The ICT-based fluorescence and colorimetric dual sensing of endogenous hypochlorite in living cells, bacteria, and zebrafish†
Xiaojun
He‡
a,
Chuchu
Xu‡
b,
Wei
Xiong‡
a,
Yuna
Qian
c,
Jinyi
Fan
b,
Feng
Ding
d,
Hui
Deng
b,
Hong
Chen
*e and
Jianliang
Shen
 *ac
*ac
aSchool of Ophthalmology & Optometry, School of Biomedical Engineering, Wenzhou Medical University, Wenzhou, Zhejiang 325035, China. E-mail: shenjl@wibe.ac.cn
bSchool of Stomatology, Wenzhou Medical University, Wenzhou, 325035, China
cWenzhou Institute, University of Chinese Academy of Sciences, Wenzhou, Zhejiang 325001, China
dDepartment of Microbiology & Immunology, School of Basic Medical Sciences, Wenzhou Medical University, Wenzhou 325035, China
eLuoyang Key Laboratory of Organic Functional Molecules, College of Food and Drug, Luoyang Normal University, Luoyang, 471934, China. E-mail: chenwexpo@sina.com
First published on 12th November 2019
Abstract
This work demonstrates a novel chemosensor, SPTPA, that exhibits fluorescence and colorimetric dual sensing of hypochlorite with an ICT ON strategy. Additionally, the chemosensor successfully detected and imaged endogenous hypochlorite in living cells, various types of bacteria, and zebrafish.
Reactive oxygen species (ROS), which are ubiquitous, are composed of oxygen in the organism and natural environment, and play a key role in physiological and pathological processes.1–4 ROS are a double-edged sword for organisms. When phagocytic cells are stimulated, a large amount of active oxygen is produced through the mechanism of respiratory burst, which is the dominant factor for phagocytic cells to exert phagocytosis and killing.5,6 Moreover, ROS could be used as a strong oxidant for disinfection, and has a wide range of microbicidal effects on bacteria, spores, viruses, and fungi.7–10 Recently, with the advancement of science and technology, there have been new developments in the application of ROS for disinfection.11–13 However, with the high content of oxygen, the organism becomes damaged at the cellular level, which is mainly caused by active oxygen.14,15 ROS also produce aging substances that damage genetic factors in excess, which initiates most diseases and aging.16–18 In addition, these materials have strong chemical reactivity and short life, which is still a global issue for their monitoring, and lack a detection method with high specificity and high sensitivity.19
Hypochlorous acid (HClO) and hypochlorite (ClO−) are ROS that are widely used as strong oxidants in our daily lives.20–22 In organisms, (HClO/ClO−) is related to the host defense system and plays a key role in combating multiple pathogens.23,24 Hence, maintaining an appropriate level of HClO/ClO− is quite important for many cellular functions. However, preternatural overproduction of HClO/ClO− is related to certain diseases, such as atherosclerosis and cancer.25,26 Consequently, the detection of HClO/ClO− has attracted wide interest, especially with fluorescence imaging techniques because of their high sensitivity, high selectivity, fast response rate, and ease of handling. Hitherto, various fluorescent probes for the detection of HClO/ClO− have been reported and employed for imaging of HClO/ClO− at cellular and tissue levels−.27–33 However, most probes may encounter problems such as poor solubility in water, weak ability to combat interference from other ROS, indistinctive Stokes shifts, and short emission wavelengths. Furthermore, the detection of exogenous HClO/ClO− has little significance in investigating subcellular structures, microorganisms, and organisms.34–36 Therefore, there is an urgent need to develop new probes with high sensitivity, fast response, and excellent biocompatibility for endogenous HClO/ClO− detection.
