lewis base to provide a large electron source. As a result, a polymer may be formed between negatively charged Qc and positively charged compounds.To verify this conclusion, the zeta potential was obtained. The zeta potential of the NPCGFs was +8.15 mV (Fig. S5†), which further confirmed the possibility of forming a polymer between Qc and the NPCGFs through electrostatic interactions, resulting in electron or energy transfer and causing FL quenching of the NPCGFs. Thus, Qc was slowly added to the NPCGFs under the excitation state, and the electron or energy, which was transferred in the Qc by the NPCGFs, was back-donated in the electron-deficient NPCGFs, leading to strong electronic interaction34 and thereby decreasing its luminescence intensity.
To further reveal the mechanism of FL quenching, time-resolved FL spectra were measured. As shown in Fig. S6,† the FL spectra of NPCGFs and the mixture of NPCGFs and Qc obey dual exponential decay kinetics, and two lifetimes were acquired, which may be attributed to the differences in the distribution of complex luminescent pathways resulting from multiple unique NPCGFs and/or sites. The average lifetime of NPCGFs in the absence and presence of Qc was approximately 8.72 ns and 7.74 ns, respectively. The relative changes in the FL lifetime of the NPCGFs can be attributed to the increase in the radiative decay rates, indicating that the formation of an extra shell partly through combination with Qc changes the surface states. Additionally, the nonlinear Stern–Volmer plot (Fig. 5d) indicates that the decrease in lifetime of the NPCGFs by Qc obeys a complex quenching mechanism. That is, the NPCGFs, like positively charged cations, coordinate to the negative electron source of the Qc and should act as an electron-deficient compound to bind electron-enriched Qc, and subsequently quench the FL of NPCGFs in the presence of Qc, which might be attributed to an electron- or energy-transfer mechanism.
Detection of Qc in real samples
On account of the complexity of real samples, an excellent sensor should not only possess high sensitivity, but also specific selectivity. As shown in Fig. S7,† some likely coexisting inorganic ions (Fe3+, Pb2+, Hg2+, Mg2+, Cu2+, Fe2+, Co2+, and Ag+) and organic compounds (histidine, cysteine, rutin, catechol, morin, and quinol) were tested to identify the specificity of Qc detection by the NPCGF probe. The results demonstrate that the FL intensity of the NPCGFs did not change when other interfering compounds were separately added, indicating that other interfering compounds have nearly no impact on the detection of Qc.The property of being highly selective enables accurate detection of Qc in water samples. Water collected from the Xuan Wu River was selected as a real sample to reveal the applicability of NPCGFs for Qc detection, and the standard addition method was used. The collected samples were filtered through a 0.22 mm membrane and then centrifuged at 8000 rpm for 15 min. As listed in Table S5,† the recoveries of Qc from samples to which Qc was added were in the range of 98.90–100.18% with RSD of 3.95–4.82%, indicating that the NPCGF probe is reliable for the detection of Qc in real water samples.
Imaging of Qc in living cells
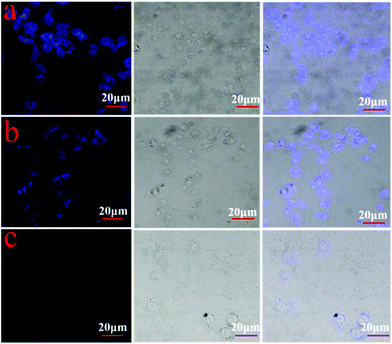
The application of NPCGFs in biological samples was also analyzed using HeLa cancer cells as a model organism to explore Qc detection in living cells. Cells were first incubated with the NPCGFs (40 μg mL−1) for 4 h, and then washed with PBS. As shown in Fig. 6a, HeLa cells showed strong blue emission when excited at 360 nm due to the strong FL from the NPCGFs, which indicates that the NPCGFs penetrated the cell membrane and translocated into cells by endocytosis. However, the intracellular FL intensity became weaker as different concentrations of Qc were added to cells, revealing that Qc diffused into the cell and reacted with the NPCGFs by an electron- or energy-transfer mechanism (Fig. 6b and c). All the above results clearly demonstrated that NPCGFs can be applied to the highly selective visual detection of Qc in living cells.Conclusion
In conclusion, we report the use of organic P precursors to create highly luminescent N and P co-doped CNMs named as NPCGFs, which possess a high QY of 32.49% without any post-treatment. The NPCGFs exhibited excellent photostability, superior aqueous dispersibility, good biocompatibility, and low toxicity. Furthermore, the unique FL properties of the NPCGFs include multifunctional sensing for detection of pH and Qc. A linear range of detection of 0.35–30 μg mL−1 with a Qc detection limit of 0.2 μg mL−1 and pH variation between 4 and 7 were readily realized. By the virtue of these properties, this sensor was further applied to detect Qc in living cells, indicating its potential practical applications for FL cellular imaging.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFF0207804) and the State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (Guangxi Normal University) (CMEMR2017-B06).Notes and references
- X. Y. Xu, R. Ray, Y. L. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS.
- Q. Xu, W. J. Li, L. Ding, W. J. Yang, H. H. Xiao and W. J. Ong, Nanoscale, 2019, 11, 1475–1504 RSC.
- W. N. Yang, H. Zhang, J. X. Lai, X. Y. Peng, Y. P. Hu, W. Gu and L. Ye, Carbon, 2018, 128, 78–85 CrossRef CAS.
- L. Zhang, Z. Y. Zhang, R. P. Liang, Y. H. Li and J. D. Qiu, Anal. Chem., 2014, 86, 4423–4430 CrossRef CAS.
- Z. C. Yang, M. Wang, A. M. Yong, S. Y. Wong, X. H. Zhang, H. Tan, A. Y. Chang, X. Li and J. Wang, Chem. Commun., 2011, 47, 11615–11617 RSC.
- Q. Xu, B. F. Li, Y. C. Ye, W. Cai, W. J. Li, C. Y. Yang, Y. S. Chen, M. Xu, N. Li, X. S. Zheng, J. Street, Y. Luo and L. L. Cai, Nano Res., 2018, 11, 3691–3701 CrossRef CAS.
- Z. X. Wang, Y. F. Gao, X. H. Yu, F. Y. Kong, W. X. Lv and W. Wang, Microchim. Acta, 2018, 185, 422–431 CrossRef.
- A. Beltran, F. Borrull, R. M. Marce and P. A. G. Cormack, TrAC, Trends Anal. Chem., 2010, 29, 1363–1375 CrossRef CAS.
- S. Xu, L. G. Chen and L. Ma, Microchim. Acta, 2018, 185, 492–501 CrossRef.
- Z. Zhang, Y. Miao, L. Lian and G. Yan, Anal. Biochem., 2015, 489, 17–24 CrossRef CAS.
- P. Xiao, F. Zhao and B. Zeng, Microchem. J., 2007, 85, 244–249 CrossRef CAS.
- P. Zuo, D. Xiao, M. Gao, J. Peng, R. Pan, Y. Xia and H. He, Microchim. Acta, 2014, 181, 1309–1316 CrossRef CAS.
- P. C. H. Hollman and I. C. M. Arts, J. Sci. Food Agric., 2000, 80, 1081–1093 CrossRef CAS.
- K. S. Abdelkawy, M. E. Balyshev and F. Elbarbry, Biomed. Chromatogr., 2017, 31, e3819–e3827 CrossRef.
- X. Kan, T. Zhang, M. Zhong and X. Lu, Biosens. Bioelectron., 2016, 77, 638–643 CrossRef CAS.
- Y. Numata and H. Tanaka, Food Chem., 2011, 126, 751–755 CrossRef CAS.