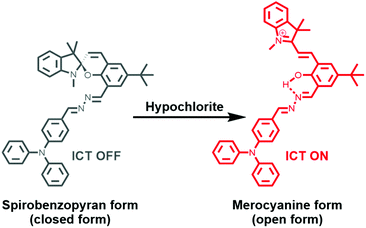
Here, we report a chemosensor, SPTPA, based on spiropyran and triphenylamine, and explored its fluorescence sensing of various ROS. Spiropyran was selected as the substance that fluoresces because it has a long emission wavelength and a large Stokes shift. Moreover, there is significant variation in the absorption and emission of spiropyran in the closed form and open form, which can greatly improve the detection efficiency. The chemosensors were prepared, and they successfully responded to hypochlorite with the intramolecular charge transfer (ICT) mechanism (Scheme 1).
To endow the synthetic host with a high selectivity and recognition ability for hypochlorite, we envisaged the structure of the multifunctional ligand triphenylamine as a bridge to the spiropyran through the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N
N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) group. As a donor center, the triphenylamine group has unique radical characteristics and large steric hindrance, which resulted in a super-conjugated electron effect for the entire molecular structure.37 Moreover, the spiropyran building block has a strong ability for discoloration, which could produce unique luminescence between the spirobenzopyran form (closed form) and the merocyanine form (open form). Additionally, spiropyran is a material that can effectively change color and has potential application in light-controlled switches and bio-imaging. Therefore, the process of light regulation is carried out by the “closing” and “opening” of the spiropyran ring.
group. As a donor center, the triphenylamine group has unique radical characteristics and large steric hindrance, which resulted in a super-conjugated electron effect for the entire molecular structure.37 Moreover, the spiropyran building block has a strong ability for discoloration, which could produce unique luminescence between the spirobenzopyran form (closed form) and the merocyanine form (open form). Additionally, spiropyran is a material that can effectively change color and has potential application in light-controlled switches and bio-imaging. Therefore, the process of light regulation is carried out by the “closing” and “opening” of the spiropyran ring.
To this end, we designed a small molecular structure of SPTPA, obtained the triphenylamine group and spiropyran through the N–N bond bridge (Scheme S1†), and characterized the small molecule by 1H NMR, 13C NMR, and mass spectrometry to confirm its molecular structure (Fig. S1–S6†). As expected, SPTPA has a strong ability to recognize hypochlorite. A yellow solution resulted when SPTPA was in the closed form without any fluorescence signal, and it changed into a deep red solution with a significant red fluorescence signal after responding to hypochlorite.
The absorption and fluorescence spectra of the SPTPA probe with and without ClO− are shown in Fig. S7,† where free SPTPA exhibits a sharp peak at 298 nm and 408 nm, and no emission signal with excitation at 408 nm. However, the reaction solution of SPTPA with ClO− shows absorption at 298, 371, and 530 nm, accompanied by a conspicuous color change from yellow to deep red (see the insets) and a prominent fluorescence enhancement at 605 nm. This apparent difference in the spectral signal is very advantageous for sensitive assay. The molecular recognition behavior of the SPTPA probe toward ClO− was confirmed by absorption and fluorescence spectroscopy. The absorption spectrum of the SPTPA probe (10 μM) in dimethylsulfoxide (DMSO)/H2O (v/v, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was obtained.
1) was obtained.
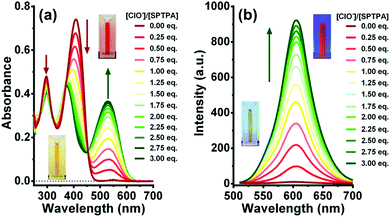
Upon addition of ClO− (0–30 μM) to the solution of SPTPA, the absorption band at 408 nm apparently decreased and a new redshifted band appeared at 530 nm with an isosbestic point at 454 nm (Fig. 1a). The colour of the solution changed from yellow to deep red, allowing colorimetric detection of hypochlorite in DMSO/H2O by the naked eye (inset). The fluorescence spectrum of SPTPA (2.0 μM) does not exhibit any emission when excited at 408 nm. This non-emissive nature of SPTPA is due to non-radiative deactivation through the spirobenzopyran form.38 Upon addition of ClO− to the solution of SPTPA, an emission band at 605 nm is observed (Fig. 1b), the fluorescence intensity of which significantly increases with increased concentration of ClO− (0–6 μM). The formation of an emission band at 605 nm is due to the intramolecular charge transfer (ICT) between the oxhydryl and nitrogen. When protons are free and near oxygen and nitrogen atoms, the ICT occurred, which results in an emission band at 605 nm when excited at 408 nm, leading to a tremendous Stokes shift (197 nm) with a calculated limit of detection (LOD) of 64.2 nM (Fig. S8†).