- Z. G. Chen, S. H. Qian, J. H. Chen and X. Chen, J. Nanopart. Res., 2012, 14, 1264–1271 CrossRef.
- Y. Zou, F. Y. Yan, T. C. Zheng, D. C. Shi, F. Z. Sun, N. Yang and L. Chen, Talanta, 2015, 135, 145–148 CrossRef CAS PubMed.
- Z. X. Wang, Y. F. Gao, X. Jin, X. H. Yu, X. Tao, F. Y. Kong, D. H. Fan and W. Wang, Analyst, 2019, 144, 2256–2263 RSC.
- X. Miao, D. Qu, D. X. Yang, B. Nie, Y. K. Zhao and H. Y. Fan, Adv. Mater., 2018, 30, 1704740–1704748 CrossRef PubMed.
- H. Y. Yang, Y. L. Liu, Z. Y. Guo, B. F. Lei, J. L. Zhuang, X. J. Zhang, Z. M. Liu and C. F. Hu, Nat. Commun., 2019, 10, 1789–1800 CrossRef PubMed.
- L. M. Liu, L. Yang, R. S. Li, B. B. Chen, H. Liu and C. Z. Huang, Green Chem., 2017, 19, 3611–3617 RSC.
- L. B. Tang, R. B. Ji, X. K. Cao, J. Y. Lin, H. X. Jiang and X. M. Li, ACS Nano, 2012, 6, 5102–5110 CrossRef CAS PubMed.
- Q. Xu, P. Pu, J. G. Zhao, C. B. Dong, C. Gao, Y. S. Chen, J. R. Chen, Y. Liu and H. J. Zhou, J. Mater. Chem. A, 2015, 3, 542–546 RSC.
- Z. H. Sheng, L. Shao, J. J. Chen, W. J. Bao, F. B. Wang and X. X. Xia, ACS Nano, 2011, 5, 4350–4358 CrossRef CAS.
- J. P. Paraknowitsch, Y. J. Zhang, B. Wienert and A. Thomas, Chem. Commun., 2013, 49, 1208–1210 RSC.
- W. Song, W. X. Duan, Y. H. Liu, Z. J. Ye, Y. L. Chen, H. L. Chen, S. D. Qi, J. Wu, D. Liu, L. H. Xiao, C. L. Ren and X. G. Chen, Anal. Chem., 2017, 89, 13626–13633 CrossRef CAS.
- D. Qu, M. Zheng, P. Du, Y. Zhou, L. Zhang, D. Li, H. Tan, Z. Zhao, Z. Xie and Z. Sun, Nanoscale, 2013, 5, 12272–12277 RSC.
- X. Liu, H. Jiang, J. Ye, C. Zhao, S. Gao, C. Wu, C. Li, J. Li and X. M. Wang, Adv. Funct. Mater., 2016, 26, 8694–8706 CrossRef CAS.
- X. Wang, L. Cao, S. T. Yang, F. Lu, M. J. Meziani, L. Tian, K. W. Sun, M. A. Bloodgood and Y. P. Sun, Angew. Chem., Int. Ed., 2010, 49, 5310–5314 CrossRef CAS PubMed.
- L. L. Pan, S. Sun, L. Zhang, K. Jiang and H. W. Lin, Nanoscale, 2016, 8, 17350–17356 RSC.
- W. F. Niu, L. Fan, M. Nan, Z. B. Li, D. T. Lu, M. S. Wong, S. M. Shuang and C. Dong, Anal. Chem., 2015, 87, 2788–2793 CrossRef CAS PubMed.
- S. J. Xiao, X. J. Zhao, P. P. Hu, Z. J. Chu, C. Z. Huang and L. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 8184–8191 CrossRef CAS PubMed.
- D. Xiao, D. Yuan, H. He and M. Gao, J. Lumin., 2013, 140, 120–125 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9an02077b |
| This journal is © The Royal Society of Chemistry 2020 |