In order to further understand the molecular structure of the probe and the sensing mechanism of fluorescence and absorption spectroscopy, the theoretical calculation of SPTPA before and after the coordination of ClO− was carried out to obtain insight into their electronic transition using the density functional theory (DFT) method.39 As shown in Fig. 2, before and after the reaction of SPTPA with ClO−, molecular orbital diagrams are presented, and the optimized structures visualized show that SPTPA can recognize ClO− with C–O–C to form an –OH group, which is consistent with the results of the above spectral experiments. Additionally, the electron atmosphere of SPTPA at the ground state was mainly located in the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N
N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) group of the molecular structure, but it was transferred and dispersed to the entire conjugated system and surrounding atoms upon excitation, which indicated a moderate intramolecular charge transfer (ICT) effect in the molecule. Additionally, the energy levels of the closed/open form were 0.092 eV/0.084 eV from the level of HOMO (−0.150/−0.195 eV) to LUMO (−0.058/−0.111 eV), and the other orbital diagrams of the closed/open form are shown in Fig. S9.† It is noteworthy that the end-capped spirobenzopyran ring was non-coplanar with the other part of the molecule, and there was participation in electron rearrangement, indicating that it had an influence on the charge transfer effect.
group of the molecular structure, but it was transferred and dispersed to the entire conjugated system and surrounding atoms upon excitation, which indicated a moderate intramolecular charge transfer (ICT) effect in the molecule. Additionally, the energy levels of the closed/open form were 0.092 eV/0.084 eV from the level of HOMO (−0.150/−0.195 eV) to LUMO (−0.058/−0.111 eV), and the other orbital diagrams of the closed/open form are shown in Fig. S9.† It is noteworthy that the end-capped spirobenzopyran ring was non-coplanar with the other part of the molecule, and there was participation in electron rearrangement, indicating that it had an influence on the charge transfer effect.
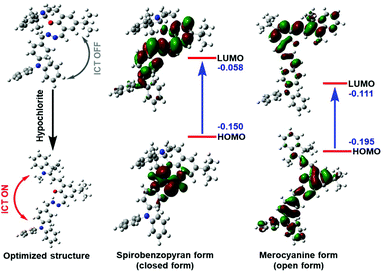 | ||
| Fig. 2 Proposed mechanism showing how SPTPA senses hypochlorite, and frontier orbital energy representations of the spirobenzopyran form (closed form) and merocyanine form (open form). | ||
To explore whether the SPTPA probe could specifically recognize ClO− over other ROS and reactive nitrogen species (RNS) including F−, Cl−, NO2−, ClO4−, HCO3−, H2PO4−, SO42−, S2O32−, CO3−, H2O2, I−, O2, NO3−, S2−, P2O72−, P3O105−, NO˙, 1O2, ˙OH, O2−, ONOO−, and TBHP, various ROS and RNS were added to a solution of SPTPA (2.0 μM) in DMSO/H2O. In Fig. S10a,† it is shown that the fluorescent signal of the solution of SPTPA was very weak with λex at 408 nm. With the addition of 6.0 μM ClO−, a prominent fluorescent signal with λem at 605 nm was induced. Moreover, the fluorescence intensity at 605 nm was significantly enhanced only when ClO− was added (Fig. S10b and 10c†) in the midst of other ROS species, and the photographic changes in the fluorescence of SPTPA with various cations were also visualized with a UV lamp at 365 nm, as shown in the Fig. S6b† insert. The above results revealed that high sensitivity of SPTPA toward ClO− could be used for specific ClO− detection under physiological conditions.
To apply sensors in complicated systems, such as a living organism and the natural environment, the fluorescence signal profile of the curve showed remarkable and rapid enhancement with the addition of ClO− (Fig. S11a†). As time passed, the fluorescence signal of SPTPA towards ClO− increased over a period of approximately 50 s, and then remained unchanged for approximately 3 min. In addition, the fluorescence signal of SPTPA was examined over a wide pH range with or without ClO−, as shown in Fig. S11b.† Free SPTPA exhibited a weak and changeless fluorescence signal at pH from 2.0 to 9.0, and the signal increased at pH from 9.0 to 12.0. However, upon addition of ClO−, the fluorescence signal of SPTPA remarkably increased and stabilized in wide pH values from 2.0 to 12.0. Collectively, the results proved the time-dependency and pH stability of SPTPA for a wide range of potential biological applications. Spiropyran is normally sensitive to light, which will initiate the opening of the spiropyran ring. As shown in Fig. S12,† the fluorescence signal of SPTPA barely changed with 1 min irradiation with different light sources, which was due to the large spatial structure of the triphenylamine group that further stabilized the molecular skeleton. The results indicate that SPTPA has good stability without being affected by light irradiation.
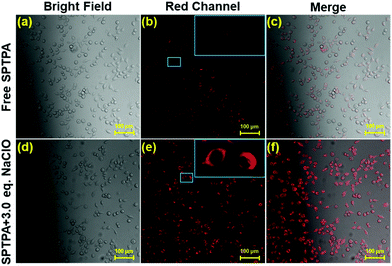
To explore the potential biological usability of SPTPA for the subcellular labeling and detection of ClO− in living cells, cell bioimaging was executed in fetal lung fibroblast cell line MRC-5. First, the cytotoxicity of SPTPA was evaluated by traditional MTT assay,40 which indicated that approximately 80% of cells still remained alive even though 80 μM SPTPA was normalized for 24 h (Fig. S13†), revealing that SPTPA has low cytotoxicity and can be further used for cell imaging experiments. Subsequently, to demonstrate the capability of this probe to sense endogenous ClO− in cells, we performed an imaging assay with negative and positive controls to obtain powerful evidence. Negligible fluorescence was found in the red channel when the cells were pretreated with N-acetylcysteine (NAC), which inhibited production of endogenous ClO− (Fig. S15†). In contrast, a visible fluorescence signal was observed when the cells were incubated with free SPTPA, as shown in Fig. 3. These pretreated cells were subsequently incubated with SPTPA for 20 min, and remarkable fluorescence signals in the red channel were immediately observed with the addition of ClO− (6.0 μM). These results clearly indicate that the SPTPA probe meets the requirements for the monitoring of exogenous ClO− in living cells.
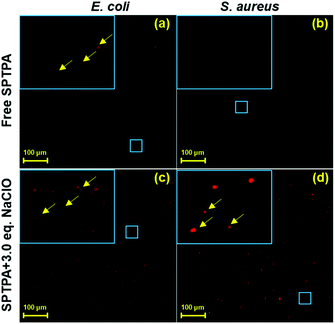
ROS are closely related to the aerobic metabolism and the formation of enzymes in bacteria, and the enzyme-catalyzed reaction is mainly carried out on the cell membrane,41,42 Therefore, the focus shifted to the membrane of bacteria, where ROS is produced. Recently, it has been reported that ROS have a direct relationship with the growth of bacteria, and there is great significance in exploring the content of ROS in bacteria for the development of new antibacterial drugs, clinical medicine, and food safety. Hence, fluorescence monitoring of ROS (ClO−) in bacteria would be useful to obtain insight into this antimicrobial mechanism. To explore its application further, SPTPA were employed to sense ClO− in Escherichia coli and Staphylococcus aureus bacteria using fluorescence bioimaging. As illustrated in Fig. 4, E. coli and S. aureus stained by 2.0 μM SPTPA alone showed minimal fluorescence in the red channel in E. coli and no fluorescence in S. aureus. With the addition of ClO−, the fluorescence of both E. coli and S. aureus in the red channel became more apparent, and the “rhabditiform” and “spherical” shape of E. coli and S. aureus, respectively, could be clearly visualized from the fluorescent pattern in the enlarged area. These results proved that SPTPA could be applied in tracing ClO− in E. coli and S. aureus, which could be used as a potential tool to study the biotransformation of ClO− in bacteria.
In normal metabolic processes, organs of an organism could spontaneously produce various ROS and RNS. Thus far, the majority of reported studies demonstrated the possibility of fluorescence imaging of ROS either by introducing additional ROS via feeding/injecting or by the addition of ROS-induced chemicals into the organism in advance. However, bioimaging of spontaneous ROS by fluorescent probes during normal organisms is still limited by the sensitivity and stability of the probe and its ability to pass various biological barriers. Numerous biological processes are produced in the purtenance of living animals, causing ROS (ClO−) to self-generate there. Therefore, we used five-day-old zebrafish, a popular vertebrate model, to investigate the potential biological application of SPTPA responding to endogenous and exogenous ClO−.
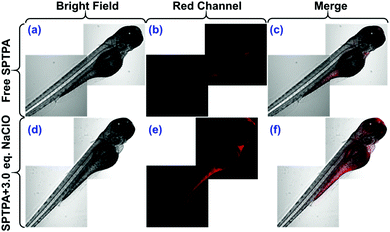
Fig. 5 shows that when the zebrafish were cultured in embryo medium and incubated with free SPTPA probe (2.0 μM) for 20 min to allow SPTPA to permeate into all the tissues of the zebrafish, they exhibited a weak but visible red fluorescence in their abdominal area. This indicated that the probe responded to endogenous ClO− by emitting fluorescence. There was almost no fluorescence found in the red channel when the zebrafish were pretreated with N-acetylcysteine, which inhibits production of endogenous ClO− (Fig. S16†). In addition, when the zebrafish were pretreated with SPTPA and washed three times with phosphate-buffered saline (PBS), and then further incubated with ClO− (6.0 μM), they displayed remarkable red fluorescence in the head and abdomen. Thus, these results convincingly indicated that SPTPA possesses high tissue penetration capacity and could be successfully used to visualize ClO− in zebrafish.
In summary, a novel fluorescence probe, SPTPA, was designed and synthesized, and it functions as a highly hypochlorite-selective sensor based on an “ICT ON” strategy. SPTPA exhibits excellent spirobenzopyran/merocyanine form conversion properties as a hypochlorite-specific fluorescence probe over other ROS and RNS species with high selectivity and sensitivity. Moreover, we successfully used SPTPA to monitor and image exogenous and endogenous hypochlorite in living cells, various types of bacteria, and zebrafish with low cytotoxicity and good permeability. Therefore, we expect that SPTPA will be a potentially powerful biological tool for investigations into the functions of hypochlorite.
Live subject statement
All animal manipulations (fluorescence imaging in zebrafish) were performed in accordance with the guidelines for laboratory animal care and use at Wenzhou Medical University and approved by the Animal Ethics Committee of Wenzhou Medical University.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31800833 and 21977081), the Zhejiang Provincial Natural Science of Foundation of China (Z19H180001), and the Wenzhou Medical University and Wenzhou Institute of Biomaterials & Engineering (WIBEZD2017001-03).Notes and references
- T. Finkel and N. J. Holbrook, Nature, 2000, 408, 239–247 CrossRef CAS PubMed.
- C. C. Winterbourn, Nat. Chem. Biol., 2008, 4, 278–286 CrossRef CAS PubMed.
- F.-J. Huo, J.-J. Zhang, Y.-T. Yang, J.-B. Chao, C.-X. Yin, Y.-B. Zhang and T.-G. Chen, Sens. Actuators, B, 2012, 166, 44–49 CrossRef.
- J. Kang, F. Huo, Y. Zhang, J. Chao, R. M. Strongin and C. Yin, Sens. Actuators, B, 2018, 273, 1532–1538 CrossRef CAS.
- J. Geng, X. Sun, P. Wang, S. Zhang, X. Wang, H. Wu, L. Hong, C. Xie, X. Li, H. Zhao, Q. Liu, M. Jiang, Q. Chen, J. Zhang, Y. Li, S. Song, H. R. Wang, R. Zhou, R. L. Johnson, K. Y. Chien, S. C. Lin, J. Han, J. Avruch, L. Chen and D. Zhou, Nat. Immunol., 2015, 16, 1142–1152 CrossRef CAS PubMed.
- T. C. Morais, L. C. de Abreu, O. B. de Quental, R. S. Pessoa, M. Fujimori, B. E. G. Daboin, E. L. Franca and A. C. Honorio-Franca, Cells, 2019, 8(6), 519 CrossRef PubMed.
- Q. Shen, W. Zhou, H. Li, L. Hu and H. Mo, PLoS One, 2016, 11, e0155647 CrossRef PubMed.
- B. Ezraty, A. Gennaris, F. Barras and J. F. Collet, Nat. Rev. Microbiol., 2017, 15, 385–396 CrossRef CAS PubMed.
- J. Jansen, K. Jansen, E. Neven, R. Poesen, A. Othman, A. van Mil, J. Sluijter, J. Sastre Torano, E. A. Zaal, C. R. Berkers, D. Esser, H. J. Wichers, K. van Ede, M. van Duursen, S. Burtey, M. C. Verhaar, B. Meijers and R. Masereeuw, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 16105–16110 CrossRef CAS PubMed.
- M. Schulte, K. Frick, E. Gnandt, S. Jurkovic, S. Burschel, R. Labatzke, K. Aierstock, D. Fiegen, D. Wohlwend, S. Gerhardt, O. Einsle and T. Friedrich, Nat. Commun., 2019, 10, 2551 CrossRef PubMed.
- R. Mittler, Trends Plant Sci., 2017, 22, 11–19 CrossRef CAS PubMed.
- J. N. Moloney and T. G. Cotter, Semin. Cell Dev. Biol., 2018, 80, 50–64 CrossRef CAS PubMed.
- Y. Huang, Y. Zhang, F. Huo, J. Chao and C. Yin, Sens. Actuators, B, 2019, 287, 453–458 CrossRef CAS.
- G. Zerbi, A. Barbon, R. Bengalli, A. Lucotti, T. Catelani, F. Tampieri, M. Gualtieri, M. D'Arienzo, F. Morazzoni and M. Camatini, Nanoscale, 2017, 9, 13640–13650 RSC.
- O. G. Lyublinskaya, J. S. Ivanova, N. A. Pugovkina, I. V. Kozhukharova, Z. V. Kovaleva, A. N. Shatrova, N. D. Aksenov, V. V. Zenin, Y. A. Kaulin, I. A. Gamaley and N. N. Nikolsky, Redox Biol., 2017, 12, 758–769 CrossRef CAS PubMed.
- P. Davalli, T. Mitic, A. Caporali, A. Lauriola and D. D'Arca, Oxid. Med. Cell. Longevity, 2016, 2016, 3565127 Search PubMed.
- A. Umeno, V. Biju and Y. Yoshida, Free Radical Res., 2017, 51, 413–427 CrossRef CAS PubMed.
- K. Xiong, F. Huo, Y. Zhang, Y. Wen and C. Yin, Anal. Methods, 2019, 11, 1751–1756 RSC.
- K. Xiong, F. Huo, C. Yin, Y. Chu, Y. Yang, J. Chao and A. Zheng, Sens. Actuators, B, 2016, 224, 307–314 CrossRef CAS.
- Q. Wu, Z. He, X. Wang, Q. Zhang, Q. Wei, S. Ma, C. Ma, J. Li and Q. Wang, Nat. Commun., 2019, 10, 240 CrossRef PubMed.
- Y. Yu, Y. Cui, L. J. Niedernhofer and Y. Wang, Chem. Res. Toxicol., 2016, 29, 2008–2039 Search PubMed.
- Y. Yue, F. Huo, C. Yin, J. Chao, Y. Zhang and X. Wei, RSC Adv., 2015, 5, 77670–77672 RSC.
- L. A. C. Carvalho, J. Lopes, G. H. Kaihami, R. P. Silva, A. Bruni-Cardoso, R. L. Baldini and F. C. Meotti, Redox Biol., 2018, 16, 179–188 CrossRef CAS PubMed.
- Y. Yue, F. Huo, C. Yin, J. O. Escobedo and R. M. Strongin, Analyst, 2016, 141, 1859–1873 RSC.
- U. Forstermann, N. Xia and H. Li, Circ. Res., 2017, 120, 713–735 CrossRef PubMed.
- M. Smith, R. Hunter, N. Stellenboom, D. A. Kusza, M. I. Parker, A. N. Hammouda, G. Jackson and C. H. Kaschula, Biochim. Biophys. Acta, 2016, 1860, 1439–1449 CrossRef CAS PubMed.
- L. Wu, I. C. Wu, C. C. DuFort, M. A. Carlson, X. Wu, L. Chen, C. T. Kuo, Y. Qin, J. Yu, S. R. Hingorani and D. T. Chiu, J. Am. Chem. Soc., 2017, 139, 6911–6918 CrossRef CAS PubMed.
- H. Zhu, Z. Zhang, S. Long, J. Du, J. Fan and X. Peng, Nat. Protoc., 2018, 13, 2348–2361 CrossRef CAS PubMed.
- Y. L. Pak, S. J. Park, D. Wu, B. Cheon, H. M. Kim, J. Bouffard and J. Yoon, Angew. Chem., Int. Ed., 2018, 57, 1567–1571 CrossRef CAS PubMed.
- D. Shi, S. Chen, B. Dong, Y. Zhang, C. Sheng, T. D. James and Y. Guo, Chem. Sci., 2019, 10, 3715–3722 RSC.
- C. Zhang, Q. Nie, I. Ismail, Z. Xi and L. Yi, Chem. Commun., 2018, 54, 3835–3838 RSC.
- J. Kang, F. Huo, Y. Yue, Y. Wen, J. Chao, Y. Zhang and C. Yin, Dyes Pigm., 2017, 136, 852–858 CrossRef CAS.
- J. Li, P. Li, F. Huo, C. Yin, T. Liu, J. Chao and Y. Zhang, Dyes Pigm., 2016, 130, 209–215 CrossRef CAS.
- Y. Yang, F. Huo, C. Yin, M. Xu, Y. Hu, J. Chao, Y. Zhang, T. E. Glass and J. Yoon, J. Mater. Chem. B, 2016, 4, 5101–5104 RSC.
- F. Huo, Y. Zhang and C. Yin, Sens. Actuators, B, 2018, 269, 180–188 CrossRef.
- K. Xiong, F. Huo, Y. Zhang, Y. Wen, J. Chao and C. Yin, Sens. Actuators, B, 2018, 255, 2378–2383 CrossRef CAS.
- N. Shao, J. Y. Jin, H. Wang, Y. Zhang, R. H. Yang and W. H. Chan, Anal. Chem., 2008, 80, 3466–3475 CrossRef CAS PubMed.
- S. Goswami, A. K. Das, A. K. Maity, A. Manna, K. Aich, S. Maity, P. Saha and T. K. Mandal, Dalton Trans., 2014, 43, 231–239 RSC.
- X. He, C. Wu, Y. Qian, Y. Li, F. Ding, Z. Zhou and J. Shen, Talanta, 2019, 205, 120118 CrossRef CAS PubMed.
- A. van Tonder, A. M. Joubert and A. D. Cromarty, BMC Res. Notes, 2015, 8, 47 CrossRef PubMed.
- A. T. Dharmaraja, J. Med. Chem., 2017, 60, 3221–3240 CrossRef CAS PubMed.
- M. L. Odyniec, A. C. Sedgwick, A. H. Swan, M. Weber, T. M. S. Tang, J. E. Gardiner, M. Zhang, Y. B. Jiang, G. Kociok-Kohn, R. B. P. Elmes, S. D. Bull, X. P. He and T. D. James, Chem. Commun., 2018, 54, 8466–8469 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9an02226k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |